High-Temperature Solid Lubricants and Self-Lubricating Composites: A Critical Review
Abstract
1. Introduction
2. Mechanisms of Solid Lubrication
3. Characteristics of Solid Lubricant Materials
4. Classification of Solid Lubricants
4.1. Polytetrafluoroethylene (PTFE) and Polyimides
4.2. Soft Metals
4.3. Layer Lattice Solid Lubricants
4.3.1. Graphite
4.3.2. Hexagonal Boron Nitride (h-BN)
4.3.3. Transition Metal Dichalcogenides
4.3.4. Layered MAX Phase Materials
4.4. Chemically Stable Fluorides
4.5. Binary Metallic Oxides
4.6. Ternary Metallic Oxides
4.6.1. Molybdates
4.6.2. Tungstates
4.6.3. Vanadates
4.6.4. Tantalates
4.6.5. Alkaline Earth Metallic Chromates
4.7. Alkaline Earth Metallic Sulfates
4.8. Silicates
4.9. Cesium Oxythiomolybdate Cs2MoOS3
4.10. Synergistic Effects of Various Solid Lubricants
5. Production Technologies of Self-Lubricating Composites and Coatings
5.1. Self-Lubricating Coatings by Physical Vapor Deposition
5.2. Self-Lubricating Coatings by Thermal Spraying
5.3. Self-Lubricating Composites by Powder Metallurgy
5.4. Self-Lubricating Coatings by Electrodeposition
6. Challenges Highlighted in High Temperature Solid Lubrication Applications
6.1. Bearings for Advanced Propulsion Systems
6.2. Seals Components for Advanced Propulsion Systems
6.3. Hot Metal Forming Process
6.4. High-Speed Dry Machining
6.5. Electric Contacts for Electric Railways and Transmission in Space
6.6. Refractory Side Dams for Thin-Strip Steel Casting Process
7. Conclusions
- (1)
- The noble metals such as Ag and Au offer good lubricity due to enhanced ductility and plastic deformation over a wide temperature range. The polymer composites containing PTFE or polyimides provide lubrication with the lowest temperature capacity, up from 300 to 350 °C.
- (2)
- MoS2/WS2 are able to form a transfer film and generate excellent lubrication in a vacuum and dry N2, while a graphite-like transfer film from graphite and DLC provides lubrication in moist air. Layer-lattice solid lubricants such as graphite, MoS2, and graphite fluoride generate structural degradation such as oxidation or dissociation at certain temperatures, as well as the complex chalcogenides of Cs2MoOS3, Cs2WOS3, and ZnMoOS3.
- (3)
- CaF2 and BaF2/CaF2 eutectic are chemically stable non-layered inorganic compounds under oxidizing environments, which exhibit low shear strength and easy film-forming ability to provide good lubricity from 500 to 900 °C.
- (4)
- Alkaline earth chromates of BaCrO4 and BaCr2O4, and sulfates of BaSO4 and SrSO4 and their solid solutions show very good thermal stability and exceptional promise for lubricity over a wide temperature range.
- (5)
- For extreme temperature circumstances, oxide lubrication is the focus of future studies. A new approach to solving low-temperature brittleness in oxide lubrication is to reduce their grain size to a few nanometers. In this case, plastic deformation in large part results from grain boundary sliding or grain rotating and only a minor contribution is associated with dislocation activity in ultrafine grains.
- (6)
- Self-lubricating composites/coatings have been developed by a variety of material preparation techniques, which include powder metallurgy, physical/chemical vapor depositions, thermal spraying, electrodeposition, laser cladding, and additive manufacturing.
- (7)
- Synergistic effects of different solid lubricants are widely explored for humidity-, temperature-, vacuum- or load-adaptive tribological applications. The underlying adaptive mechanisms are associated with environmental-assisted oxidation or interfacial tribo-reaction to form easy-to-shear and low-melting-point binary and ternary compounds, temperature-activated diffusion or melting of soft metals, and thermo-mechanically induced softening or surface self-glazing.
- (8)
- The challenge associated with wide-range solid lubrication is the reversibility of the humidity-, temperature-, vacuum-, or load-adaptive tribological surfaces over multiple thermal cycles occurring in various engineering applications. Various approaches are postulated for adaptive multilayered coatings and surface multifunctional design, such as bionic compositing, tunable surface texturing, and tribo-reaction of oriented lubricants. Temperature-adaptive composites/coatings exhibiting diffusion-, melting-, oxidation-, or triboreaction-limiting lubrication are developed over multiple thermal cycles through microlaminate architectures to activate the functionality of various solid lubricants.
- (9)
- Adaptive solid lubrication design that can operate on earth and in space from room temperature to 1000 °C or even higher would be considered a breakthrough, which would increase air and space vehicle lifetime and performance. The challenges in high-temperature solid lubrication applications such as sliding and rolling contact bearings, seal components in advanced propulsion systems, hot-metal forming, high-speed dry machining, pantograph contact strips for electric railways, and side dams for thin-strip steel casting are highlighted. Microstructurally engineered combinations of solid lubricants will be of significant importance for the development of advanced lubrication systems under extreme environments of low/high temperature, high pressure, high chemical reactivity, and ultrahigh vacuum.
Author Contributions
Funding
Conflicts of Interest
References
- Sliney, H.E. Solid lubricant materials for high-temperatures: A review. Tribol. Int. 1982, 15, 303–315. [Google Scholar] [CrossRef]
- Torres, H.; Ripoll, M.R.; Prakash, B. Tribological behavior of self-lubricating materials at high temperatures. Int. Mater. Rev. 2018, 63, 309–340. [Google Scholar] [CrossRef]
- Muratore, C.; Voevodin, A.A. Chameleon coatings: Adaptive surfaces to reduce friction and wear in extreme environments. Ann. Rev. Mater. Res. 2009, 39, 297–324. [Google Scholar] [CrossRef]
- Zhu, S.; Cheng, J.; Qiao, Z.; Yang, J. High temperature solid-lubricating materials: A review. Tribol. Int. 2019, 133, 206–223. [Google Scholar] [CrossRef]
- John, M.; Menezes, P.L. Self-lubricating materials for extreme condition applications. Materials 2021, 14, 5588. [Google Scholar] [CrossRef]
- Rosado, L.; Forster, N.H.; Trivedi, H.K.; King, J.P. Solid lubrication of silicon nitride with cesium-based compounds: Part I rolling contact endurance, friction and wear. Tribol. Trans. 2000, 43, 489–497. [Google Scholar] [CrossRef]
- Dellacorte, C.; Fellenstein, J.A.; Benoy, P.A. Evaluation of advanced solid lubricant coatings for foil air bearings operating at 25 °C and 500 °C. Tribol. Trans. 1999, 42, 338–342. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Luster, B.; Kohli, P.; Muratore, C.; Voevodin, A.A. Progress in the development of adaptive nitride-based coatings for high temperature tribological application. Surf. Coat. Technol. 2009, 204, 962–968. [Google Scholar] [CrossRef]
- Skopp, A.; Woydt, M. Ceramic and ceramic composite materials with improved friction and wear properties. Tribol. Trans. 1995, 38, 233–242. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal-chemical approach to lubrication by sloid oxides. Tribol. Lett. 2000, 8, 97–102. [Google Scholar] [CrossRef]
- Allam, I.M. Solid lubricants for applications at elevated temperatures. J. Mater. Sci. 1991, 26, 3977–3984. [Google Scholar] [CrossRef]
- Dellacorte, C.; Steinetz, B. Tribological evaluation of an Al2O3-SiO2 ceramic fiber candidate for high temperature sliding seals. Tribol. Trans. 1994, 37, 369–377. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.B.; Liu, Y.F.; Luo, Y.S.; Meng, Y. Microstructure and tribological performance of Ni60-based composite coatings on Ti6Al4V alloy with different Ti3SiC2 ceramic additions by laser cladding. Ceram. Int. 2020, 46, 28996–29010. [Google Scholar] [CrossRef]
- Chen, J.F.; Zhao, W.J. Simple method for preparing nanometer thick Ti3C2TX sheets towards highly efficient lubrication and wear resistance. Tribol. Trans. 2021, 153, 106598. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Umeda, K. The friction and wear characteristics of plasma-sprayed ZrO2-BaCrO4 coating at elevated temperatures. Surf. Coat. Technol. 2002, 154, 131–139. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Umeda, K. Low-pressure plasma-sprayed ZrO2-CaF2 composite coating for high temperature tribological applications. Surf. Coat. Technol. 2001, 137, 21–30. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Umeda, K. Effects of different additives on microstructure and high temperature tribological properties of plasma-sprayed Cr2O3 ceramic coatings. Wear 2001, 249, 56–66. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Umeda, K. The friction and wear characteristics of plasma-sprayed ZrO2-Cr2O3-CaF2 from room temperature to 800 °C. J. Mater. Sci. 2001, 36, 547–555. [Google Scholar] [CrossRef]
- Bhushan, B. Principles and Applications of Tribology; John Wiley & Sons, Ltd.: New York, NY, USA, 2013. [Google Scholar]
- Kumar, R.; Hussainova, I.; Rahmani, R.; Antonov, M. Solid lubrication at high-temperature—A review. Materials 2022, 15, 1695. [Google Scholar] [CrossRef]
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology, 3rd ed.; Elsevier Butterworth-Heinemann: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Ouyang, J.H.; Murakami, T.; Sasaki, S. High-temperature tribological properties of a cathodic arc ion-plated (V,Ti)N coating. Wear 2007, 263, 1347–1353. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal chemical approach to the formulation of self-lubricating nanocomposite coatings. Surf. Coat. Technol. 2005, 200, 1792–1796. [Google Scholar] [CrossRef]
- Gulbinski, W.; Suszko, T. Thin films of Mo2N/Ag nanocomposite- the structure, mechanical and tribological properties. Surf. Coat. Technol. 2006, 201, 1469–1476. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Paudel, Y.; Voevodin, A.A. Adaptive Mo2N/MoS2/Ag tribological nanocomposite coatings for aerospace applications. Tribol. Lett. 2008, 29, 95–103. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Liang, X.S. High-temperature solid lubricating materials. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: New York, NY, USA, 2013; pp. 1671–1681. [Google Scholar]
- Stone, D.; Liu, J.; Singh, D.P.; Muratore, C.; Voevodin, A.A.; Mishra, S.; Rebholz, C.; Ge, Q.; Aouadi, S.M. Layered atomic structures of double oxides for low shear strength at high temperatures. Scr. Mater. 2010, 62, 735–738. [Google Scholar] [CrossRef]
- Ageh, V.; Mohseni, H.; Scharf, T.W. Lubricious zinc titanate coatings for high temperature applications. Surf. Coat. Technol. 2013, 237, 241–247. [Google Scholar] [CrossRef]
- Mohseni, H.; Scharf, T.W. Atomic layer deposition of ZnO/Al2O3/ZrO2 nanolaminates for improved thermal and wear resistance in carbon-carbon composites. J. Vac. Sci. Technol. A 2012, 30, 01A149. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Muratore, C.; Aouadi, S.M. Hard coatings with high temperature adaptive lubrication and contact thermal management: A review. Surf. Coat. Technol. 2014, 257, 247–265. [Google Scholar] [CrossRef]
- Franz, R.; Mitterer, C. Vanadium containing self-adaptive low-friction hard coatings for high-temperature applications: A review. Surf. Coat. Technol. 2013, 228, 1–13. [Google Scholar] [CrossRef]
- Glieter, H. Nanocrystalline Materials. Prog. Mater. Sci. 1989, 33, 223–315. [Google Scholar] [CrossRef]
- Karch, J.; Birringer, R.; Glieter, H. Ceramics ductile at low temperature. Nature 1987, 330, 556–558. [Google Scholar] [CrossRef]
- Kumar, K.N.P.; Keizer, K.; Burggraaf, A.J.; Okubo, T.; Nagamoto, H.; Morooka, S. Densification of nanostructured titania assisted by a phase transformation. Nature 1992, 358, 48–51. [Google Scholar] [CrossRef]
- Yin, M.H.; Zhang, Y.C.; Zhou, R.M.; Zhai, Z.Y.; Wang, J.L.; Cui, Y.H.; Li, D.S. Friction mechanism and application of PTFE coating in finger seals. Tribol. Trans. 2022, 65, 260–269. [Google Scholar] [CrossRef]
- Khedkar, J.; Negulescu, I.; Meletis, E.I. Sliding wear behavior of PTFE composites. Wear 2002, 252, 361–369. [Google Scholar] [CrossRef]
- Sliney, H.E. Evaluation of two polyimides and of an improved liner retention design for self-lubricating bushings. In Proceedings of the Joint Lubrication Conference; San Diego, CA, USA, 22 October 1984. Available online: https://ntrs.nasa.gov/citations/19840019818 (accessed on 30 April 2022).
