Assessment of Stability and Thermophysical Properties of Jojoba Nanofluid as a Metal-Cutting Fluid: Experimental and Modelling Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanofluid Preparation
2.2. Stability and Thermophysical Properties Measurement
2.2.1. Stability Assessment
2.2.2. Viscosity Measurement
2.2.3. Thermal Conductivity
3. Results and Discussion
3.1. Stability Assessment
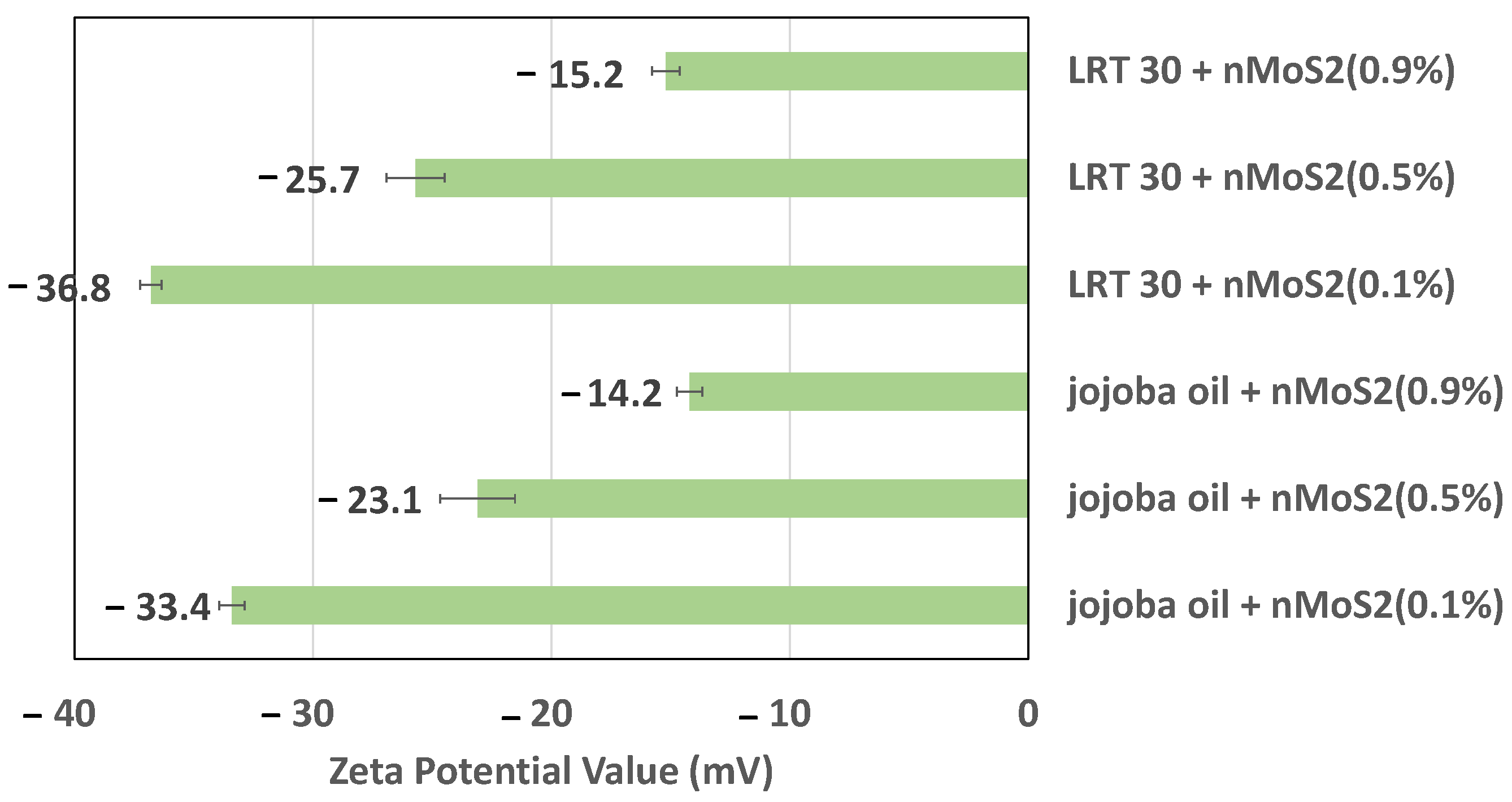
3.1.1. Zeta Potential Test
3.1.2. Sedimentation Test
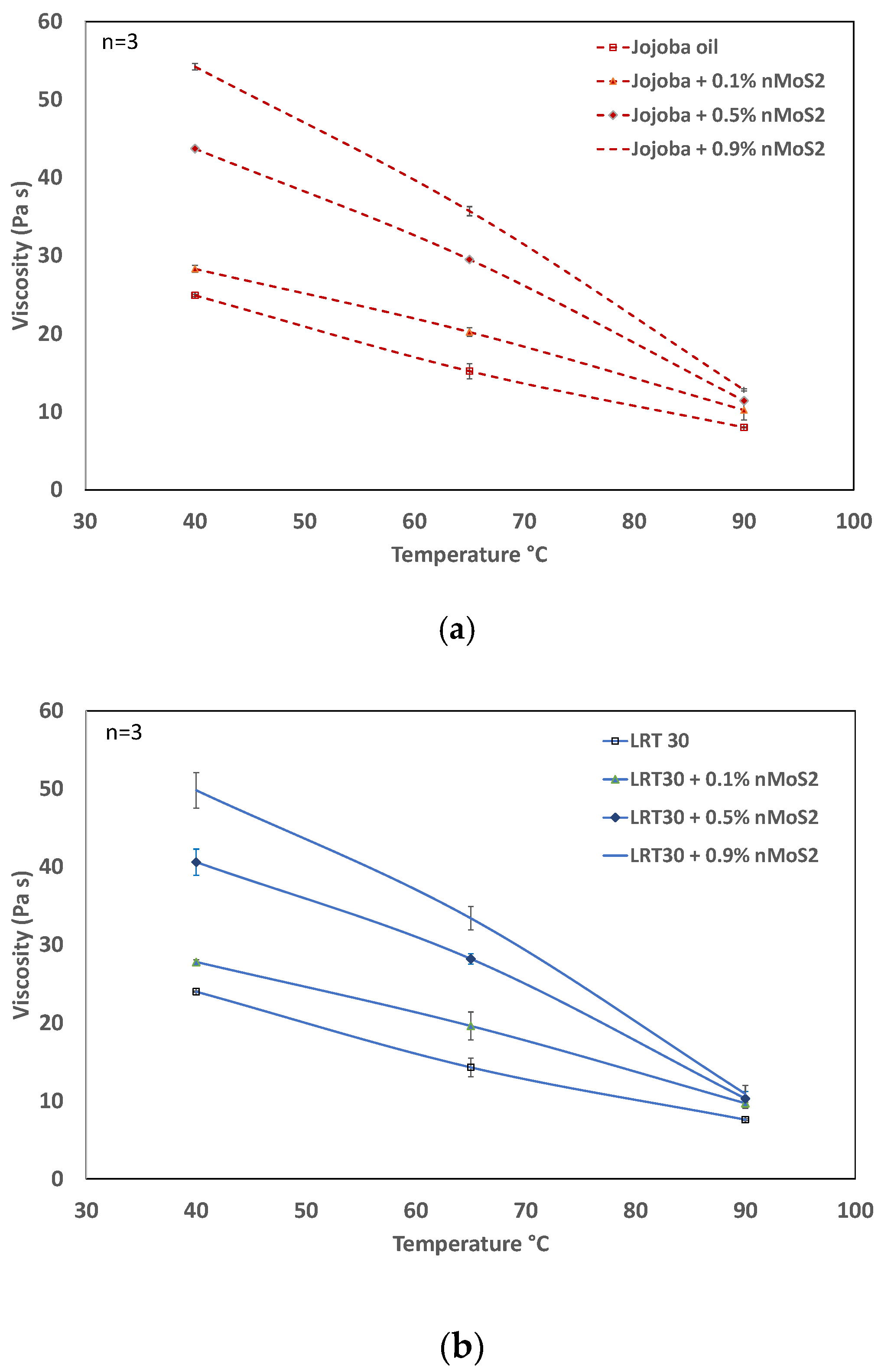
