Friction and Wear Properties of a Nanoscale Ionic Liquid-like GO@SiO2 Hybrid as a Water-Based Lubricant Additive
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
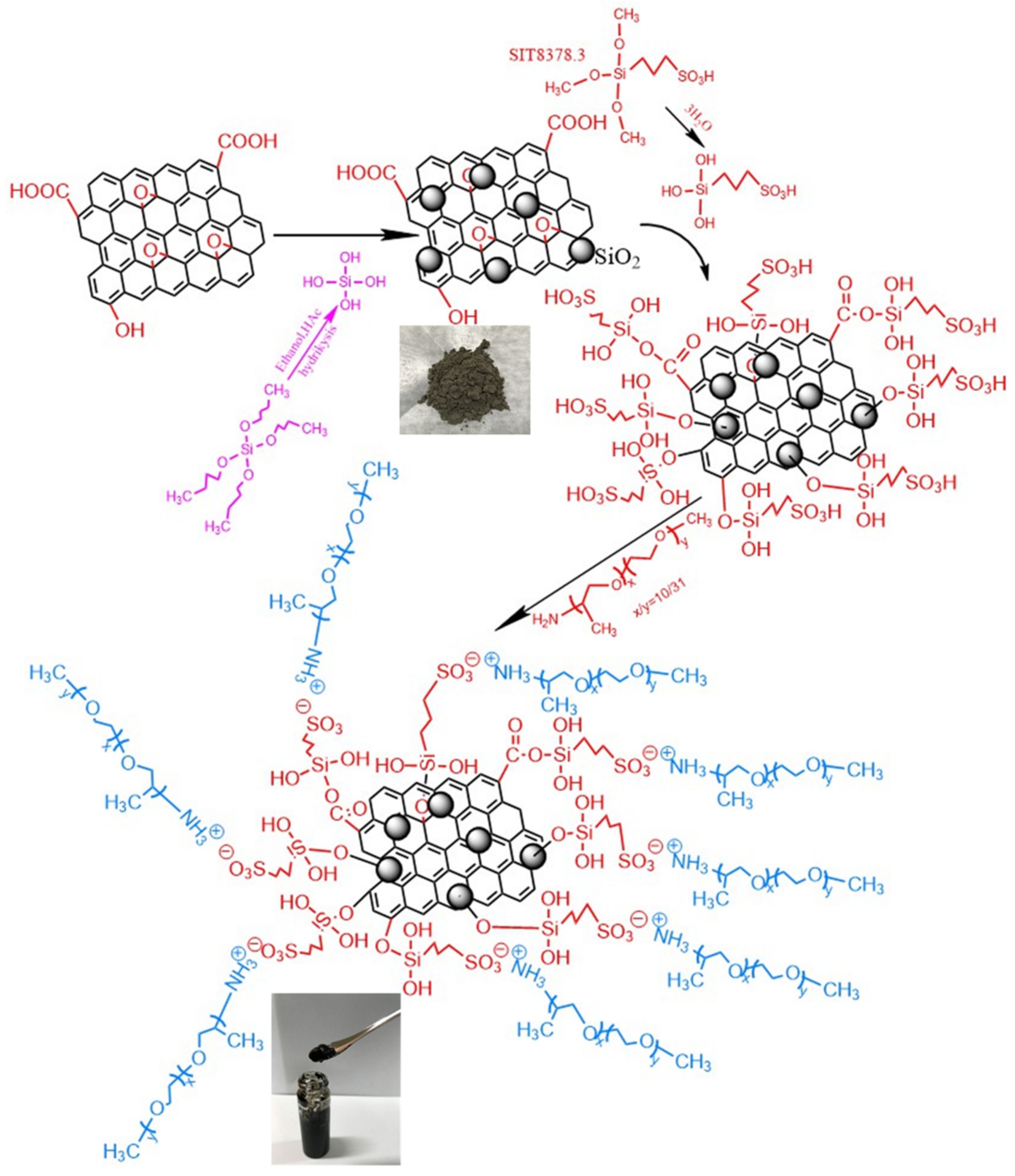