- Guo, Y.; Xu, J.; Yan, C.; Chen, Y.; Zhang, X.; Jia, X.; Liu, Y.; Wang, X.; Zhou, F. Direct ink writing of high performance architectured polyimides with low dimensional shrinkage. Adv. Eng. Mater. 2019, 21, 1801314. [Google Scholar] [CrossRef]
- Yao, X.; Liu, S.; Ji, Z.; Guo, R.; Sun, C.; Guo, Y.; Wang, X.; Wang, Q. 3D printing of PTFE-filled polyimide for programmable lubricating in the region where lubrication is needed. Tribol. Int. 2022, 167, 107405. [Google Scholar] [CrossRef]
- Guleryuz, C.G.; Krzanowski, J.E.; Veldhuis, S.C.; Fox-Rabinovich, G.S. Machining performance of TiN coatings incorporating indium as a solid lubricant. Surf. Coat. Technol. 2009, 203, 3370–3376. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Zhou, H.; Chen, J.; An, Y.; Yan, F. HVOF-sprayed adaptive low friction NiMoAl-Ag coating for tribological application from 20 to 800 °C. Tribol. Lett. 2014, 56, 55–66. [Google Scholar] [CrossRef]
- Zhong, H.; Feng, X.; Jia, J.; Yi, G. Tribological characteristics and wear mechanisms of NiMoAl composite coatings in reversible temperature cycles from RT to 900 °C. Tribol. Int. 2017, 114, 48–56. [Google Scholar] [CrossRef]
- Baker, C.C.; Hu, J.J.; Voevodin, A.A. Preparation of Al2O3/DLC/Au/MoS2 chameleon coatings for space and ambient environments. Surf. Coat. Technol. 2006, 201, 4224–4229. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Wang, J.; Li, H.; Huang, J.; Xiong, D. Frictional properties of silver overcoated on surface textured tantalum interlayer at elevated temperatures. Surf. Coat. Technol. 2019, 365, 189–199. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, Z.M.; Wang, S.R.; Yang, L.Y. Fabrication and tribological properties of HSS-based self-lubrication composites with an interpenetrating network. Lubr. Sci. 2010, 22, 453–463. [Google Scholar] [CrossRef]
- Spalvins, T.; Sliney, H.E. Frictional behavior and adhesion of Ag and Au films applied to aluminum-oxide by oxygen-ion assisted screen cage ion plating. Surf. Coat. Technol. 1994, 68, 482–488. [Google Scholar] [CrossRef]
- Liu, Z. Elevated temperature diffusion self-lubricating mechanisms of a novel cermet sinter with orderly micro-pores. Wear 2007, 262, 600–606. [Google Scholar] [CrossRef]
- Baker, C.C.; Chromik, R.R.; Wahl, K.J.; Hu, J.J.; Voevodin, A.A. Preparation of chameleon coatings for space and ambient environments. Thin Solid Film. 2007, 515, 6737–6743. [Google Scholar] [CrossRef]
- Hu, J.J.; Muratore, C.; Voevodin, A.A. Silver diffusion and high-temperature lubrication mechanisms of YSZ-Ag-Mo based nanocomposite coatings. Compos. Sci. Technol. 2007, 67, 336–347. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Costa, H.L.; Baykara, M.Z.; Martini, A. Synergetic effects of surface texturing and solid lubricants to tailor friction and wear: A review. Tribo. Inter. 2021, 155, 106792. [Google Scholar] [CrossRef]
- Basnyat, R.; Luster, B.; Muratore, C.; Voevodin, A.; Haasch, R.; Zakeri, R. Surface texturing for adaptive solid lubrication. Surf. Coat. Technol. 2008, 203, 73–79. [Google Scholar] [CrossRef]
- Huai, W.; Zhang, C.; Wen, S. Graphite-based solid lubricant for high-temperature lubrication. Friction 2021, 9, 1660–1672. [Google Scholar] [CrossRef]
- Eichler, J.; Lesniak, C. Boron nitride (BN) and BN composites for high-temperature applications. J. Eur. Ceram. Soc. 2008, 28, 1105–1109. [Google Scholar] [CrossRef]
- Marian, M.; Berman, D.; Rota, A.; Jackson, R.L.; Rosenkranz, A. Layered 2D Nanomaterials to tailor friction and wear in machine elements—A review. Adv. Mater. Interfaces 2021, 9, 2101622. [Google Scholar] [CrossRef]
- Martin, J.M.; Le Mogne, T.; Chassagnette, C.; Gardos, M.N. Friction of hexagonal boron nitride in various environments. Tribol. Trans. 1992, 35, 462–472. [Google Scholar] [CrossRef]
- Cao, Y.; Du, L.; Huang, C.; Liu, W.; Zhang, W. Wear behavior of sintered hexagonal boron nirtride under atmosphere and water vapor ambiences. Appl. Surf. Sci. 2011, 257, 10195–10200. [Google Scholar] [CrossRef]
- Pawlak, Z.; Pai, R.; Bayraktar, E.; Kaldonski, T.; Oloyede, A. Lamellar lubrication in vivo and vitro: Friction testing of hexagonal boron nitride. Biosystems 2008, 94, 202–208. [Google Scholar] [CrossRef]
- Pawlak, Z.; Kaldonski, T.; Pai, R.; Bayraktar, E.; Oloyede, A. A comparative study on the tribological behavior of hexagonal boron nitride (h-BN) as lubricating micro-particles-an additive in porous sliding bearing for a car clutch. Wear 2009, 267, 1198–1202. [Google Scholar] [CrossRef]
- Chen, B.; Bi, Q.; Yang, J.; Xia, Y.; Hao, J. Tribological properties of solid lubricants (graphite, h-BN) for Cu-based P/M friction composites. Tribol. Int. 2008, 41, 1145–1152. [Google Scholar] [CrossRef]
- Kimura, Y.; Wakabayashi, T.; Okada, K.; Wada, T.; Nishikawa, H. Born nitride as a lubricant additive. Wear 1999, 232, 199–206. [Google Scholar] [CrossRef]
- Cho, D.H.; Kim, J.S.; Kwon, S.H.; Lee, C.; Lee, Y.Z. Evaluation of hexagonal boron nitride nano-sheets as a lubricant additive in water. Wear 2013, 302, 981–986. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Wei, S.; Yang, Q. Tribological behavior of B4C-hBN ceramic composites used as pins or discs coupled with B4C ceramic under dry sliding condition. Ceram. Int. 2017, 43, 1578–1583. [Google Scholar] [CrossRef]
- Du, L.; Huang, C.; Zhang, W.; Li, T.; Liu, W. Preparation and wear performance of NiCr/Cr3C2-NiCr/hBN plasma sprayed composite coating. Surf. Coat. Technol. 2011, 205, 3722–3728. [Google Scholar] [CrossRef]
- Leon, O.A.; Staia, M.H.; Hintermann, H.E. Wear mechanism of Ni–P–BN(h) composite autocatalytic coatings. Surf. Coat. Technol. 2005, 200, 1825–1829. [Google Scholar] [CrossRef]
- Tyagi, R.; Xiong, D.S.; Li, J.; Dai, J. Elevated temperature tribological behavior of Ni based composites containing nano-silver and hBN. Wear 2010, 269, 884–890. [Google Scholar] [CrossRef]
- Skopp, A.; Woydt, M.; Habig, K.H. Tribological behavior of silicon nitride materials under unlubricated sliding between 22 °C and 1000 °C. Wear 1995, 181–183, 571–580. [Google Scholar]
- Guo, X.; Zhu, Z.; Ekevad, M.; Bao, X.; Cao, P. The cutting performance of Al2O3 and Si3N4 ceramic cutting tools in the milling plywood. Adv. Appl. Ceram. 2018, 117, 16–22. [Google Scholar] [CrossRef]
- Akhtar, S.S. A critical review on self-lubricating ceramic-composite cutting tools. Ceram. Int. 2021, 47, 20745–20767. [Google Scholar] [CrossRef]
- Podgornik, B.; Kosec, T.; Kocijan, A.; Donik, C. Tribological behavior and lubrication performance of hexagonal boron nitride (h-BN) as a replacement for graphite in aluminum forming. Tribol. Int. 2015, 81, 267–275. [Google Scholar] [CrossRef]
- Fournier, P.; Platon, F. Wear of refractory ceramics against nickel. Wear 2000, 244, 118–125. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Yao, M.; Ouyang, J.H.; Zhou, Y.; Guo, Y. Corrosion kinetics and corrosion mechanisms of BN-ZrO2-SiC composites in molten steel. Corrosion Sci. 2014, 89, 93–100. [Google Scholar] [CrossRef]