3.2. Influence of Concentration and Temperatures on Viscosity
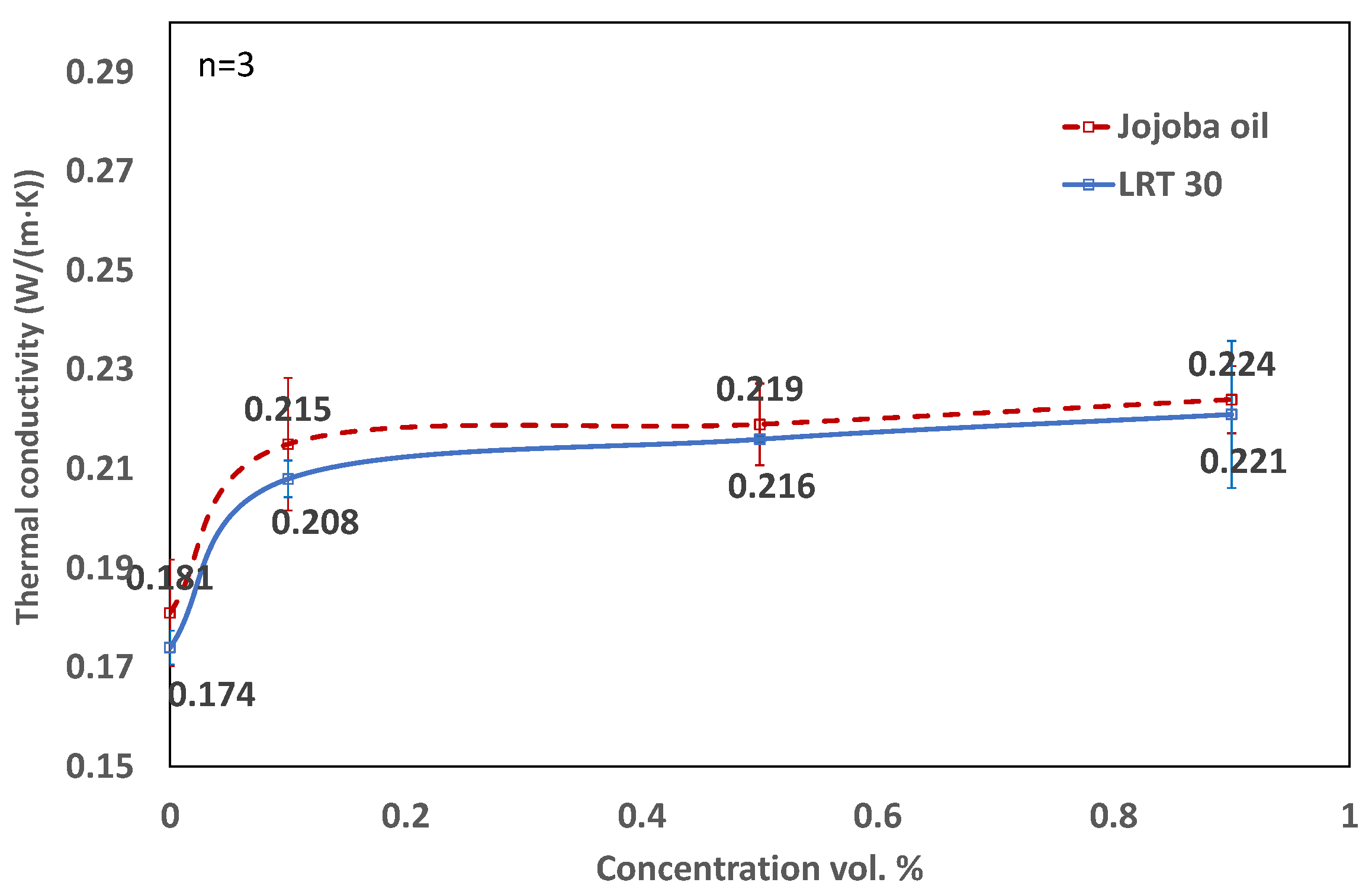
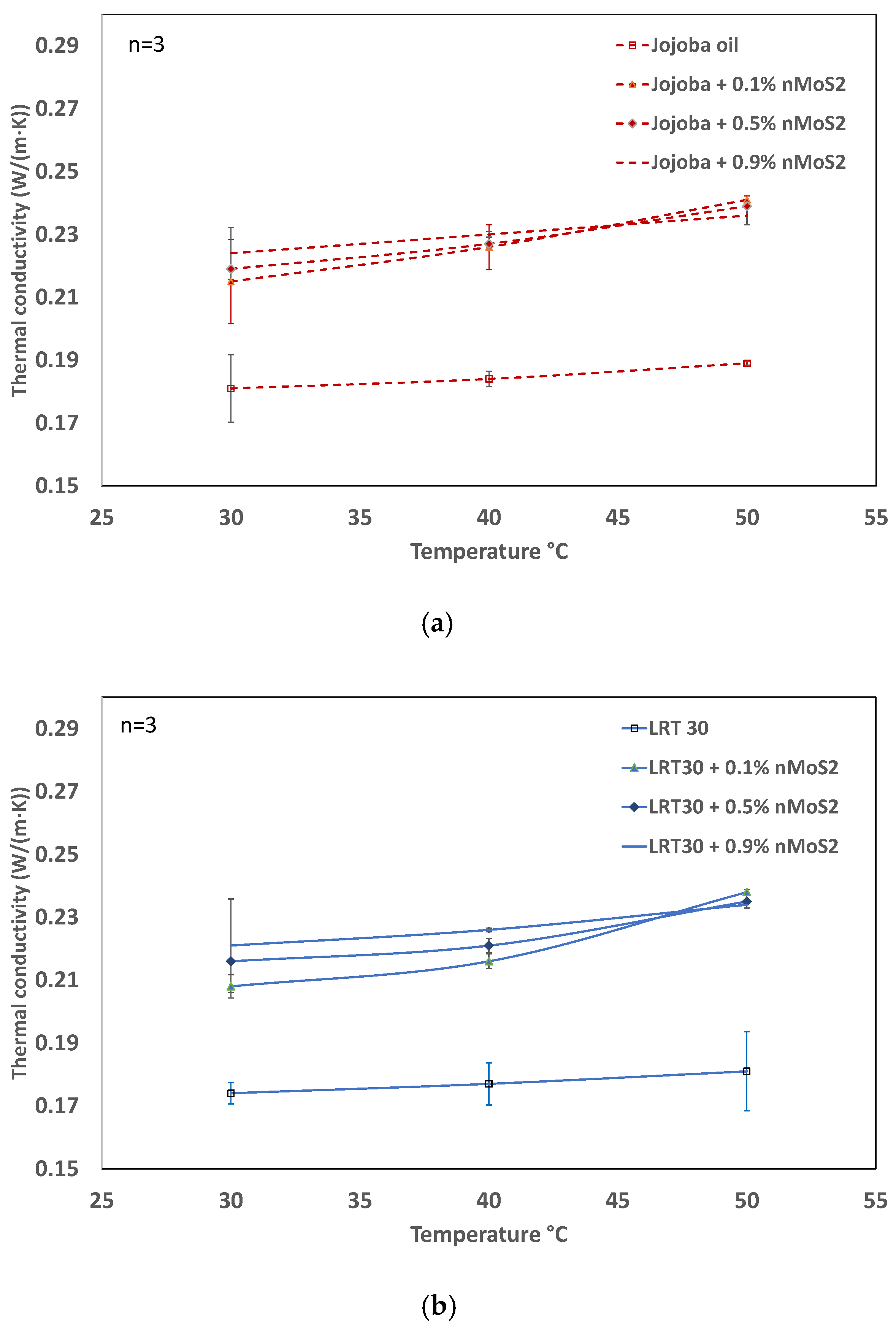
3.3. Influence of Concentration and Temperatures on Thermal Conductivity
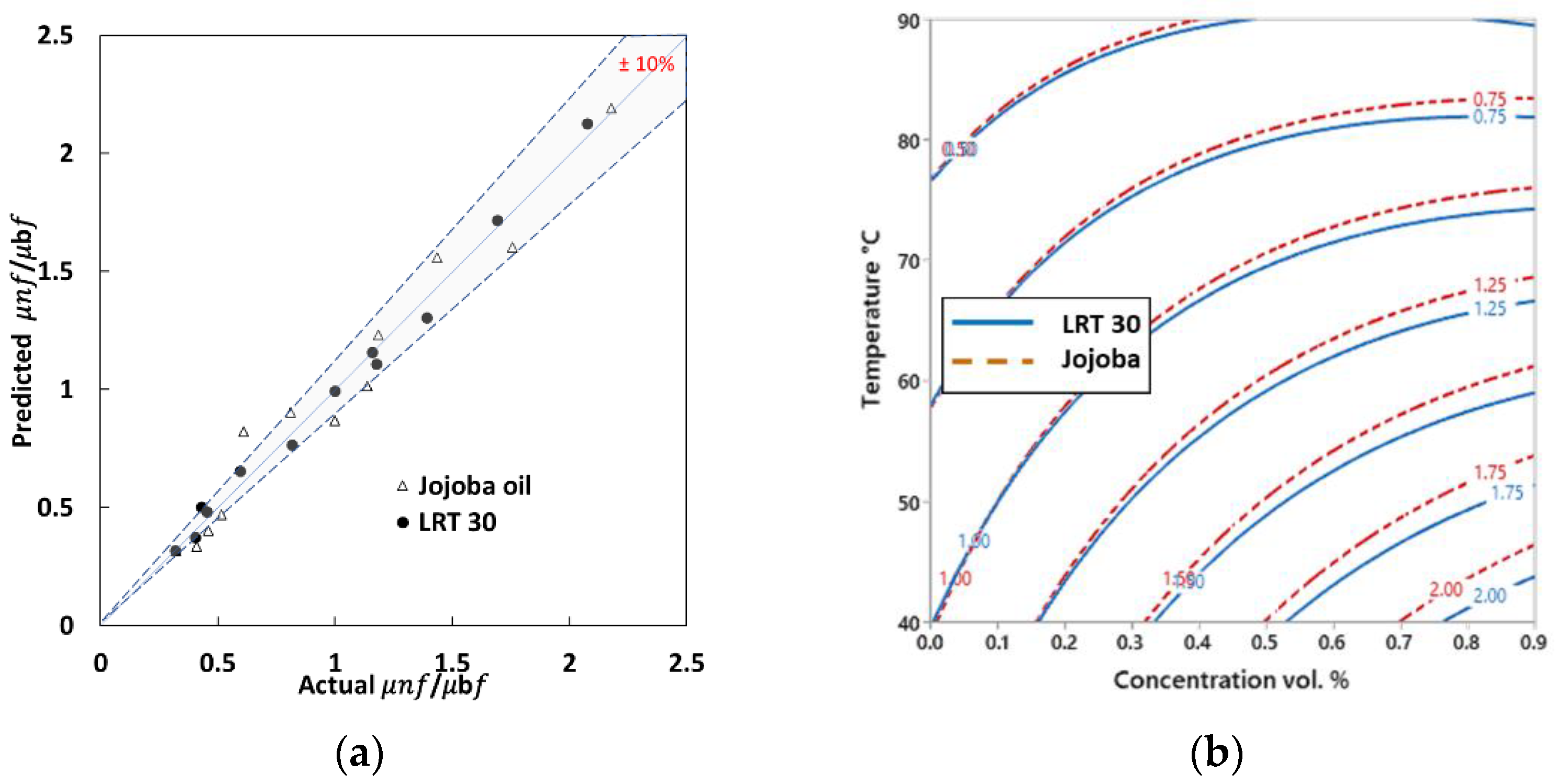