2.2. Synthesis of the GO@SiO2 Compound
2.3. Preparation of the Nanoscale Liquid-like GO@SiO2 Hybrid
2.4. Tribological Tests
2.5. Characterizations
3. Results and Discussion
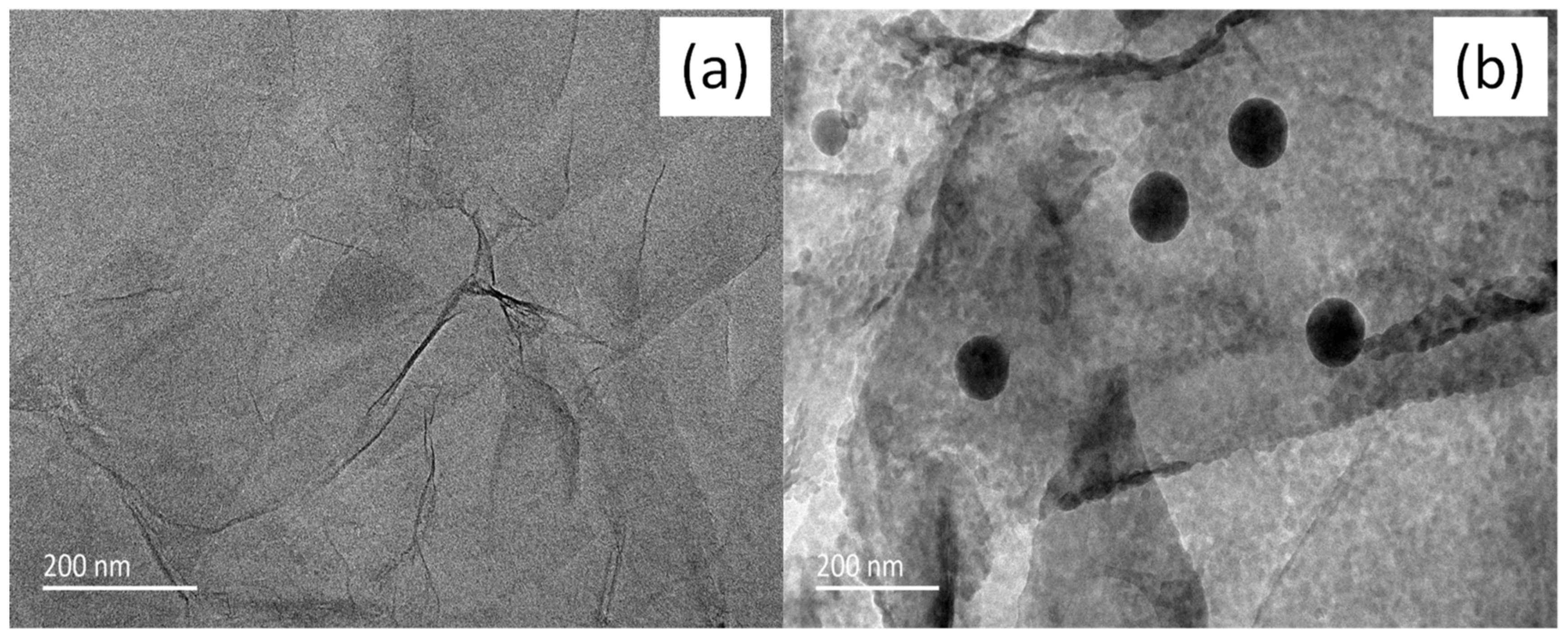
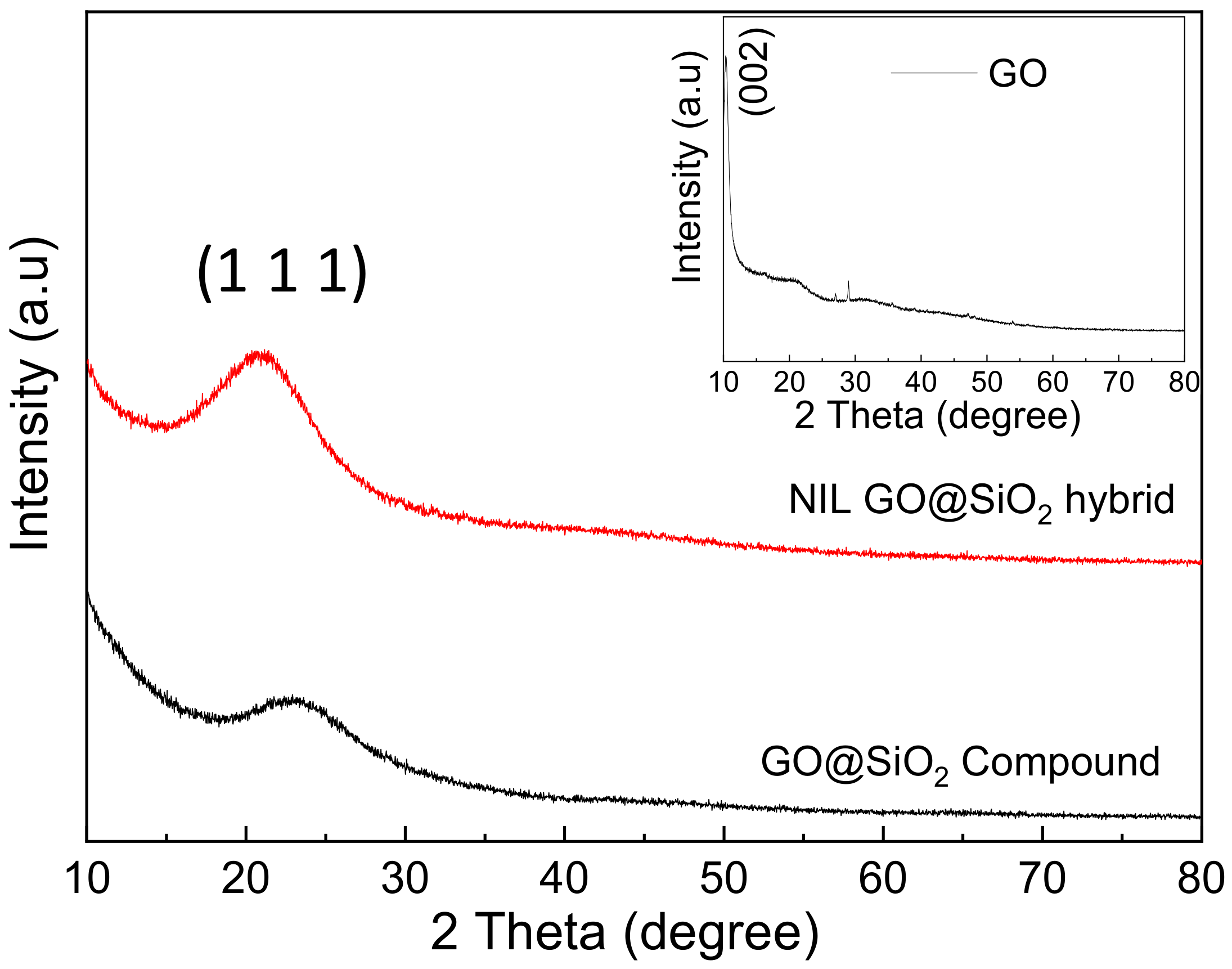
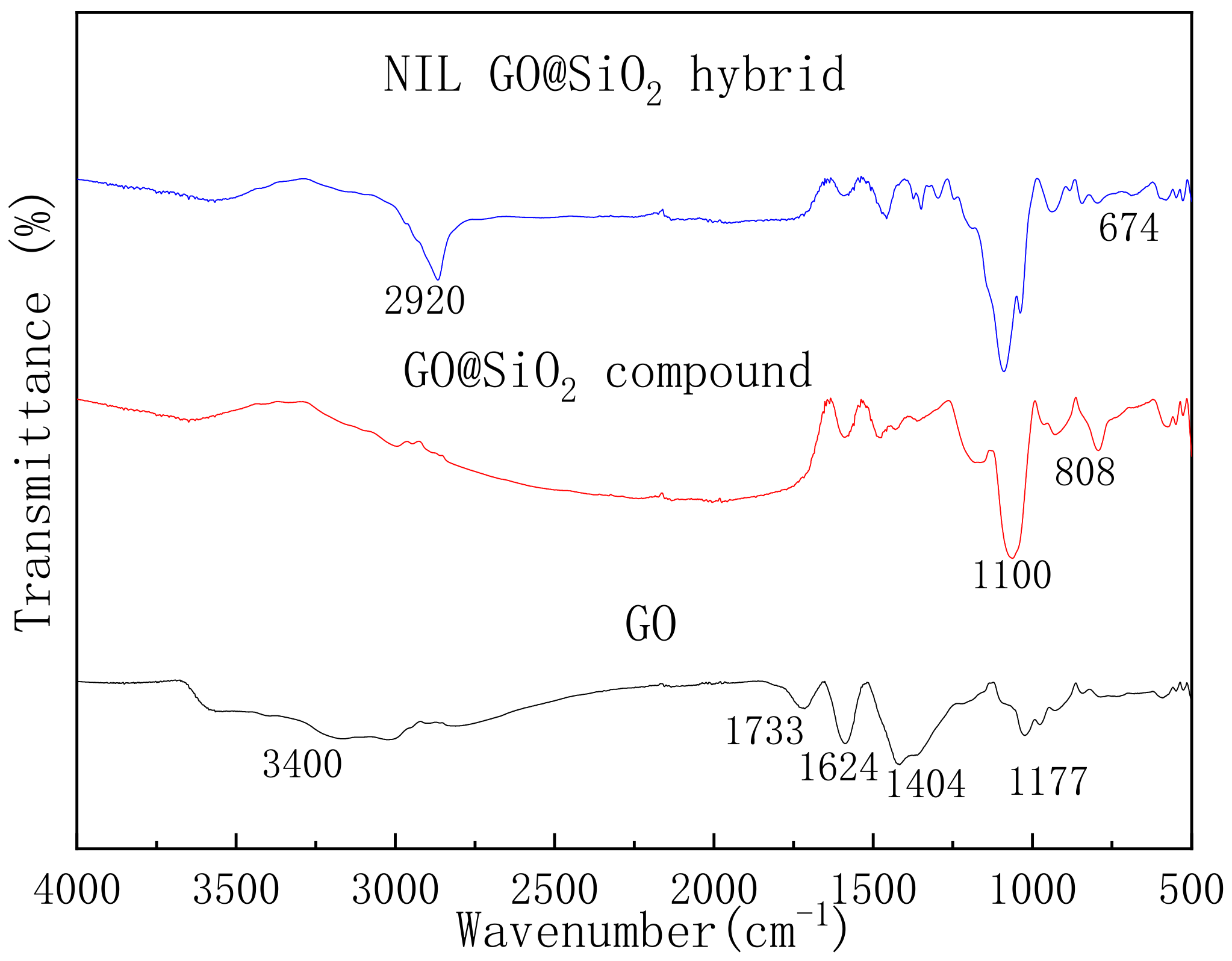