- Chen, L.; Zhen, L.; Wang, Y.; Duan, X.; Ouyang, J.H.; Zhou, Y. Corrosion behavior and microstructural evolution of BN-ZrO2-SiC composites in molten steel. Int. J. Appl. Ceram. Technol. 2017, 14, 665–674. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Ouyang, J.H.; Duan, X.; Zhou, Y. Low-temperature sintering nehavior and mechanical properties of BN-ZrO2-SiC composites. Mater. Sci. Eng. A-Struct. 2017, 681, 50–55. [Google Scholar] [CrossRef]
- Duan, X.; Yang, Z.; Chen, L.; Tian, Z.; Cai, D.; Wang, Y.; Jia, D.; Zhou, Y. Review on the properties of hexagonal boron nitride matrix composite ceramics. J. Eur. Ceram. Soc. 2016, 36, 3725–3737. [Google Scholar] [CrossRef]
- Niu, Z.B.; Chen, F.; Xiao, P.; Li, Z.; Pang, L.; Li, Y. Effect of h-BN addition on friction and wear properties of C/C-SiC composites fabricated by LSI. Int. J. Appl. Ceram. Technol. 2022, 19, 108–118. [Google Scholar] [CrossRef]
- Yuan, S.; Toury, B.; Benayoun, S. Novel chemical process for preparing h-BN solid lubricant coatings on titanium-based substrates for high temperature tibological applications. Surf. Coat. Technol. 2015, 272, 366–372. [Google Scholar] [CrossRef]
- Yuan, S.; Benayoun, S.; Briouse, A.; Dezellus, O.; Beaugiraud, B.; Toury, B.J. New potential for preparation of performing h-BN coatings via polymer pyrolysis in RTA furnace. Eur. Ceram. Soc. 2013, 33, 393–402. [Google Scholar] [CrossRef]
- Miyoshi, K.; Buckley, D.H.; Pouch, J.J.; Alterovitz, S.A.; Sliney, H.E. Mechanical strength and tribological behavior of ion-beam deposited boron-nitride films on non-metallic substrates. Surf. Coat. Technol. 1987, 33, 221–233. [Google Scholar] [CrossRef][Green Version]
- Spear, J.C.; Ewers, B.W.; Batteas, J.D. 2D-nanomaterials for controlling friction and wear at interfaces. Nano Today 2015, 10, 301–314. [Google Scholar] [CrossRef]
- Vazirisereshk, M.R.; Martini, A.; Strubbe, D.A.; Baykara, M.Z. Solid lubrication with MoS2: A review. Lubricants 2019, 7, 57. [Google Scholar] [CrossRef]
- Martin, J.M.; Donnet, C.; Lemogne, T.; Epicier, T. Superlubricity of molybdenum disulfide. Phys. Rev. B 1993, 48, 10583–10586. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, Q.; Wang, Y.; Yan, C.; He, W. Nano-crystallization-driven high temperature self-lubricating properties of magnetron-sputtered WS2 coatings. Tribol. Lett. 2020, 68, 1–11. [Google Scholar] [CrossRef]
- Rapoport, L.; Fleischer, N.; Tenne, R. Fullerene-like WS2 nanoparticles: Superior lubricants for harsh conditions. Adv. Mater. 2003, 15, 651–655. [Google Scholar] [CrossRef]
- Sun, X. Solid lubricants for space mechanisms. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013. [Google Scholar]
- Roberts, E.W. Space tribology: Its role in spacecraft mechanisms. J. Phys. D Appl. Phys. 2012, 45, 503001. [Google Scholar] [CrossRef]
- Miyoshi, K. Aerospace mechanisms and tribology technology: Case study. Tribol. Int. 1999, 32, 673–685. [Google Scholar] [CrossRef]
- Lince, J.R. Effective application of solid lubricants in spacecraft mechanisms. Lubricants 2020, 8, 74. [Google Scholar] [CrossRef]
- Davis, D.; Marappan, G.; Sivalingam, Y.; Panigrahi, B.B.; Singh, S. Tribological behavior of NiMoAl-based self-lubricating composites. ACS Omega 2020, 5, 14669–14678. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Filimonov, D.; Palanisamy, T.; El-Raghy, T.; Barsoum, M.W. Ta2AlC and Cr2AlC Ag-based composites—New solid lubricant materials for use over a wide temperature range against Ni-based superalloys and alumina. Wear 2007, 262, 1479–1489. [Google Scholar] [CrossRef]
- Barsoum, M.W. The Mn+1AXn phases: A new class of solids thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–208. [Google Scholar] [CrossRef]
- Gupta, S.; Filimonov, D.; Zaitsev, V.; Palanisamy, T.; Barsoum, M.W. Ambient and 550 °C tribological behavior of select MAX phases against Ni-based superalloys. Wear 2008, 264, 270–278. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.M.; Liu, F.Y. Microstructure and tribological properties of spark plasma sintered Ti3SiC2-Pb-Ag composites at elevated temperatures. Materials 2022, 15, 1437. [Google Scholar] [CrossRef]
- Sliney, H.E.; DellaCorte, C.; Lukaszewicz, V. The tribology of PS212 coatings and PM212 composites for the lubrication of titanium 6Al-4V components of a stirling engine space power-system. Tribol. Trans. 1995, 38, 497–506. [Google Scholar] [CrossRef]
- DellaCorte, C.; Laskowski, J.A. Tribological evaluation of PS300: A new chrome oxide-based solid lubricant coating sliding against Al2O3 from 25° to 650 °C. Tribol. Trans. 1997, 40, 163–167. [Google Scholar] [CrossRef]
- Bemis, K.; Bogdanski, M.S.; DellaCorte, C.; Sliney, E. The effect of prolonged exposure to 750 °C air on the tribological performance of PM212 self-lubricating composite material. Tribol. Trans. 1995, 38, 745–756. [Google Scholar] [CrossRef]
- Mazumder, S.; Metsalaar, H.S.C.; Sukiman, N.L.; Zulkifli, N.W.M. An overview of fluoride-based solid lubricants in sliding contacts. J. Eur. Ceram. Soc. 2020, 40, 4974–4996. [Google Scholar] [CrossRef]
- Ding, C.H.; Li, P.L.; Ran, G.; Tian, Y.W.; Zhou, J.N. Tribological property of self-lubricating PM304 composite. Wear 2007, 262, 575–581. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Bi, Q.L.; Yang, J.; Liu, W.M.; Xue, Q.J. Ni3Al matrix high temperature self-lubricating composites. Tribol. Int. 2011, 44, 445–453. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Bi, Q.L.; Yang, J.; Liu, W.M.; Xue, Q.J. Effect of particle size on tribological behavior of Ni3Al matrix high temperature self-lubricating composites. Tribol. Int. 2011, 44, 1800–1809. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Bi, Q.L.; Kong, L.Q.; Yang, J.; Liu, W.M. Influence of Cr content on tribological properties of Ni3Al matrix high temperature self-lubricating composites. Tribol. Int. 2011, 44, 1182–1187. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Li, Y.F.; Wang, Y.M.; Zhou, Y.; Murakami, T.; Sasaki, S. Microstructure and tribological properties of ZrO2(Y2O3) matrix composites doped with different solid lubricants from room temperature to 800 °C. Wear 2009, 267, 1353–1360. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Murakami, T.; Umeda, K. The synergistic effects of CaF2 and Au lubricants on tribological properties of spark-plasma-sintered ZrO2(Y2O3) matrix composites. Mater. Sci. Eng. A Struct. 2004, 386, 234–243. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Murakami, T.; Umeda, K. Tribological properties of spark plasma sintered (SPS) ZrO2-CaF2-Ag composites at elevated temperatures. Wear 2005, 258, 1444–1454. [Google Scholar] [CrossRef]
- Gong, H.; Yu, C.; Zhang, L.; Xie, G.; Guo, D.; Luo, J. Intelligent lubricating materials: A review. Compos. Pt. B 2020, 202, 108450. [Google Scholar] [CrossRef]
- Dimitrov, V.; Komatsu, T. Classification of simple oxides: A polarizability approach. J. Solid State Chem. 2002, 163, 100–112. [Google Scholar] [CrossRef]