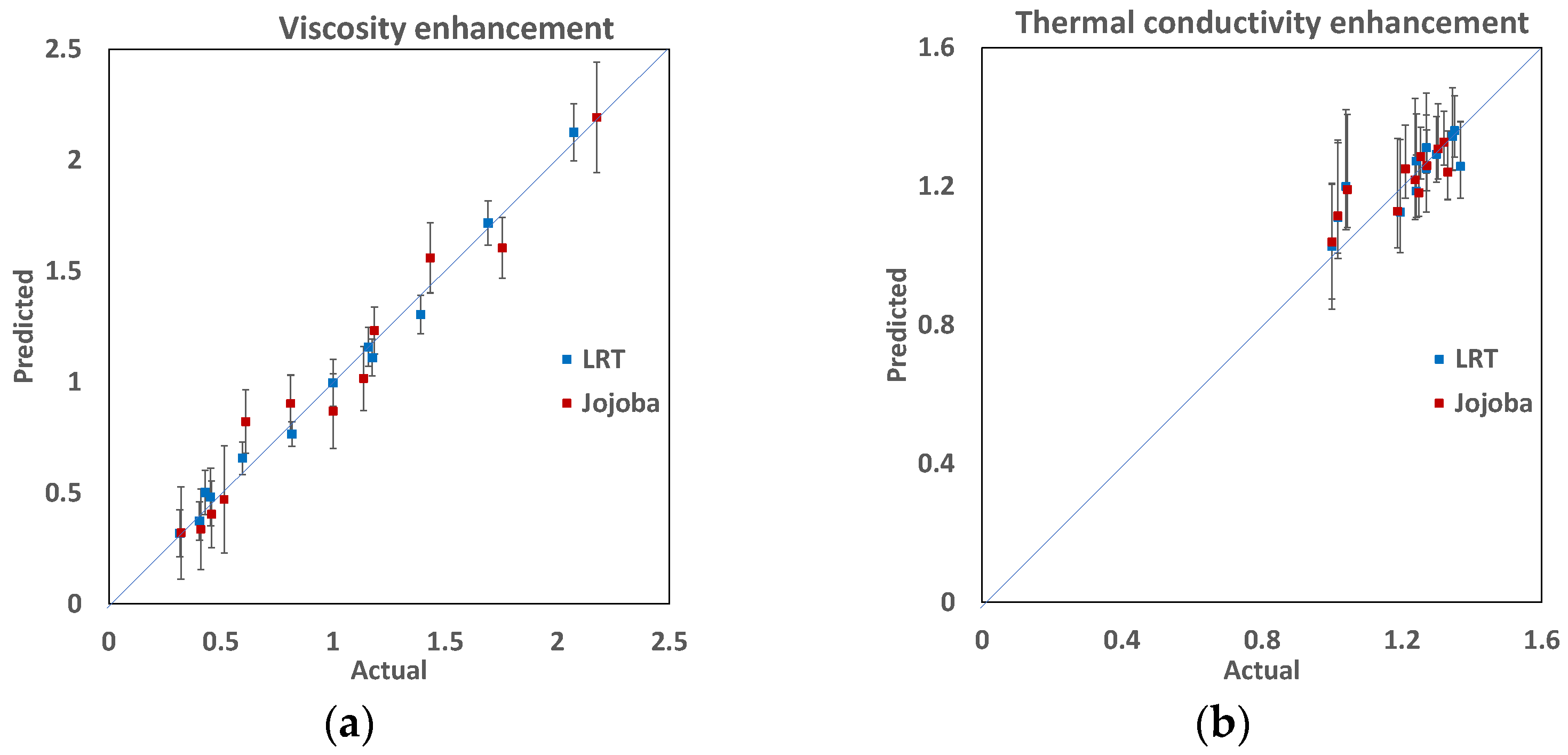
3.4. Predictive Models
3.4.1. Viscosity Model
3.4.2. Thermal Conductivity Model
4. Conclusions
- The nanoparticle concentration affects the stability of the nanofluids jojoba + nMoS2 and LRT 30 + nMoS2. The as-prepared nanofluid shows higher Zeta potential at lower concentrations (e.g., 0.1%) than at higher concentrations (e.g., 0.9%). Increases in concentration lead to a condition in which the attractive force is greater than the repulsive force vis-à-vis the particles colliding and aggregating.