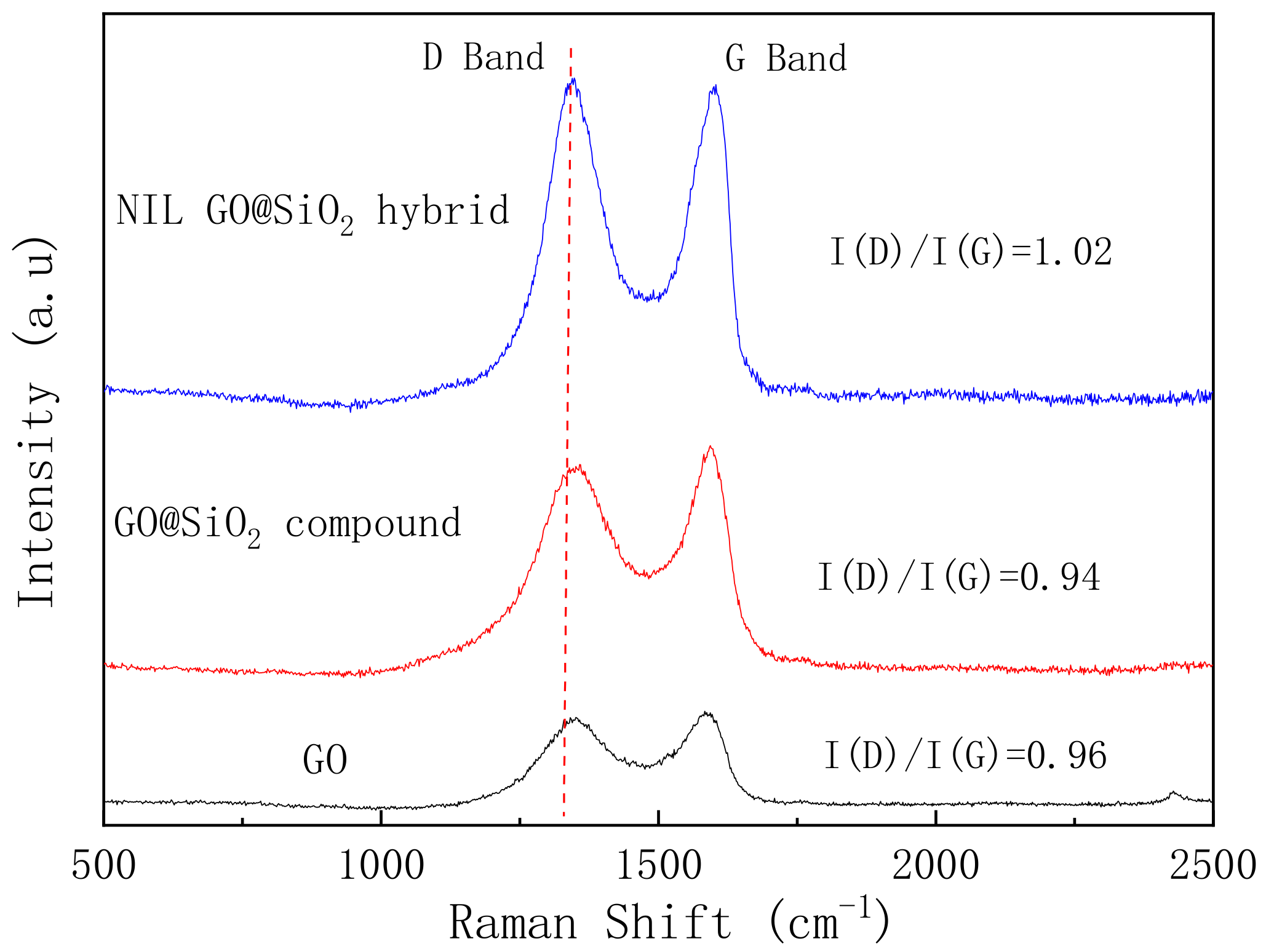
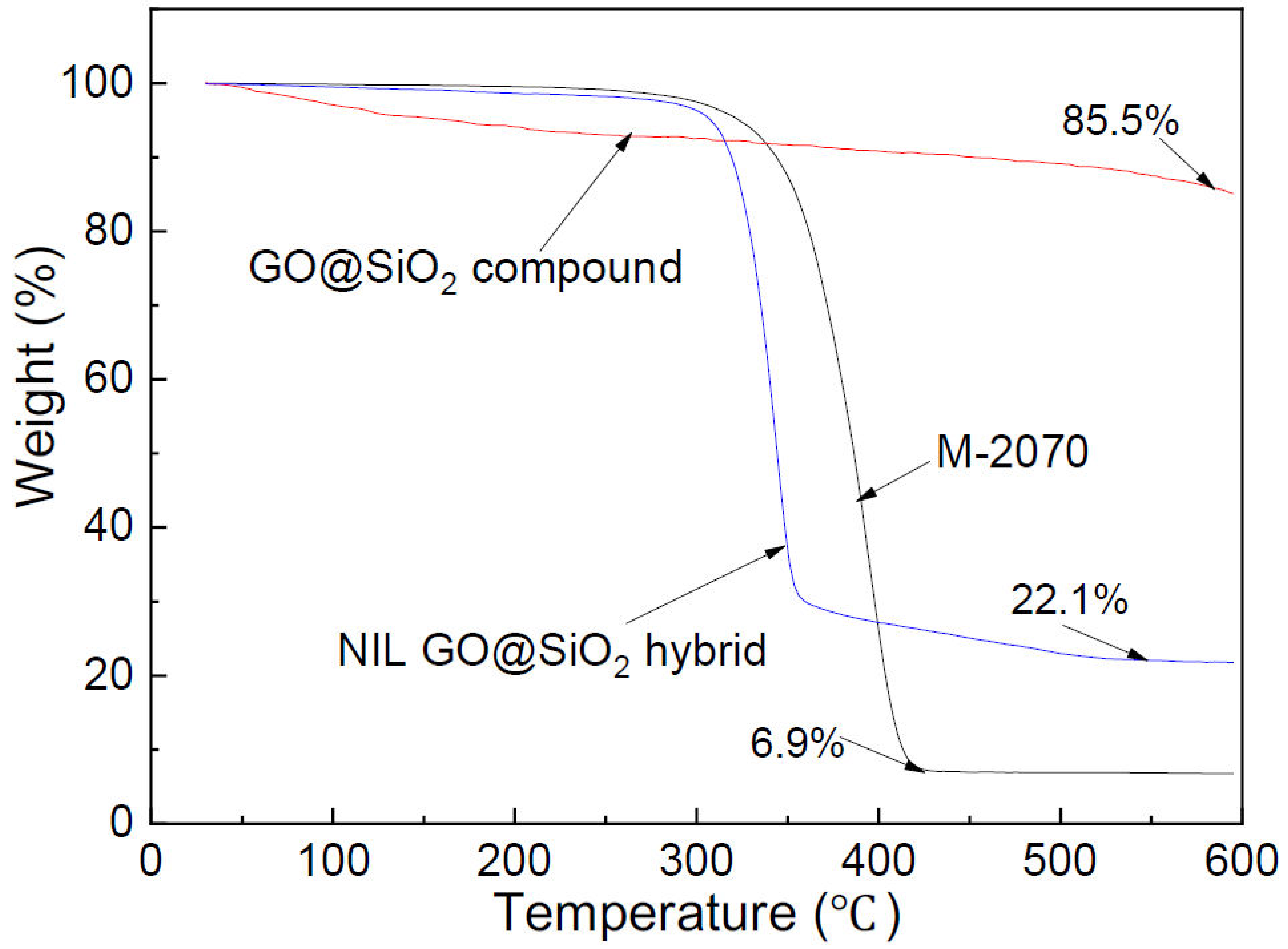