- Prakash, B.; Celis, J.P. The lubricity of oxides revised based on a polarizability approach. Tribol. Lett. 2007, 27, 105–112. [Google Scholar] [CrossRef]
- Reeswinkel, T.; Music, D.; Schneider, J.M. Coulomb-potential-dependent decohesion of Magneli phases. J. Phys. Condens. Matter 2010, 22, 292203–292207. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.M.; Stahr, C.C.; Saaro, S.; Thiele, S.; Woydt, M.; Kelling, N. Dry sliding up to 7.5 m/s and 800 °C of thermally sprayed coatings of the TiO2-Cr2O3 system and (Ti,Mo)(C,N)-Ni(Co). Wear 2009, 267, 954–964. [Google Scholar] [CrossRef]
- Gulbinski, W.; Suszko, T.; Sienicki, W.; Warcholinski, B. Tribological properties of silver- and copper-doped transition metal oxide coatings. Wear 2003, 254, 129–135. [Google Scholar] [CrossRef]
- Fateh, N.; Fontalvo, G.A.; Mitterer, C. Tribological properties of reactive magnetron sputtered V2O5 and VN-V2O5 coatings. Tribol. Lett. 2008, 30, 21–26. [Google Scholar] [CrossRef]
- Heo, S.J.; Kim, K.H.; Kang, M.C.; Suh, J.H.; Park, C.G. Synthesis and mechanical properties of Mo-S-N coatings by a hybrid coating system. Surf. Coat. Technol. 2006, 201, 4180–4184. [Google Scholar] [CrossRef]
- Cavaleiro, A.; Trindade, B.; Vieira, M.T. Influence of Ti addition on the properties of W-Ti-C/N sputtered films. Surf. Coat. Technol. 2003, 174, 68–75. [Google Scholar] [CrossRef]
- Ding, Q.D.; Li, C.S.; Dong, L.R.; Wang, M.L.; Peng, Y.; Yan, X.H. Preparation and properties of YBa2Cu3O7-delta/Ag self-lubricating composites. Wear 2008, 265, 1136–1141. [Google Scholar] [CrossRef]
- Zhou, Z.; Rainforth, W.M.; Luo, Q.; Hovsepian, P.E.; Ojeda, J.J.; Romero-Gonzalez, M.E. Wear and Friction of TiAlN/VN Coatings Against Al2O3 in Air at Room and Elevated Temperatures. Acta Mater. 2010, 58, 2912–2925. [Google Scholar] [CrossRef]
- Fateh, N.; Fontalvo, G.A.; Gassner, G.; Mitterer, C. The beneficial effect of high-temperature oxidation on the tribological behavior of V and VN coatings. Tribol. Lett. 2007, 28, 1–7. [Google Scholar] [CrossRef]
- Peterson, M.B.; Li, S.Z.; Murray, S.F. Wear-Resisting Oxide Films for 900 °C. Mater. Sci. Technol. 1997, 13, 99–106. Available online: https://www.jmst.org/EN/Y1997/V13/I2/99 (accessed on 30 April 2022).
- Zabinski, J.S.; Day, A.E.; Donley, M.S.; DellaCorte, C.; McDevitt, N.T. Synthesis and characterization of a high-temperature oxide lubricant. J. Mater. Sci. 1994, 29, 5875–5879. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Shi, C.C.; Liu, Z.G.; Wang, Y.M.; Wang., Y.J. Fabrication and high-temperature tribological properties of self-lubricating NiCr-BaMoO4 composites. Wear 2015, 330–331, 272–279. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Paudel, Y.; Simonson, W.J.; Ge, Q.; Kohli, P.; Muratore, C.; Voevodin, A.A. Tribological investigation of adaptive Mo2N/MoS2/Ag coatings with high sulfur content. Surf. Coat. Technol. 2009, 203, 1304–1309. [Google Scholar] [CrossRef]
- Lei, M.; Ye, C.X.; Ding, S.S.; Bi, K.; Xiao, H.; Sun, Z.B.; Fan, D.Y.; Yang, H.J.; Wang, Y.G. Controllable route to barium molybdate crystal and their photoluminescence. J. Alloy. Compd. 2015, 639, 102–105. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Singh, D.P.; Stone, D.S.; Polychronopoulou, K.; Nahif, F.; Rebholz, C.; Muratore, C.; Voevodin, A.A. Adaptive VN/Ag Nanocomposite Coatings with Lubricious Behavior from 25 to 1000 °C. Acta Mater. 2010, 58, 5326–5331. [Google Scholar] [CrossRef]
- Hao, E.; An, Y.; Chen, J.; Zhao, X.; Hou, G.; Chen, J.M.; Gao, M.; Yan, F. In-situ formation of layer-like Ag2MoO4 induced by high-temperature oxidation and its effect on the self-lubricating properties of NiCoCrAlYTa/Ag/Mo coatings. J. Mater. Sci. Technol. 2021, 75, 164–173. [Google Scholar] [CrossRef]
- Chen, J.; An, Y.L.; Yang, J.; Zhao, X.Q.; Yan, F.Y.; Zhou, H.D.; Chen, J.M. Tribological properties of adaptive NiCrAlY-Ag-Mo coatings prepared by atmospheric plasma spraying. Surf. Coat. Technol. 2013, 235, 521–528. [Google Scholar] [CrossRef]
- Zhu, S.; Li, F.; Ma, J.; Cheng, J.; Yin, B.; Yang, J.; Qiao, Z.; Liu, W. Tribological properties of Ni3Al matrix composites with addition of silver and barium salt. Tribol. Int. 2015, 84, 118–123. [Google Scholar] [CrossRef]
- Wenda, E. High temperature reactions in the MoO3-Ag2O system. J. Therm. Anal. Calorim. 1998, 53, 861–870. [Google Scholar] [CrossRef]
- John, P.J.; Prasad, S.V.; Voevodin, A.A.; Zabinski, J.S. Calcium sulfate as a high temperature solid lubricant. Wear 1998, 219, 155–161. [Google Scholar] [CrossRef]
- Prasad, S.; McDevitt, N.; Zabinski, J. Tribology of tungsten disulfide-nanocrystalline zinc oxide adaptive lubricant films from ambient to 500 °C. Wear 2000, 237, 186–196. [Google Scholar] [CrossRef]
- Zhu, S.; Bi, Q.; Yang, J.; Liu, W.M. Ni3Al matrix composite with lubricious tungstate at high temperatures. Tribol. Lett. 2012, 45, 251–255. [Google Scholar] [CrossRef]
- Xie, B.; Wu, Y.; Jiang, Y.; Li, F.; Wu, J.; Yuan, S.; Yu, W.; Qian, Y.T. Shape-controlled synthesis of BaWO4 crystals under different surfactants. J. Cryst. Growth 2002, 235, 283–286. [Google Scholar] [CrossRef]
- Shi, H.T.; Qi, L.M.; Ma, J.M.; Cheng, H.M. Polymer-directed synthesis of penniform BaWO4 nanostructures in reverse micelles. J. Am. Chem. Soc. 2003, 125, 3450–3451. [Google Scholar] [CrossRef]
- Shi, H.T.; Wang, X.H.; Zhao, N.; Qi, L.M.; Ma, J.M. Growth mechanism of penniform BaWO4 nanostructures in catanionic reverse micelles involving polymers. J. Phys. Chem. B 2006, 110, 748–753. [Google Scholar] [CrossRef]
- Guo, H.J.; Han, M.M.; Chen, W.Y.; Lu, C.; Li, B.; Wang, W.Z.; Jia, J.H. Microstructure and properties of VN/Ag composite films with various silver content. Vacuum 2017, 137, 97–103. [Google Scholar] [CrossRef]
- Xin, B.B.; Yu, Y.J.; Zhou, J.S.; Wang, L.Q.; Ren, S.F.; Zhen, L. Effect of silver vanadate on the lubricating proeprties of NiCrAlY laser cladding coating at elevated temperatures. Surf. Coat. Technol. 2016, 307, 136–145. [Google Scholar] [CrossRef]
- Song, J.M.; Lin, Y.Z.; Yao, H.B.; Fan, F.J.; Li, X.G.; Yu, S.H. Superlong beta-AgVO3 nanoribbons: High-yield synthesis by a pyridine-assisted solution approach, their stability, electrical and electrochemical properties. ACS Nano 2009, 3, 653–660. [Google Scholar] [CrossRef]
- Wang, G.; Ren, Y.; Zhou, G.J.; Wang, J.P.; Cheng, H.F.; Wang, Z.Y. Synthesis of highly efficient visible light Ag@Ag3VO4 plasmonic photocatalysts. Surf. Coat. Technol. 2013, 228, S283–S286. [Google Scholar] [CrossRef]
- Su, Y.; Hu, L.; Fan, H.; Song, J.; Zhang, Y. Surface engineering design of alumina/molybdenum fibrous monolithic ceramic to achieve continuous lubrication from room temperature to 800 °C. Tribol. Lett. 2017, 65, 47. [Google Scholar] [CrossRef]
- Stone, D.S.; Harbin, S.; Mohseni, H.; Mogonye, J.E.; Scharf, T.W.; Muratore, C.; Voevodin, A.A.; Martini, A.; Aouadi, S.M. Lubricious silver tantalate films for extreme temperature applications. Surf. Coat. Technol. 2013, 217, 140–146. [Google Scholar] [CrossRef]