- The nanofluids remain stable for the first 24 h. Subsequently, the nanofluids with a higher concentration of nanoparticles help to increase the density and viscosity; thus, nanofluids with a concentration of 0.1% sediment before those at 0.5 and 0.9% concentrations.
- The viscosity of jojoba- and LRT 30-based nanofluids (at a reference temperature of 40 °C) increases linearly with the addition of MoS2 nanoparticles. With increases in temperature, the viscosity falls. At higher temperatures (e.g., 90 °C), the effects of changes in the nanoparticle concentration are not visible, i.e., the viscosity at 0.1 to 0.9% concentration at 90 °C remains almost the same.
- The thermal conductivity shows a sharp rise when a small amount of nanoparticle (0.1 vol.%) is added to the base fluids. Subsequently, significant gains are not observed. At higher concentrations, the expected increase in thermal conductivity due to the increase in density is counter-balanced by the increase in attractive forces, which hampers the mobility of nanoparticles and the corresponding change in thermal conductivity.
- The viscosity of jojoba nanoliquid is nearly the same as that of LRT 30, especially at higher temperatures. Jojoba nanofluid at higher concentrations reaches a higher thermal conductivity, even at lower temperatures, than LRT 30 nanofluid.
- Viscosity and thermal conductivity can be accurately predicted with respect to nanoparticle concentrations and temperatures. The viscosity’s relation to nanoparticle concentrations and temperatures fits well with a second-order function. The thermal conductivity relationship is more complicated and follows a third-order interaction function.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishna, P.V.; Srikant, R.R.; Rao, D.N. Experimental investigation on the performance of nanoboric acid suspensions in SAE-40 and coconut oil during turning of AISI 1040 steel. Int. J. Mach. Tools Manuf. 2010, 50, 911–916. [Google Scholar] [CrossRef]
- Danish, M.; Gupta, M.K.; Rubaiee, S.; Ahmed, A.; Sarikaya, M. Influence of graphene reinforced sunflower oil on thermo-physical, tribological and machining characteristics of inconel 718. J. Mater. Res. Technol. 2021, 15, 135–150. [Google Scholar] [CrossRef]
- Cui, X.; Cao, P.; Guo, J.; Ming, P. Use and performance of soybean oil based bio-lubricant in reducing specific cutting energy during biomimetic machining. J. Manuf. Process. 2021, 62, 577–590. [Google Scholar] [CrossRef]
- Ni, J.; Cui, Z.; Wu, C.; Sun, J.; Zhou, J. Evaluation of MQL broaching AISI 1045 steel with sesame oil containing nanoparticles under best concentration. J. Clean. Prod. 2021, 320, 128888. [Google Scholar] [CrossRef]
- Puttaswamy, J.T.; Ramachandra, J.S. Experimental investigation on the performance of vegetable oil based cutting fluids in drilling AISI 304L using Taguchi technique. Tribol. Online 2018, 13, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Olawale, K.B.; Aji, I.S.; Ejilah, R.I. Lubricity assessment of neem and castor oils and their blends in machining mild steel. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 48, 128–137. [Google Scholar]
- Wang, Y.; Li, C.; Zhang, Y.; Yang, M.; Li, B.; Jia, D.; Hou, Y.; Mao, C. Experimental evaluation of the lubrication properties of the wheel/workpiece interface in minimum quantity lubrication (MQL) grinding using different types of vegetable oils. J. Clean. Prod. 2016, 127, 487–499. [Google Scholar] [CrossRef]
- Sajeeb, A.; Rajendrakumar, P.K. Comparative evaluation of lubricant properties of biodegradable blend of coconut and mustard oil. J. Clean. Prod. 2019, 240, 118255. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Jia, D.; Zhang, D.; Zhang, X. Experimental evaluation of MoS2 nanoparticles in jet MQL grinding with different types of vegetable oil as base oil. J. Clean. Prod. 2015, 87, 930–940. [Google Scholar] [CrossRef]
- Taha-Tijerina, J.; Aviña, K.; Diabb, J.M. Tribological and thermal transport performance of SiO2-based natural lubricants. Lubricants 2019, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Akincioğlu, S.; Şirin, Ş. Evaluation of the tribological performance of the green hBN nanofluid on the friction characteristics of AISI 316L stainless steel. Ind. Lubr. Tribol. 2021, 73, 1176–1186. [Google Scholar] [CrossRef]