3.1. Structural Analysis
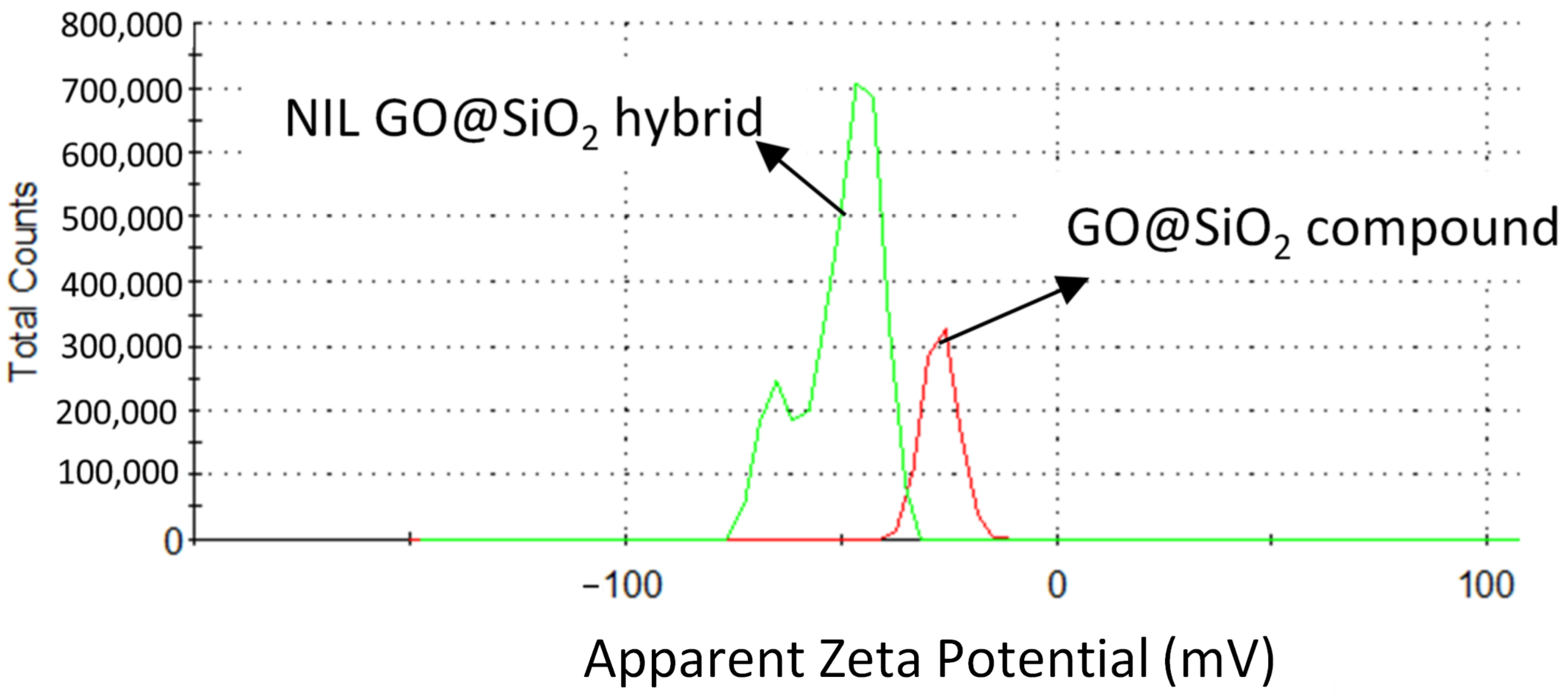
3.2. Dispersion Stability