- Valant, M.; Axelsson, A.K.; Zou, B.; Alford, N. Oxygen transport during formation and decomposition of AgNbO3 and AgTaO3. J. Mater. Res. 2007, 22, 1650–1655. [Google Scholar] [CrossRef]
- Gao, H.; Stone, D.S.; Mohseni, H.; Aouadi, S.M.; Scharf, T.W.; Martini, A. Mechanistic studies of high temperature friction reduction in silver tantalate. Appl. Phys. Lett. 2013, 102, 121603–121605. [Google Scholar] [CrossRef]
- Gao, H.; Otero-de-la-Roza, A.; Gu, J.; Stone, D.; Aouadi, S.M.; Johnson, E.R.; Martini, A. (Ag,Cu)-Ta-O ternaries as high-temperature solid-lubricant coatings. ACS Appl. Mater. Interfaces 2015, 7, 15422–15429. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Otero-de-la-Roza, A.; Aouadi, S.M.; Johnson, E.R.; Martini, A. An empirical model for silver tantalite. Model. Simul. Mater. Sci. Eng. 2013, 21, 055002. [Google Scholar] [CrossRef]
- Liang, X.S.; Ouyang, J.H.; Liu, Z.G.; Yang, Z.L. Friction and wear characteristics of BaCr2O4 ceramics at elevated temperature in sliding against sintered alumina ball. Tribol. Lett. 2012, 47, 203–209. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Murakami, T.; Umeda, K. Spark-plasma-sintered ZrO2(Y2O3)-BaCrO4 self-lubricating composites for high temperature tribological applications. Ceram. Int. 2005, 31, 543–553. [Google Scholar] [CrossRef]
- Murakami, T.; Ouyang, J.; Korenaga, A.; Umeda, K.; Sasaki, S.; Yoneyama, Y. High temperature tribological properties of Al2O3-X (X: BaCrO4, BaSO4 and CaSO4) spark-plasma-sintered composites containing sintering additives. Mater. Trans. 2004, 45, 2614–2617. [Google Scholar] [CrossRef]
- Liang, X.S.; Ouyang, J.H.; Liu, Z.G. Preparation of BaCrO4 particles in the presence of EDTA from aqueous solutions. J. Coord. Chem. 2012, 65, 2432–2441. [Google Scholar] [CrossRef]
- Muller-Buschbaum, H.K. The crystal chemistry of AM2O4 oxometallates. J. Alloy. Compd. 2003, 349, 49–104. [Google Scholar] [CrossRef]
- Liang, X.S.; Ouyang, J.H.; Liu, Z.G. Influences of temperature and atmosphere on thermal stability of BaCrO4. J. Therm. Anal. Calorim. 2013, 111, 371–375. [Google Scholar] [CrossRef]
- Gontarz, Z. Analysis of the steps of thermal decomposition of oxo-compounds of the dsp block elements. J. Therm. Anal. Calorim. 1995, 43, 57–68. [Google Scholar] [CrossRef]
- Azad, A.M.; Sudha, R.; Sreedharan, O.M. The standard Gibbs energies of formation of ACrO4 (A = Ca, Sr or Ba) from EMF measurements. Thermochim. Acta 1992, 194, 129–136. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Liang, X.S.; Wen, J.; Liu, Z.G.; Yang, Z.L. Electrodeposition and tribological properties of self-lubricating Ni-BaCr2O4 composite coatings. Wear 2011, 271, 2037–2045. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Liang, X.S.; Liu, Z.G.; Yang, Z.L.; Wang, Y.J. Friction and wear properties of hot-pressed NiCr-BaCr2O4 high temperature self-lubricating composites. Wear 2013, 301, 820–827. [Google Scholar] [CrossRef]
- Li, Y.F.; Ouyang, J.H.; Zhou, Y.; Liang, X.S.; Zhong, J.Y. Facile fabrication of SrSO4 nanocrystals with different crystallographic morphologies via a simple surfactant-free aqueous solution route. Mater. Lett. 2008, 62, 4417–4420. [Google Scholar] [CrossRef]
- Li, Y.F.; Ouyang, J.H.; Zhou, Y. Novel fabrication of monodispersed peanut-type celestine particles using Sr-EDTA chelating precursors. Mater. Chem. Phys. 2008, 111, 508–512. [Google Scholar] [CrossRef]
- Li, Y.F.; Ouyang, J.H.; Zhou, Y.; Liang, X.S.; Murakami, T.; Sasaki, S. Room temperature template-free synthesis of dumbbell-like SrSO4 nanostructures with a hierarchical architecture. J. Cryst. Growth. 2010, 312, 1886–1890. [Google Scholar] [CrossRef]
- Li, Y.F.; Ouyang, J.H.; Zhou, Y.; Liang, X.S.; Zhong, J.Y. Synthesis and characterization of nano-sized BaxSr1−xSO4 (0 ≤ x ≤ 1) solid solution by a simple surfactant-free aqueous solution route. Bull. Mat. Sci. 2009, 32, 149–153. [Google Scholar] [CrossRef]
- Murakami, T.; Ouyang, J.H.; Sasaki, S.; Umeda, K.; Yoneyama, Y. High temperature tribological properties of spark-plasma-sintered Al2O3 composites containing barite-type structure sulfates. Tribol. Int. 2007, 40, 246–253. [Google Scholar] [CrossRef]
- Li, Y.F.; Ouyang, J.H.; Sasaki, S. Tribological properties of spark plasma sintered ZrO2(Y2O3)-Al2O3-BaxSr1-xSO4 (x = 0.25, 0.5, 0.75) composites at elevated temperature. Tribol. Lett. 2012, 45, 291–300. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, J.; Niu, M.; Tan, H.; Liu, W.; Yang, J. Microstructure and high temperature tribological behavior of Fe3Al-Ba0.25Sr0.75SO4 self-lubricating composites. Tribol. Int. 2016, 101, 81–87. [Google Scholar] [CrossRef]
- Liang, X.S.; Ouyang, J.H.; Li, Y.F.; Wang, Y.M. Electrodeposition and tribological properties of Ni-SrSO4 composite coatings. Appl. Surf. Sci. 2009, 255, 4316–4321. [Google Scholar] [CrossRef]
- Murakami, T.; Umeda, K.; Sasaki, S.; Ouyang, J.H. High-temperature tribological properties of strontium sulfate films formed on zirconia-alumina, alumina and silicon nitride substrates. Tribol. Int. 2006, 39, 1576–1583. [Google Scholar] [CrossRef]
- Borawski, A. Conventional and unconventional materials used in the production of brake pads—Review. Sci. Eng. Compos. Mater. 2020, 27, 374–396. [Google Scholar] [CrossRef]
- Menapace, C.; Leonardi, M.; Matejka, V.; Gialanella, S.; Straffelini, G. Dry sliding behavior and frictional layer formation in copper-free barite containing friction materials. Wear 2018, 398–399, 191–200. [Google Scholar] [CrossRef]
- Li, Y.F.; Ouyang, J.H.; Zhou, Y.; Wang, Y.M.; Murakami, T.; Sasaki, S. High temperature tribological properties of spark plasma sintered Al2O3-SrSO4 self-lubricating nanocomposites incorporated with and without Ag addition. Int. J. Mod. Phys. B 2009, 23, 1425–1431. [Google Scholar] [CrossRef]
- Murakami, T.; Ouyang, J.H.; Umeda, K.; Sasaki, S. High-temperature friction properties of BaSO4 and SrSO4 powder films formed on Al2O3 and stainless steel substrates. Mat. Sci. Eng. A Struct. 2006, 432, 52–58. [Google Scholar] [CrossRef]
- Carter, C.B.; Norton, M.G. Ceramic Materials: Science and Engineering; Springer: New York, NY, USA, 2007. [Google Scholar]
- Calas, G.; Henderson, G.S.; Stebbins, J.F. Glasses and melts: Linking geochemistry and materials science. Elements 2006, 2, 265–268. [Google Scholar] [CrossRef]
- Yue, W.; Wang, C.B.; Liu, Y.D.; Huang, H.P.; Wen, Q.F.; Liu, J.J. Study of the regenerated layer on the worn surface of a cylinder liner lubricated by a novel silicate additive in lubricating oil. Tribol. Trans. 2010, 53, 288–295. [Google Scholar] [CrossRef]
- Nan, F.; Xu, Y.; Xu, B.S.; Gao, F.; Wu, Y.X.; Li, Z.G. Tribological behaviors and wear mechanisms of ultrafine magnesium aluminum silicate powders as lubricant additive. Tribol. Int. 2015, 81, 199–208. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, B.; Wang, C.B. Long-term surface restoration effect introduced by advanced silicate based lubricant additive. Tribol. Int. 2013, 57, 31–37. [Google Scholar] [CrossRef]