- Su, Y.; Gong, L.; Chen, D. Dispersion stability and thermophysical properties of environmentally friendly graphite oil–based nanofluids used in machining. Adv. Mech. Eng. 2016, 8, 1687814015627978. [Google Scholar] [CrossRef]
- Khan, M.S.; Sisodia, M.S.; Gupta, S.; Feroskhan, M.; Kannan, S.; Krishnasamy, K. Measurement of tribological properties of Cu and Ag blended coconut oil nanofluids for metal cutting. Eng. Sci. Technol. Int. J. 2019, 22, 1187–1192. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Zhang, Y.; Wang, Y.; Yang, M.; Jia, D.; Zhang, N.; Wu, Q. Effect of the physical properties of different vegetable oil-based nanofluids on MQLC grinding temperature of Ni-based alloy. Int. J. Adv. Manuf. 2017, 89, 3459–3474. [Google Scholar] [CrossRef]
- Li, Y.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Techno. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Zhang, Y.; Wang, Y.; Jia, D.; Yang, M.; Zhang, N.; Wu, Q.; Han, Z.; Sun, K. Heat transfer performance of MQL grinding with different nanofluids for Ni-based alloys using vegetable oil. J. Clean. Prod. 2017, 154, 1–11. [Google Scholar] [CrossRef]
- Chinchanikar, S.; Kore, S.S.; Hujare, P. A review on nanofluids in minimum quantity lubrication machining. J. Manuf. Process. 2021, 68, 56–70. [Google Scholar] [CrossRef]
- Krajnik, P.; Pusavec, F.; Rashid, A. Nanofluids: Properties, Applications and Sustainability Aspects in Materials Processing Technologies. In Advances in Sustainable Manufacturing; Seliger, G., Khraisheh, M., Jawahir, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 107–113. [Google Scholar]
- Su, Y.; Gong, L.; Li, B.; Liu, Z.; Chen, D. Performance evaluation of nanofluid MQL with vegetable-based oil and ester oil as base fluids in turning. Int. J. Adv. Manuf. 2016, 83, 2083–2089. [Google Scholar] [CrossRef]
- Naik, B.A.K.; Vinod, A.V. Rheological behavior and effective thermal conductivity of non-Newtonian nanofluids. J. Test. Eval. 2017, 46, 445–456. [Google Scholar]
- Breki, A.; Nosonovsky, M. Einstein’s viscosity equation for nanolubricated friction. Langmuir 2018, 34, 12968–12973. [Google Scholar] [CrossRef]
- Dardan, E.; Afrand, M.; Isfahani, A.M. Effect of suspending hybrid nano-additives on rheological behavior of engine oil and pumping power. Appl. D Therm. Eng. 2016, 109, 524–534. [Google Scholar] [CrossRef]
- Babar, H.; Sajid, M.U.; Ali, H.M. Viscosity of hybrid nanofluids: A critical review. Therm. Sci. 2019, 23, 1713–1754. [Google Scholar] [CrossRef] [Green Version]
- Mechiri, S.K.; Vasu, V.; Venu Gopal, A. Investigation of thermal conductivity and rheological properties of vegetable oil based hybrid nanofluids containing Cu–Zn hybrid nanoparticles. Exp. Heat Transf. 2017, 30, 205–217. [Google Scholar] [CrossRef]
- Banerjee, N.; Sharma, A. A review on localised and multi-point aerosol application in minimum quantity lubrication machining. Int. J. Preci. Technol. 2020, 9, 95–117. [Google Scholar] [CrossRef]
- Banerjee, N.; Sharma, A. Improving machining performance of Ti-6Al-4V through multi-point minimum quantity lubrication method. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2019, 233, 321–336. [Google Scholar] [CrossRef]
- Banerjee, N.; Sharma, A. Identification of a friction model for minimum quantity lubrication machining. J. Clean. Prod. 2014, 83, 437–443. [Google Scholar] [CrossRef]
- Sikdar, S.; Rahman, M.H.; Menezes, P.L. Synergistic Study of Solid Lubricant Nano-Additives Incorporated in canola oil for Enhancing Energy Efficiency and Sustainability. Sustainability 2021, 14, 290. [Google Scholar] [CrossRef]
- Rubbi, F.; Das, L.; Habib, K.; Aslfattahi, N.; Saidur, R.; Rahman, M.T. State-of-the-art review on water-based nanofluids for low temperature solar thermal collector application. Sol. Energy Mater. Sol. Cells 2021, 230, 111220. [Google Scholar] [CrossRef]
- Nobrega, G.; de Souza, R.R.; Gonçalves, I.M.; Moita, A.S.; Ribeiro, J.E.; Lima, R.A. Recent Developments on the Thermal Properties, Stability and Applications of Nanofluids in Machining, Solar Energy and Biomedicine. Appl. Sci. 2022, 12, 1115. [Google Scholar] [CrossRef]
- Khan, S.A.; Tariq, M.; Khan, A.A.; Alamri, B.; Mihet-Popa, L. Assessment of Thermophysical Performance of Ester-Based Nanofluids for Enhanced Insulation Cooling in Transformers. Electronics 2022, 11, 376. [Google Scholar] [CrossRef]
- Zeagham, M.; Jadoon, T.M.; Qureshi, M.I.; Qureshi, B.; Sabir, S. In Search of a “Stable Green Nanofluid” for Applications in High Voltage Equipment. Eng.Proc. 2021, 12, 58. [Google Scholar]
- Gaurav, G.; Sharma, A.; Dangayach, G.S.; Meena, M.L. Assessment of jojoba as a pure and nanofluid base oil in minimum quantity lubrication (MQL) hard-turning of Ti–6Al–4V: A step towards sustainable machining. J. Clean. Prod. 2020, 272, 122553. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Pal, A.; Chatha, S.S.; Singh, K. Performance evaluation of minimum quantity lubrication technique in grinding of AISI 202 stainless steel using nano-MoS2 with vegetable-based cutting fluid. Int. J. Adv. Manuf. 2020, 110, 125–137. [Google Scholar] [CrossRef]
- Gugulothu, S.; Pasam, V.K. Experimental investigation to study the performance of CNT/MoS2 hybrid nanofluid in turning of AISI 1040 steel. Aust. J. Mech. Eng. 2020, 1–11. [Google Scholar] [CrossRef]
- Sahasrabudhe, S.N.; Rodriguez-Martinez, V.; O’Meara, M.; Farkas, B.E. Density, viscosity, and surface tension of five vegetable oils at elevated temperatures: Measurement and modeling. Int. J. Food Prop. 2017, 20, 1965–1981. [Google Scholar] [CrossRef] [Green Version]
- Nwoguh, T.O.; Okafor, A.C.; Onyishi, H.A. Enhancement of viscosity and thermal conductivity of soybean vegetable oil using nanoparticles to form nanofluids for minimum quantity lubrication machining of difficult-to-cut metals. Int. J. Adv. Manuf. 2021, 113, 3377–3388. [Google Scholar] [CrossRef]
- Banerjee, N.; Sharma, A. A comprehensive assessment of minimum quantity lubrication machining from quality, production, and sustainability perspectives. Sustain. Mater. Technol. 2018, 17, e00070. [Google Scholar] [CrossRef]





| Specification | Value |
|---|---|
| APS | 80–100 nm |
| Purity | 99.9% |
| Colour | Black |
| Density | 4.8 g/cm³ |
| Molecular Weight | 160.07 g/mol |
| Melting Point | 1185 °C |
| Morphology | Flaky Plates |
| Properties | Jojoba Oil | LRT 30 Oil |
|---|---|---|
| Viscosity cP at 40 °C | 24.9 | 24 |
| Viscosity cP at 90 °C | 8.0 | 7.6 |
| Density (kg/m3) | 867 | 953 |
| Flammability point °C | 295 | 220 |
| Solubility | Not soluble in water | Not soluble in water |
| Specific gravity at 25 °C | 0.86 | 0.9 |
| Appearance | Clear golden yellow | Clear golden yellow |
| Nanofluid | nMoS2 Concentration (% vol.) | Density (kg/m3) |
|---|---|---|
| nMoS2 + jojoba oil | 0.1 | 870 |
| 0.5 | 896 | |
| 0.9 | 912 | |
| nMoS2 + LRT 30 | 0.1 | 968 |
| 0.5 | 979 | |
| 0.9 | 987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaurav, G.; Dangayach, G.S.; Meena, M.L.; Sharma, A. Assessment of Stability and Thermophysical Properties of Jojoba Nanofluid as a Metal-Cutting Fluid: Experimental and Modelling Investigation. Lubricants 2022, 10, 126. https://doi.org/10.3390/lubricants10060126
Gaurav G, Dangayach GS, Meena ML, Sharma A. Assessment of Stability and Thermophysical Properties of Jojoba Nanofluid as a Metal-Cutting Fluid: Experimental and Modelling Investigation. Lubricants. 2022; 10(6):126. https://doi.org/10.3390/lubricants10060126
Chicago/Turabian StyleGaurav, Gaurav, Govind Sharan Dangayach, Makkhan Lal Meena, and Abhay Sharma. 2022. "Assessment of Stability and Thermophysical Properties of Jojoba Nanofluid as a Metal-Cutting Fluid: Experimental and Modelling Investigation" Lubricants 10, no. 6: 126. https://doi.org/10.3390/lubricants10060126
APA StyleGaurav, G., Dangayach, G. S., Meena, M. L., & Sharma, A. (2022). Assessment of Stability and Thermophysical Properties of Jojoba Nanofluid as a Metal-Cutting Fluid: Experimental and Modelling Investigation. Lubricants, 10(6), 126. https://doi.org/10.3390/lubricants10060126