- Wang, L.; Tieu, A.K.; Cui, S.; Deng, G.; Wang, P.; Zhu, H.; Yang, J. Lubrication mechanism of sodium metasilicate at elevated temperatures through tribo-interface observation. Tribol. Int. 2020, 142, 105972. [Google Scholar] [CrossRef]
- Wang, B.; Gao, K.; Chang, Q.; Berman, D.; Tian, Y. Magnesium silicate hydroxide-MoS2-Sb2O3 coating nanomaterials for high-temperature superlubricity. ACS Appl. Nano. Mater. 2021, 4, 7097–7106. [Google Scholar] [CrossRef]
- Gao, K.; Wang, B.; Shirani, A.; Chang, Q.; Berman, D. Macroscale superlubricity accomplished by Sb2O3-MSH/C under high-temperature. Front. Chem. 2021, 9, 226. [Google Scholar] [CrossRef]
- Strong, K.L.; Zabinski, J.S. Characterization of annealed pulsed laser deposited (PLD) thin films of cesium oxythiomolybdate (Cs2MoOS3). Thin Solid Films 2002, 406, 164–173. [Google Scholar] [CrossRef]
- Strong, K.L.; Zabinski, J.S. Tribology of pulsed laser deposited thin films of cesium oxythiomolybdate (Cs2MoOS3). Thin Solid Films 2002, 406, 174–184. [Google Scholar] [CrossRef]
- Rosado, L.; Forster, N.H.; Whittberg, T.N. Solid lubrication of silicon nitride with cesium-based compounds: Part II-Surface analysis. Tribol. Trans. 2000, 43, 521–527. [Google Scholar] [CrossRef]
- Wan, S.; Tieu, A.K.; Xia, Y.; Zhu, H.; Tran, B.H.; Cui, S. An overview of inorganic polymer as potential lubricant additive for high temperature tribology. Tribol. Int. 2016, 102, 620–635. [Google Scholar] [CrossRef]
- Zabinski, J.S.; Sanders, J.H.; Nainaparampil, J.; Prasad, S.V. Lubrication using a microstructurally engineered oxide: Performance and mechanisms. Tribol. Lett. 2000, 8, 103–116. [Google Scholar] [CrossRef]
- Zabinski, J.S.; Donley, M.S.; Dyhouse, V.J.; McDevitt, N.T. Chemical and tribological characterization of PbO-MoS2 films grown by pulsed laser deposition. Thin Solid Films 1992, 214, 156–163. [Google Scholar] [CrossRef]
- Muratore, C.; Voevodin, A.A. Molybdenum disulfide as a lubricant and catalyst in adaptive nanocomposite coatings. Surf. Coat. Technol. 2006, 201, 4125–4130. [Google Scholar] [CrossRef]
- Gautam, R.K.S.; Rao, U.S.; Tyagi, R. Influence of load on friction and wear behavior of Ni-based self-lubricating coatings deposited by atmospheric plasma spray. J. Mater. Eng. Perform. 2019, 28, 7398–7406. [Google Scholar] [CrossRef]
- Yang, J.F.; Jiang, Y.; Hardell, J.; Prakash, B.; Fang, Q.F. Influence of service temperature on tribological characteristics of self-lubricant coatings: A review. Front. Mater. Sci. 2013, 7, 28–39. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gao, H.; Martini, A.; Scharf, T.W.; Muratore, C. Lubricious oxide coatings for extreme temperature applications: A review. Surf. Coat. Technol. 2014, 257, 266–277. [Google Scholar] [CrossRef]
- Etsion, I. Improving tribological performance of mechanical components by laser surface texturing. Tribol. Lett. 2004, 17, 733–737. [Google Scholar] [CrossRef]
- Etsion, I. State of the art in laser surface texturing. J. Tribol. Trans. ASME 2005, 127, 248–253. [Google Scholar] [CrossRef]
- Wang, X.L.; Kato, K.; Adachi, K.; Aizawa, K. Loads carrying capacity map for the surface texture design of SiC thrust bearing sliding in water. Tribol. Int. 2003, 36, 189–197. [Google Scholar] [CrossRef]
- Schreck, S.; Zum Gahr, K.H. Laser-assisted structuring of ceramic and steel surfaces for improving tribological properties. Appl. Surf. Sci. 2005, 247, 616–622. [Google Scholar] [CrossRef]
- Moshkovith, A.; Perfiliev, V.; Gindin, D.; Parkansky, N.; Boxman, R.; Rapoport, L. Surface texturing using pulsed air arc treatment. Wear 2007, 163, 1467–1469. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Zabinski, J.S. Laser surface texturing for adaptive solid lubrication. Wear 2006, 261, 1285–1292. [Google Scholar] [CrossRef]
- Mitterer, C.; Lenhart, H.; Mayhofer, P.H.; Kathrein, M. Sputter-deposited Al-Au coatings. Intermetallics 2004, 12, 579–587. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J.W.; Duh, J.G. Mechanical strengthening in self-lubricating CrAlN/VN multilayer coatings for improved high-temperature tribological characteristics. Surf. Coat. Technol. 2016, 303, 12–17. [Google Scholar] [CrossRef]
- Stone, D.S.; Gao, H.; Chantharangsi, C.; Paksunchai, C.; Bischof, M.; Martini, A.; Aouadi, S.M. Reconstruction mechanisms of tantalum oxide coatings with low concentrations of silver for high temperature tribological applications. Appl. Phys. Lett. 2014, 105, 4901817. [Google Scholar] [CrossRef]
- Velasco, S.C.; Cavaleiro, A.; Carvalho, S. Functional properties of ceramic-Ag nanocomposite coatings produced by magnetron sputtering. Prog. Mater. Sci. 2016, 84, 158–191. [Google Scholar] [CrossRef]
- Tejero-Martin, D.; Rad, M.R.; McDonald, A.; Hussain, T. Beyond traditional coatings: A review on thermal-sprayed functional and smart coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef]
- DellaCorte, C.; Zaldana, A.R.; Radil, K.C. A systems approach to the solid lubrication of foil air bearings for oil-free turbomachinery. J. Tribol. Trans. ASME 2004, 126, 200–207. [Google Scholar] [CrossRef]
- Yuan, J.H.; Zhu, Y.C.; Ji, H.; Zheng, X.B.; Ruan, Q.C.; Niu, Y.R.; Liu, Z.W.; Zheng, Y. Microstructures and tribological properties of plasma sprayed WC-Co-Cu-BaF2/CaF2 self-lubricating wear resistant coating. Appl. Surf. Sci. 2010, 256, 4938–4944. [Google Scholar] [CrossRef]
- Li, B.; Jia, J.H.; Gao, Y.M.; Han, M.M.; Wang, W.Z. Microstructural and tribological characterization of NiAl matrix self-lubricating composite coatings by atmospheric plasma spraying. Tribol. Int. 2017, 109, 563–570. [Google Scholar] [CrossRef]
- Chen, J.M.; Hou, G.L.; Chen, J.; An, Y.L.; Zhou, H.D.; Zhao, X.Q.; Yang, J. Composition versus friction and wear behavior of plasma sprayed WC-(W,Cr)2C-Ni/Ag/BaF2-CaF2 self-lubricating composite coatings for use up to 600 °C. Appl. Surf. Sci. 2012, 261, 584–592. [Google Scholar] [CrossRef]
- Sliney, E. Sliding contact PM212 bearings for service to 700 °C. Tribol. Trans. 1997, 40, 579–588. [Google Scholar] [CrossRef]
- Dellacorte, C. The evaluation of a modified chrome oxide based high temperature solid lubricant coating for foil gas bearings. Tribol. Trans. 2000, 43, 257–262. [Google Scholar] [CrossRef]
- Dellacorte, C. The effects of substrate material and thermal processing atmosphere on the strength of PS304: A high temperature solid lubricant coating. Tribol. Trans. 2003, 46, 361–368. [Google Scholar] [CrossRef]
- Dellacorte, C.; Edmonds, B.J.; Benoy, P.A. Thermal processing effects on the adhesive strength of PS304 high temperature solid lubricant coatings. Tribol. Trans. 2002, 45, 499–505. [Google Scholar] [CrossRef]
- Dellacorte, C.; Lukaszewicz, V.; Valco, M.J.; Radil, K.C.; Heshmat, H. Performance and durability of high temperature foil air bearings for oil-free turbomachinery. Tribol. Trans. 2000, 43, 774–780. [Google Scholar] [CrossRef]
- Radil, K.C.; Dellacorte, C. The effect of journal roughness and foil coatings on the performance of heavily loaded foil air bearings. Tribol. Trans. 2002, 45, 199–204. [Google Scholar] [CrossRef]
- Blanchet, T.A.; Kim, J.H.; Calabrese, S.J.; Dellacorte, C. Thrust-washer evaluation of self-lubricating PS304 composite coatings in high temperature sliding contact. Tribol. Trans. 2002, 45, 491–498. [Google Scholar] [CrossRef]
- Balic, E.E.; Blanchet, T.A. Thrust-washer tribological evaluation of PS304 coatings against Rene 41. Wear 2005, 259, 876–881. [Google Scholar] [CrossRef]
- Wang, W. Application of a high temperature self-lubricating composite coating on steam turbine components. Surf. Coat.Technol. 2004, 177–178, 12–17. [Google Scholar] [CrossRef]
- Radil, K.; DellaCorte, C. The performance of PS400 subjected to sliding contact at temperatures from 260 to 927 °C. Tribol. Trans. 2017, 60, 957–964. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Umeda, K. Microstructure and tribological properties of low pressure plasma-sprayed ZrO2-CaF2-Ag2O surface composite at elevated temperature. Wear 2001, 249, 440–451. [Google Scholar] [CrossRef]
- Su, W.M.; Niu, S.P.; Huang, Y.C.; Wang, C.; Wen, Y.Y.; Li, X.; Deng, C.M.; Deng, C.G.; Liu, M. Friction and wear properties of plasma-sprayed Cr2O3-BaCrO4 coating at elevated temperatures. Ceram. Int. 2022, 48, 8696–8705. [Google Scholar] [CrossRef]
- Chourasiya, S.K.; Gautam, G.; Singh, D. Mechanical and tribological behavior of warm rolled Al-6Si-3Graphite self lubricating composite synthesized by spray forming process. Silicon 2019, 12, 831–842. [Google Scholar] [CrossRef]
- Jin, Y.; Kato, K.; Umehara, N. Effects of sintering aids and solid lubricants on tribological behaviors of CMC/Al2O3 pair at 650 °C. Tribol. Lett. 1999, 6, 15–21. [Google Scholar] [CrossRef]
- Jin, Y.; Kato, K.; Umehara, N. Tribological properties of self-lubricating CMC/Al2O3 pairs at high temperature in air. Tribol. Lett. 1998, 4, 243–250. [Google Scholar] [CrossRef]
- Jin, Y.; Kato, K.; Umehara, N. Further investigation on the tribological behavior of Al2O3–20Ag20CaF2 composite at 650 °C. Tribol. Lett. 1999, 6, 225–232. [Google Scholar] [CrossRef]
- Charoo, M.S.; Srivyas, P.D. Friction and wear characterization of spark plasma sintered hybrid aluminum composite under different sliding conditions. J. Tribol. 2020, 142, 121701. [Google Scholar]
- Ibrahim, A.M.M.; Shi, X.; Zhang, A.; Yang, K.; Zhai, W. Tribological characteristics of NiAl matrix composites with 1.5 wt% graphene at elevated temperatures: An experimental and theoretical study. Tribol. Trans. 2015, 58, 1076–1083. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Shi, X.; Zhai, W.; Yang, K. Tribological properties of TiAl matrix self-lubricating composites containing multilayer graphene and Ti3SiC2 at high temperatures. Tribol. Trans. 2015, 58, 1131–1141. [Google Scholar] [CrossRef]
- Yan, Z.; Shi, X.; Huang, Y.; Deng, X.; Yang, K.; Liu, X. Tribological performance of Ni3Al matrix self-lubricating composites containing multilayer graphene and Ti3SiC2 at elevated temperatures. J. Mater. Eng. Perform. 2017, 26, 4605–4614. [Google Scholar] [CrossRef]
- Fernandez, J.B.; Macias, E.J.; Muro, J.S.-D.; Caputi, L.; Miriello, D.; De Luca, R.; Roca, A.S.; Fals, H.C. Tribological behavior of AA1050H24-Graphene nanocomposite obtained by friction stir processing. Metals 2018, 8, 113. [Google Scholar] [CrossRef]
- Kerr, C.; Barker, D.; Walsh, F.; Archer, J. The electrodeposition of composite coatings based on metal matrix-included particle deposits. Trans. Inst. Metal Finish. 2000, 78, 171–178. [Google Scholar] [CrossRef]
- Sangeetha, S.; Kalaignan, G.P.; Anthuvan, J.T. Pulse electrodeposition of self-lubricating Ni-W/PTFE nanocomposite coatings on mild steel surface. Appl. Surf. Sci. 2015, 359, 412–419. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.C.; Walsh, F.C.; Chiu, Y.L.; Reed, P.A.S. Self-lubricating Ni-P-MoS2 composite coatings. Surf. Coat. Technol. 2016, 307, 926–934. [Google Scholar] [CrossRef]
- He, Y.; Sun, W.T.; Wang, S.C.; Reed, P.A.S.; Walsh, F.C. An electrodeposited Ni-P-WS2 coating with combined super-hydrophobicity and self-lubricating properties. Electrochim. Acta 2017, 245, 872–882. [Google Scholar] [CrossRef]
- Zhao, H.J.; Liu, L.; Hu, W.B.; Shen, B. Friction and wear behavior of Ni-graphite composites prepared by electroforming. Mater. Des. 2007, 28, 1374–1378. [Google Scholar] [CrossRef]
- Cao, T.K.; Xiao, Z.J. Tribological behaviors of self-lubricating coatings prepared by electrospark deposition. Tribol. Lett. 2014, 56, 231–237. [Google Scholar] [CrossRef]
- Dogan, F.; Duru, E.; Uysal, M.; Akbulut, H.; Aslan, S. Tribology study of pulse electrodeposited Ni-B-SWCNT composite coating. JOM 2022, 74, 574–583. [Google Scholar] [CrossRef]
- Baiocco, G.; Menna, E.; Rubino, G.; Ucciardello, N. Mechanical characterization of functional Cu-GnP composite coatings for lubricant applications. Surf. Eng. 2022, 38, 72–78. [Google Scholar] [CrossRef]
- Mai, Y.J.; Li, Y.G.; Li, S.L.; Zhang, L.Y.; Liu, C.S.; Jie, X.H. Self-lubricating Ti3C2 nanosheets copper composite coatings. J. Alloy. Compd. 2019, 770, 1–5. [Google Scholar] [CrossRef]
- Ying, L.X.; Fu, Z.; Wu, K.; Wu, C.X.; Zhu, T.F.; Xie, Y.; Wang, G.X. Effect of TiO2 sol and PTFE emulsion on properties of Cu-Sn antiwear and friction reduction coatings. Coatings 2019, 9, 59. [Google Scholar] [CrossRef]
- Zhang, X.; Luster, B.; Church, A.; Muratore, C.; Voevodin, A.A.; Kohli, P.; Aouadi, S.; Talapatra, S. Carbon nanotube-MoS2 composites as solid lubricants. ACS Appl. Mater. Inter. 2009, 1, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Caminaga, C.; Neves, F.O.; Gentile, F.C.; Button, S.T. Study of alternative lubricants to the cold extrusion of steel shafts. J. Mater. Process. Technol. 2007, 182, 432–439. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Hovsepian, P.E.; Mitterer, C.; Munz, W.D. Calorimetric evidence for friction self-adaptation of TiAlN/VN superlattice coatings. Surf. Coat. Technol. 2004, 177–179, 341–347. [Google Scholar] [CrossRef]
- Gassner, G.; Mayrhofer, P.H.; Kutschej, K.; Mitterer, C.; Katherein, M. Magneli phase formation of PVD Mo-N and W-N coatings. Surf. Coat. Technol. 2006, 201, 3335–3341. [Google Scholar] [CrossRef]
- Cai, Q.; Bai, X.; Pu, J. Adaptive VAlCN-Ag composite and VAlCN/VN-Ag multilayer coatings intended for applications at elevated temperature. J. Mater. Sci. 2022, 57, 8113–8126. [Google Scholar] [CrossRef]
- Song, W.L.; Wang, S.J.; Lu, Y.; Xia, Z.X. Tribological performance of microhole-textured carbide tool filled with CaF2. Materials 2018, 11, 1643. [Google Scholar] [CrossRef]
- Yi, M.; Wang, J.; Li, C.; Bai, X.; Wei, G.; Zhang, J.; Xiao, G.; Chen, Z.; Zhou, T.; Wang, L.; et al. Friction and wear behavior of Ti(C,N) self-lubricating cermet materials with multilayer core-shell microstructure. Int. J. Refract. Met. Hard Mat. 2021, 100, 105629. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Guo, R.; Ji, L.; Guo, N.; Li, Q.; Xu, C. Preparation of Al2O3/Ti(C,N)/ZrO2/CaF2@Al(OH)3 ceramic tools and cutting performance in turning. Materials 2019, 12, 3820. [Google Scholar] [CrossRef]
- Shu, S.C.; Zhang, Q.; Ihde, J.; Yuan, Q.L.; Dai, W.; Wu, M.L.; Dai, D.; Yang, K.; Wang, B.; Xue, C.; et al. Surface modification on copper particles toward graphene reinforced copper matrix composites for electrical engineering application. J. Alloy. Compd. 2022, 891, 162058. [Google Scholar] [CrossRef]

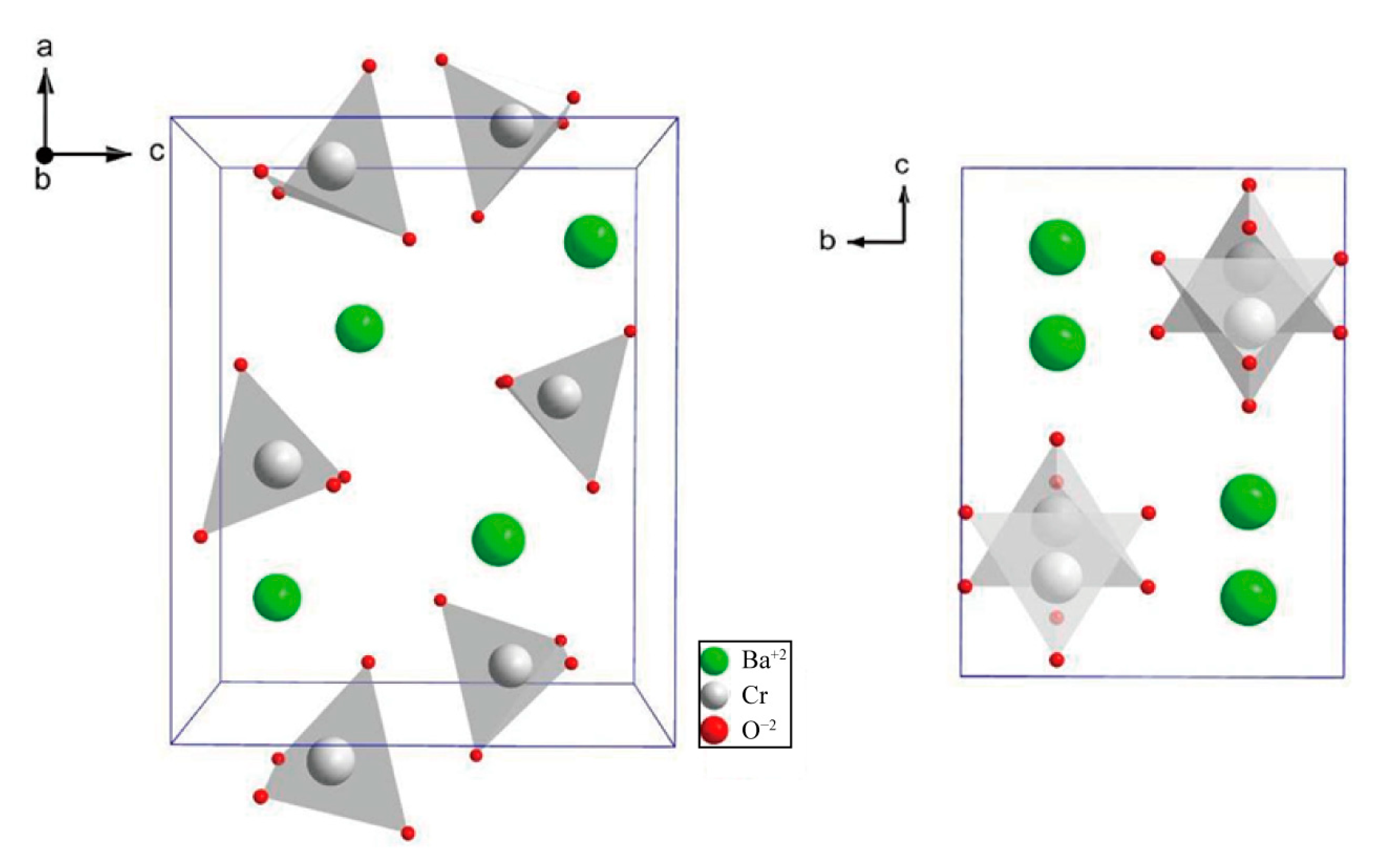




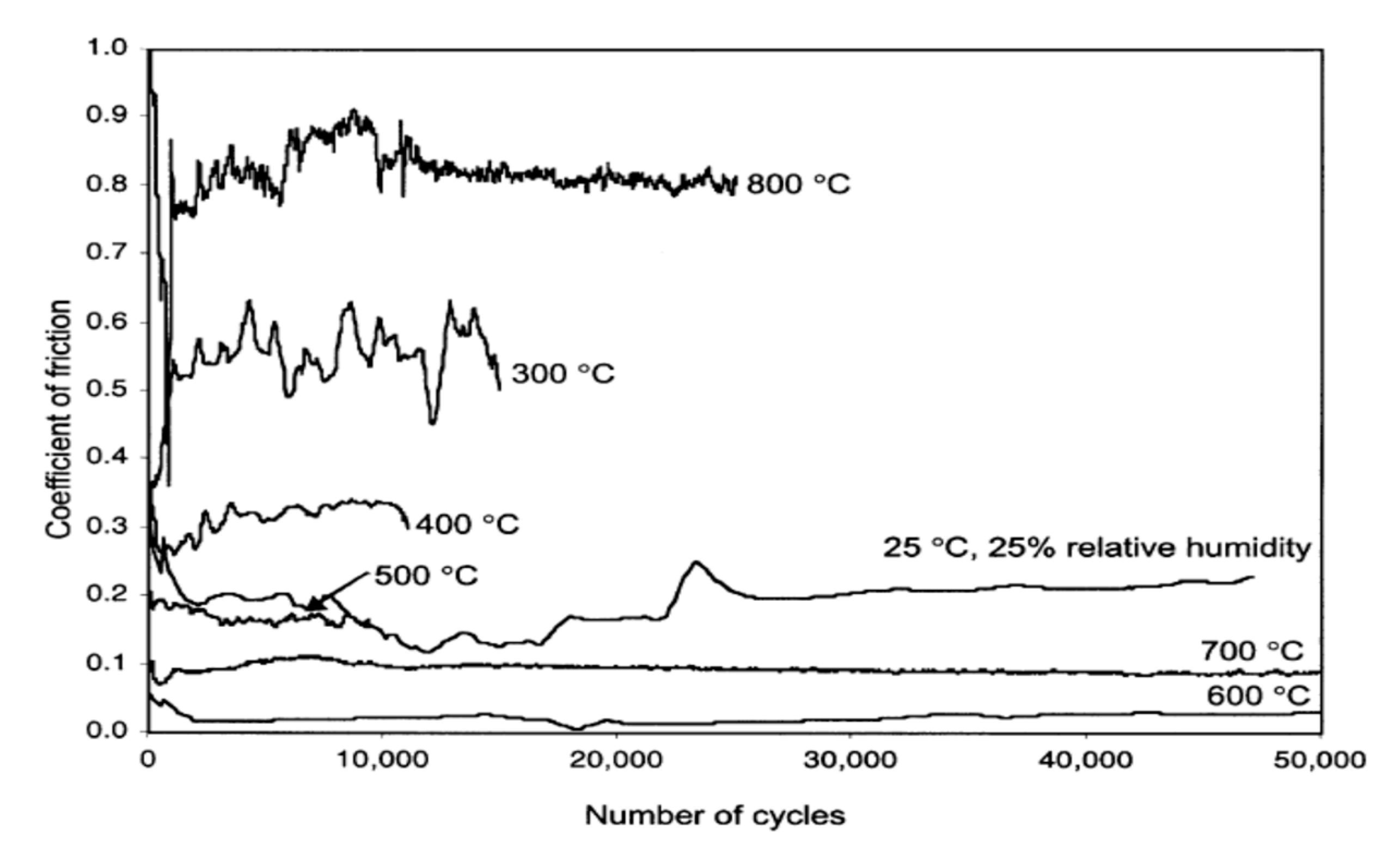

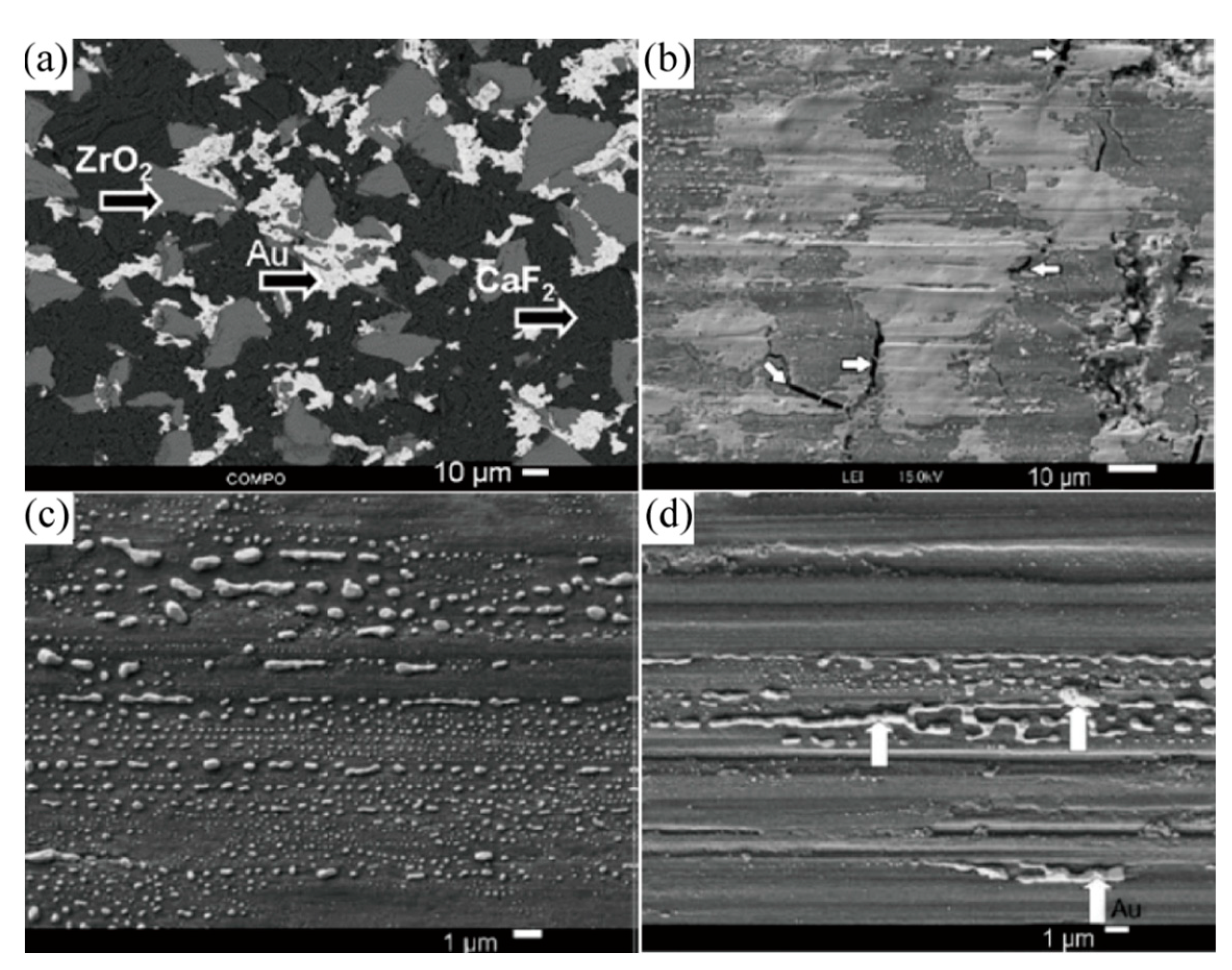
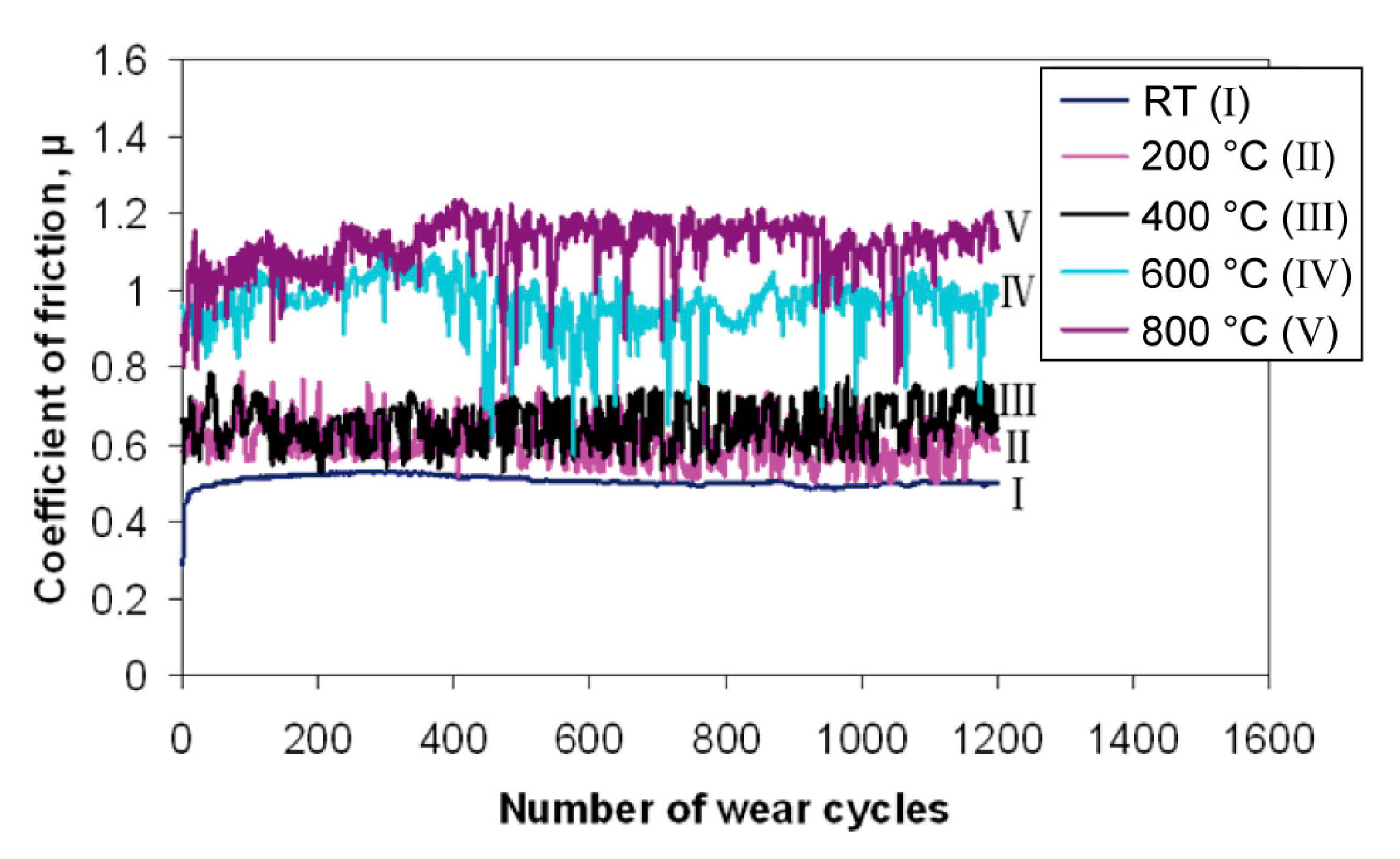
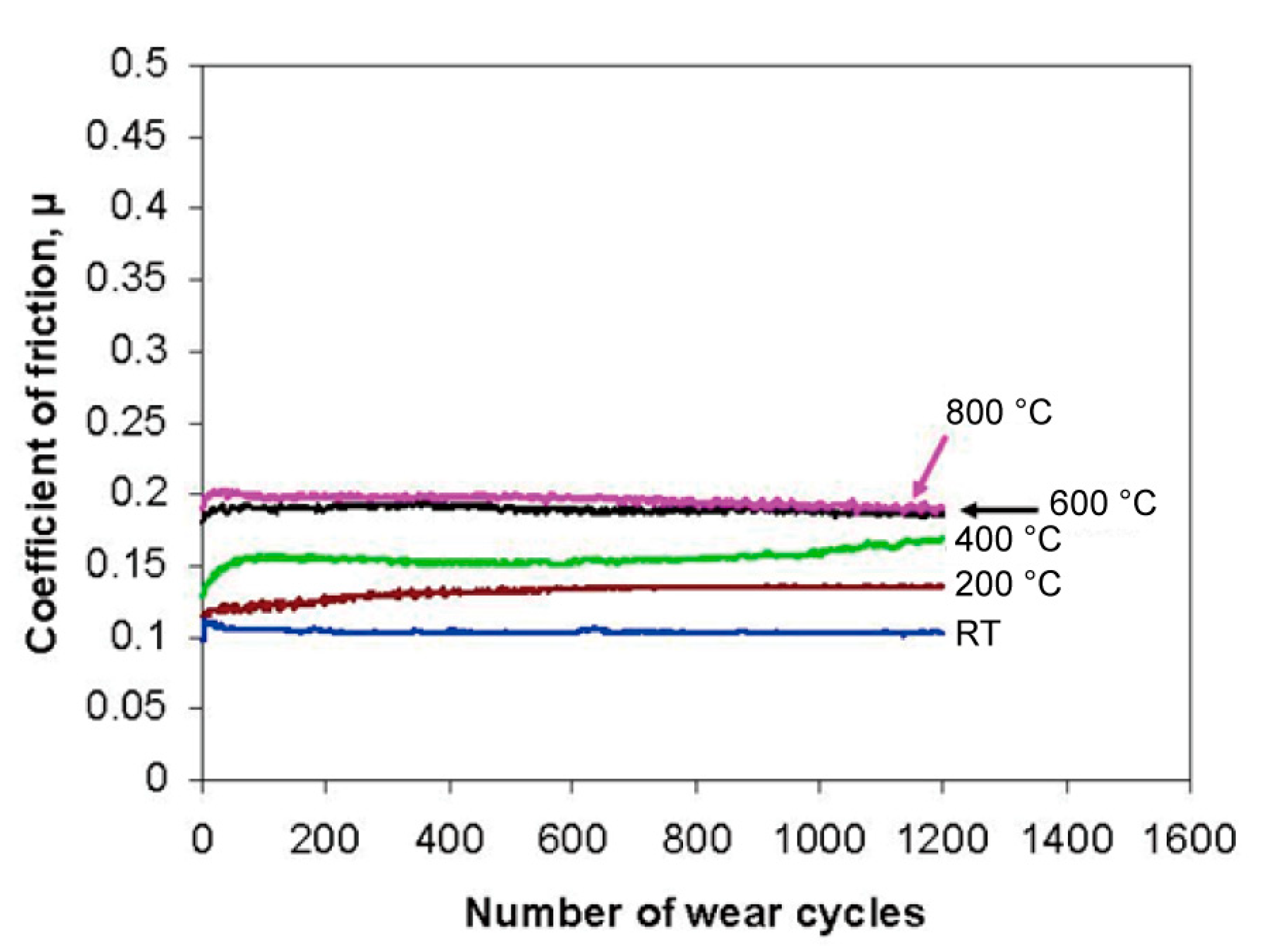
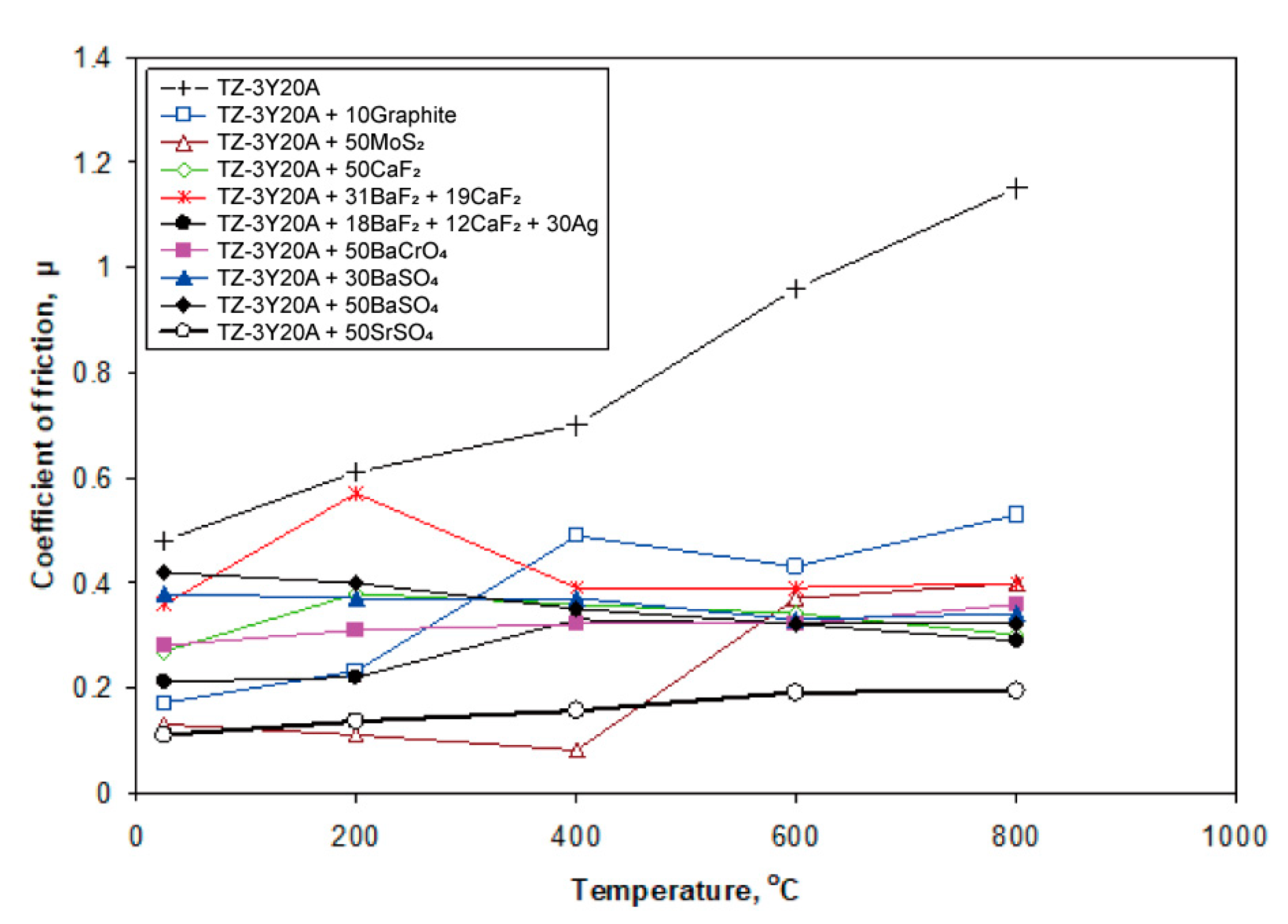
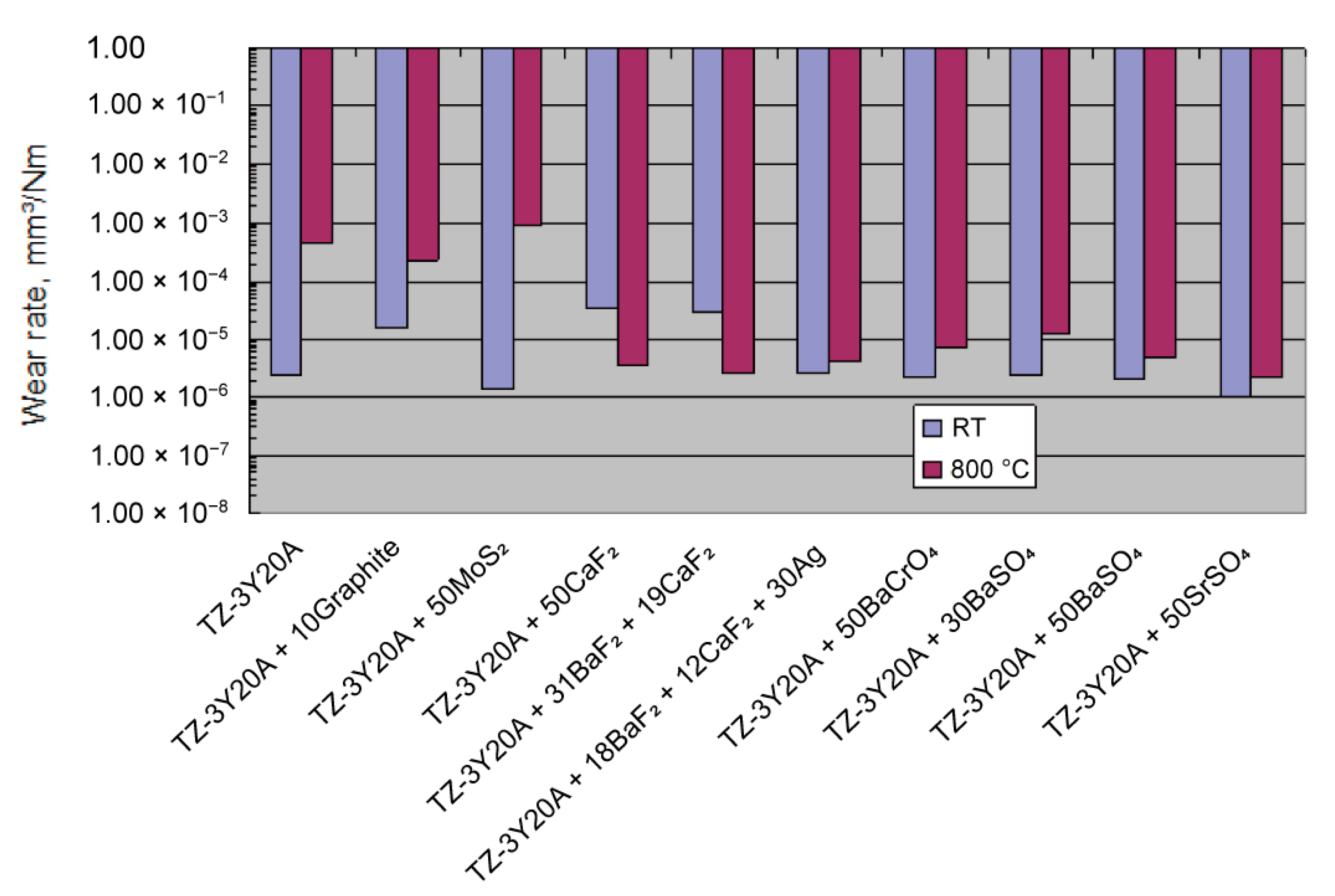
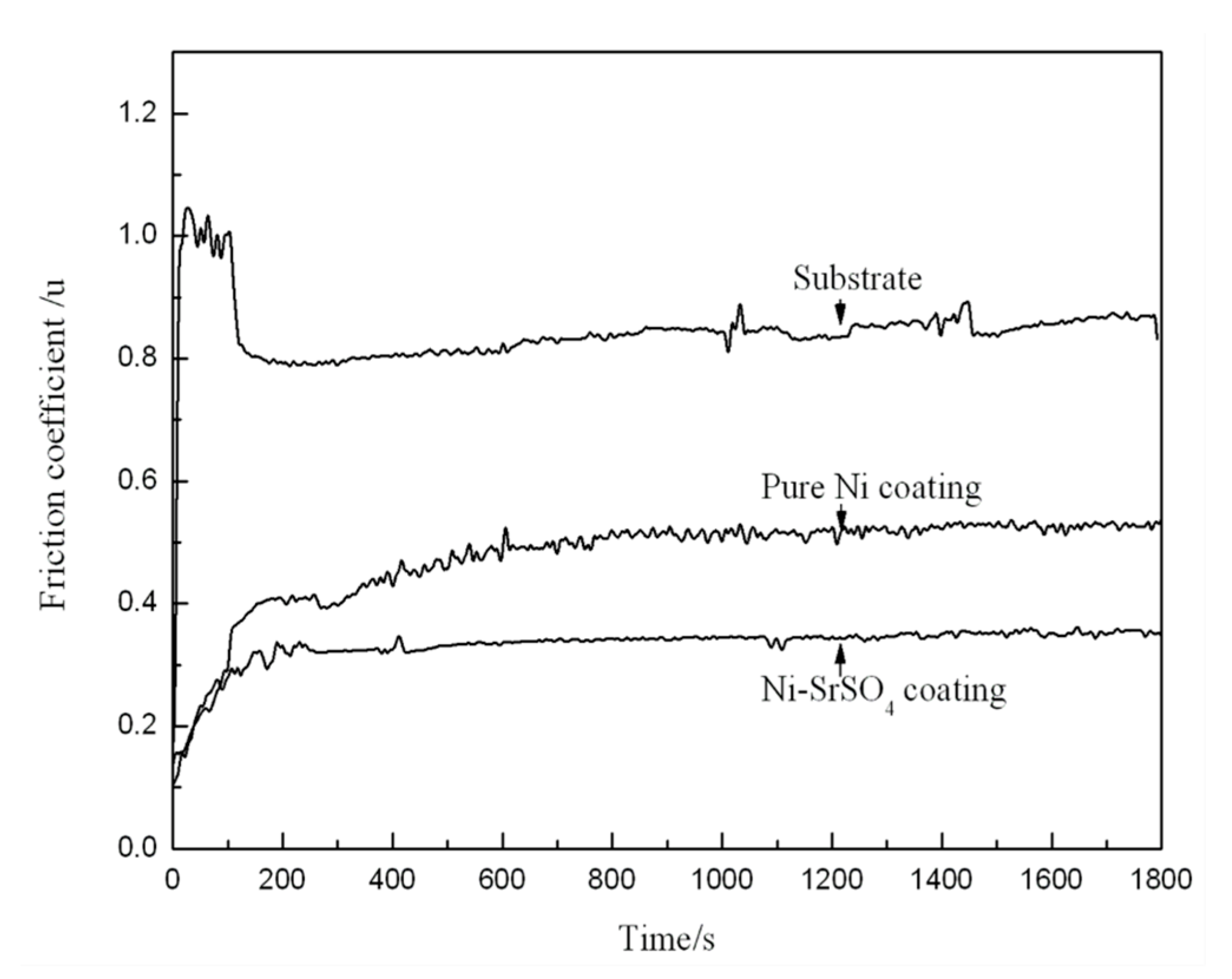
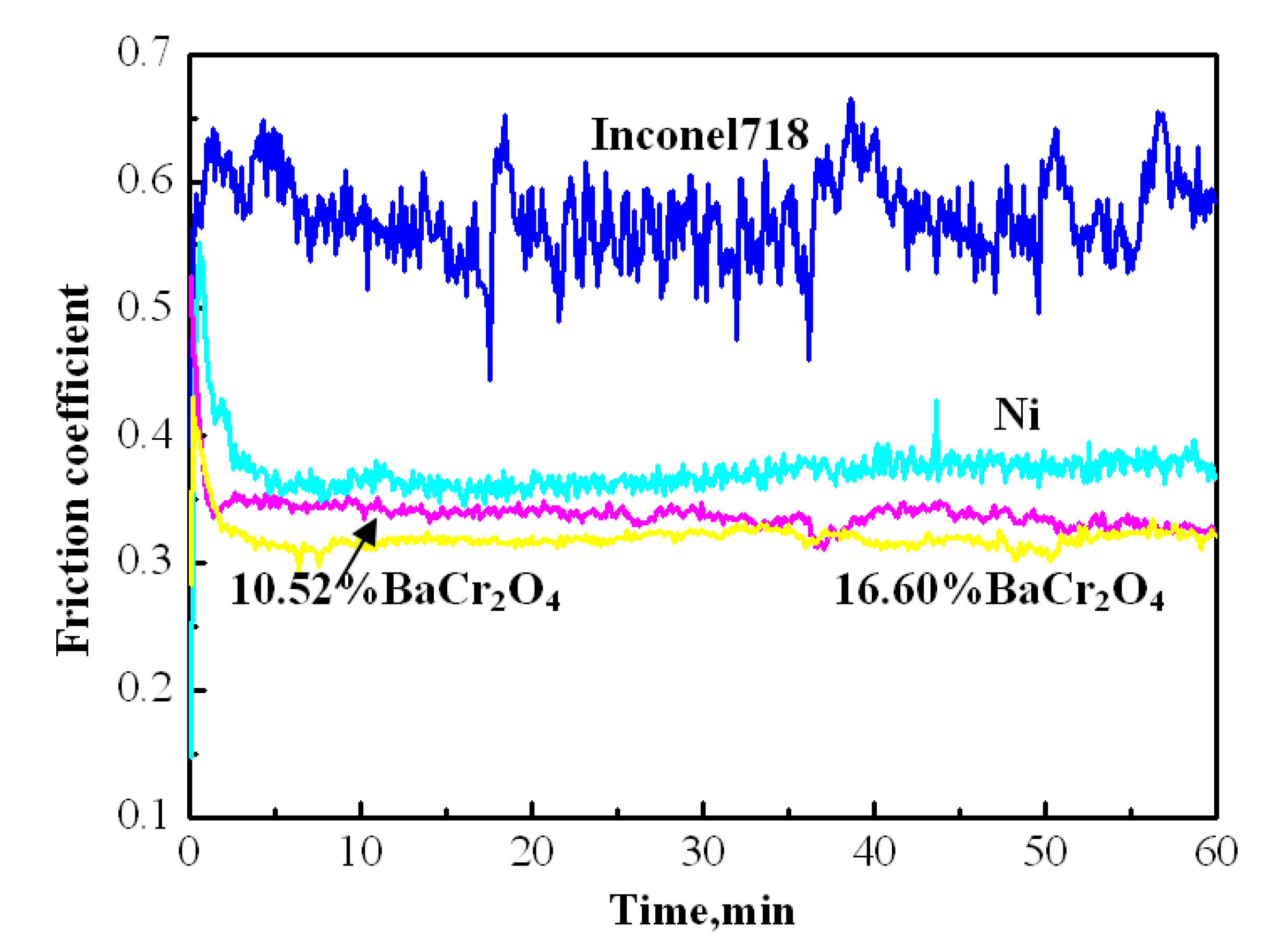
| Material | Melting Point | Mohs Hardness |
|---|---|---|
| In | 155 °C | 1.0 |
| Sn | 232 °C | 1.8 |
| Pb | 328 °C | 1.5 |
| Zn | 419 °C | 2.5 |
| Ag | 961 °C | 2.5 |
| Au | 1063 °C | 2.5 |
| Pt | 1755 °C | 4.3 |
| Materials | Fabrication Method | Tested Conditions | Results/Observations |
|---|---|---|---|
| TiN-In [40] | Sputtering deposition | Pin-on-disk; Al2O3 ball; load 1 N; 0.1 m/s; 150–1200 °C |
|
| NiMoAl-Ag [41] | High-velocity oxy-fuel spraying | Ball-on-disk Si3N4 ball; load 5 N; 0.1 m/s; 20–800 °C |
|
| NiMoAl-Al2O3-Ag [42] | Plasma spraying | Ball-on-disk Al2O3 ball; load 12 N; 0.1 m/s; RT-900 °C |
|
| Al2O3-DLC-Au-MoS2 [43] | Magnetron-assisted pulsed laser deposition | Ball-on-disk; M50 steel ball (RT); Si3N4 ball (500 °C); load 100 g; 0.2 m/s; air at 40% RH and N2 at <1% RH |
|
| Ta-Ag [44] | Magnetron sputtering deposition | Ball-on-disk; Si3N4 ball; load 2 N; 0.128 m/s; 25–600 °C |
|
| Materials | Fabrication Method | Tested Conditions | Results/Observations |
|---|---|---|---|
| h-BN h-BN-10 wt.% CaB2O4 [56] | Hot pressing | Ball-on-disk; Si3N4 ball; load 1.5 N; 0.188 m/s; RT-800 °C |
|
| Cu-based composites (Cu-Sn-Al-Fe-h-BN-Graphite-SiC) [59] | Hot pressing | Block-on-ring; AISI52100 bearing steel; load 50–125 N; 1.04–2.6 m/s; RT |
|
| B4C-h-BN [62] | Hot pressing | Pin-on-disk B4C pin; load 10 N; 0.656 m/s; 25 °C |
|
| NiCr/Cr3C2-NiCr/h-BN [63] | Plasma spraying | Ball-on-disk; Si3N4 ball; load 9.8 N; 0.188 m/s; 20–800 °C |
|
| Ni-P-h-BN [64] | Electroless plating | Pin-on-disk; AISI52100 steel ball; load 2 N; 0.1 m/s; RT |
|
| NiCrWMoAlTi-h-BN-Ag [65] | Hot pressing | Ring-on-disk; AISI52100 steel ball; load 20 N; 1 m/s; RT-600 °C |
|
| Materials | Fabrication Method | Tested Conditions | Results/Observations |
|---|---|---|---|
| PbMoO4 [117] | Pulsed laser deposition | Ball-on-flat (RT); Pin-on-disc (700 °C) 440C steel ball; 1 N; 0.6 m/s; RT, 700 °C |
|
| NiCr-BaMoO4 [118] | Hot pressing | Ball-on-disk; Si3N4 ball; load 5 N; 0.126 m/s; RT-600 °C |
|
| Mo2N-MoS2-Ag [119] | Magnetron sputtering deposition | Ball-on-disk; Si3N4 ball; load 1 N; 0.11 m/s; RT-600 °C |
|
| NiCoCrAlYTa-Ag-Mo [122] | High-velocity oxy-fuel spraying | Ball-on-disk; Al2O3 ball; load 5 N; 0.1 m/s; 25–1000 °C |
|
| NiCrAlY-Ag-Mo [123] | Atmospheric plasma spraying | Ball-on-disk; Si3N4 ball; load 5 N; 0.3 m/s; 20–800 °C |
|
| Ni3Al-Ag-BaMoO4 [124] | Hot pressing | Ball-on-disk; Si3N4 ball; 20 N; 0.19 m/s; 20–800 °C |
|
| Materials | Fabrication Method | Tested Conditions | Results/Observation |
|---|---|---|---|
| VN-Ag [121] | Magnetron sputtering deposition | Ball-on-disk; Si3N4 ball; load 2 N; 0.11 m/s; RT-1000 °C |
|
| VN-Ag [132] | Pulsed laser deposition | Ball-on-disk; Al2O3 ball; load 10 N; 0.063 m/s; RT-900 °C |
|
| NiCrAlY-Cr3C2(NiCr)-V2O5-Ag2O [133] | Laser cladding | Ball-on-disk; Si3N4 ball; load 3 N; 0.188 m/s; 25–800 °C |
|
| Materials | Fabrication Method | Tested Conditions | Results/Observations |
|---|---|---|---|
| ZrO2(Y2O3)-BaCrO4 [15] | Low-pressure plasma spraying | Ball-on-block; Al2O3 ball; load 50 N; frequency of 10 Hz with stroke of 1 mm; RT-800 °C |
|
| BaCr2O4 [142] | Hot pressing | Ball-on-disk Al2O3 ball; load 5 N; 0.126 m/s; RT-800 °C |
|
| ZrO2(Y2O3)-BaCrO4 [143] | Spark plasma sintering | Ball-on-block; Al2O3 ball; load 30 N; frequency of 10 Hz with stroke of 1 mm; RT-800 °C |
|
| Al2O3-BaCrO4 [144] | Spark plasma sintering | Ball-on-block; Al2O3 ball; load 10 N; frequency of 10 Hz with stroke of 1 mm; RT-800 °C |
|
| Al2O3-BaCrO4-SiO2 [144] | Spark plasma sintering |
| |
| Al2O3-BaCrO4-Ag [144] | Spark plasma sintering |
| |
| NiCr-BaCr2O4 [151] | Hot pressing | Ball-on-disk Al2O3 ball; load 5 N; 0.126 m/s; RT-800 °C |
|
| Ni-16.6 vol.% BaCr2O4 [150] | Electrodeposition | Ball-on-disk Al2O3 ball; load 2 N; rotating speed 400 rpm; rotating radius 3 mm; RT |
|
| Materials | Fabrication Method | Tested Conditions | Results/Observations |
|---|---|---|---|
| Al2O3-Mo-BaSO4 [136] | BaSO4 was burnished onto textured surface of hot pressed Al2O3-Mo. | Pin-on-disk; Al2O3 pin; load 70 N; frequency of 10 Hz with stroke of 1 mm; RT-800 °C |
|
| ZrO2(Y2O3)-BaSO4 [101] | Spark plasma sintering | Ball-on-block; Al2O3 ball; load 5 N; frequency of 1 Hz with stroke of 10 mm; RT-800 °C |
|
| Al2O3-SrSO4 [156] | Spark plasma sintering | Ball-on-block; Al2O3 ball; load 5 N; frequency of 1 Hz with stroke of 10 mm; RT-800 °C |
|
| Al2O3-PbSO4-SiO2 [156] | Spark plasma sintering |
| |
| Al2O3-BaSO4-Ag [156] | Spark plasma sintering |
| |
| ZrO2(Y2O3)-Al2O3-Ba0.5Sr0.5SO4 [157] | Spark plasma sintering | Ball-on-block; Al2O3 ball; load 5 N; frequency of 1 Hz with stroke of 10 mm; RT, 760 °C |
|
| Fe3Al–Ba0.25Sr0.75SO4 [158] | Hot pressing | Ball-on-disk; Si3N4 ball; load 10 N; 0.01 m/s; RT-800 °C |
|
| Ni-6.83 vol.% SrSO4 [159] | Electrodeposition | Ball-on-disk; SAE52100 bearing ball; load 0.5 N; rotating speed 50 rpm; rotating radius 5mm; RT |
|
| SrSO4-Ag [160] | Chemical precipitation | Ball-on-block; Al2O3 ball; load 5 N; frequency of 1 Hz with stroke of 10 mm; RT-800 °C |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.-H.; Li, Y.-F.; Zhang, Y.-Z.; Wang, Y.-M.; Wang, Y.-J. High-Temperature Solid Lubricants and Self-Lubricating Composites: A Critical Review. Lubricants 2022, 10, 177. https://doi.org/10.3390/lubricants10080177
Ouyang J-H, Li Y-F, Zhang Y-Z, Wang Y-M, Wang Y-J. High-Temperature Solid Lubricants and Self-Lubricating Composites: A Critical Review. Lubricants. 2022; 10(8):177. https://doi.org/10.3390/lubricants10080177
Chicago/Turabian StyleOuyang, Jia-Hu, Yu-Feng Li, Yun-Zhuo Zhang, Ya-Ming Wang, and Yu-Jin Wang. 2022. "High-Temperature Solid Lubricants and Self-Lubricating Composites: A Critical Review" Lubricants 10, no. 8: 177. https://doi.org/10.3390/lubricants10080177
APA StyleOuyang, J.-H., Li, Y.-F., Zhang, Y.-Z., Wang, Y.-M., & Wang, Y.-J. (2022). High-Temperature Solid Lubricants and Self-Lubricating Composites: A Critical Review. Lubricants, 10(8), 177. https://doi.org/10.3390/lubricants10080177