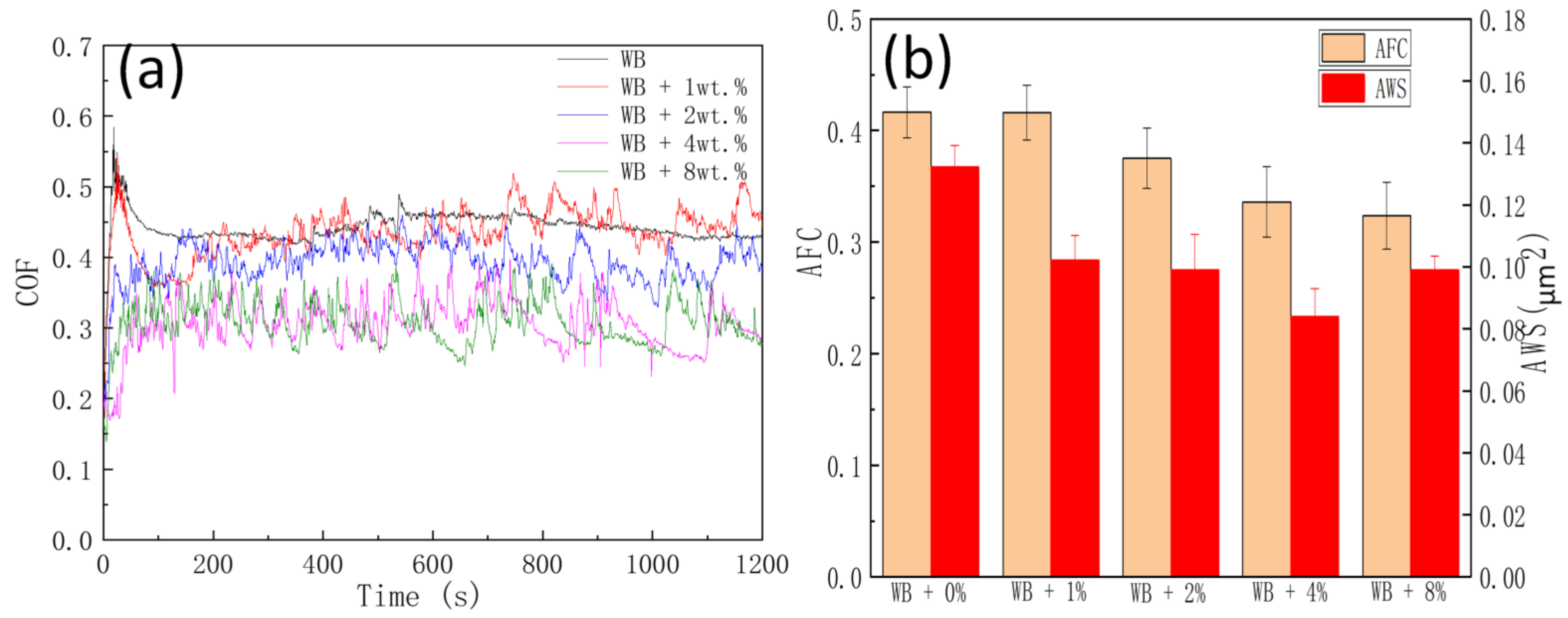
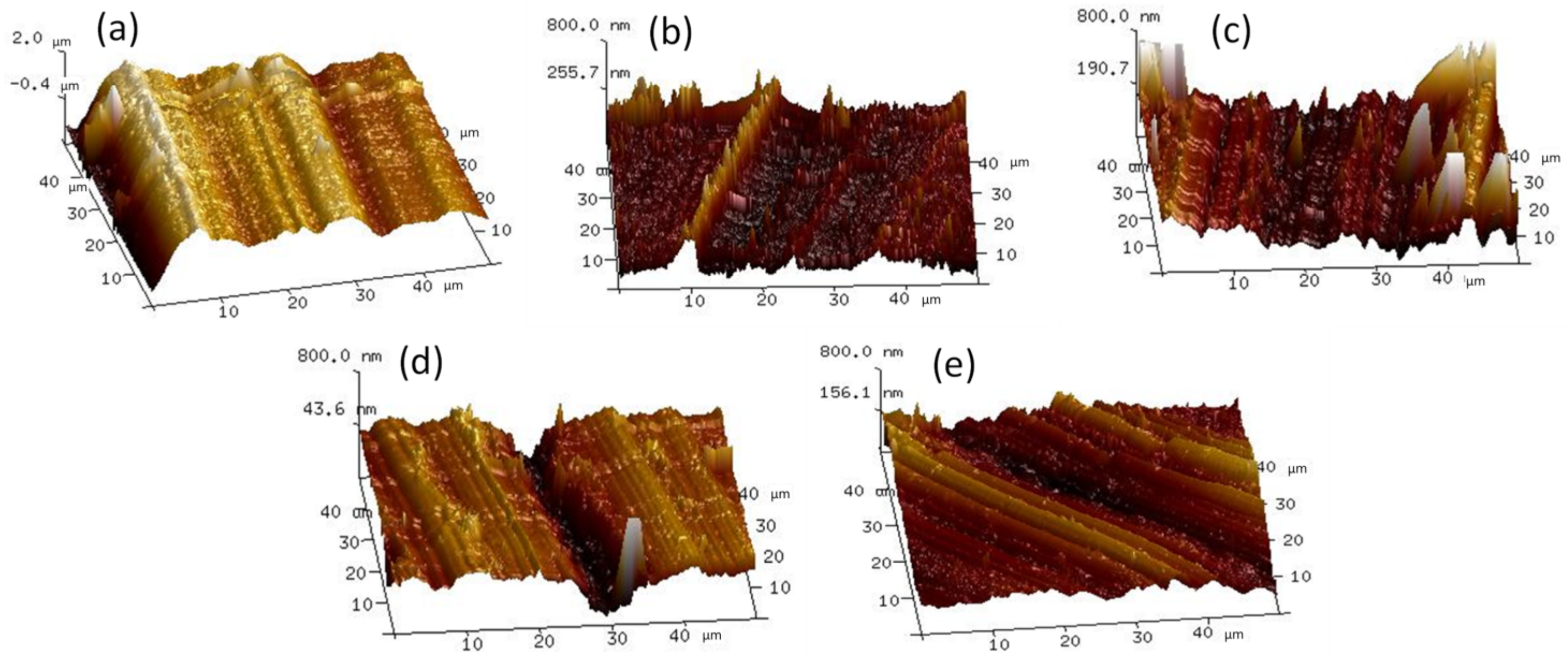
3.3. Tribological Properties
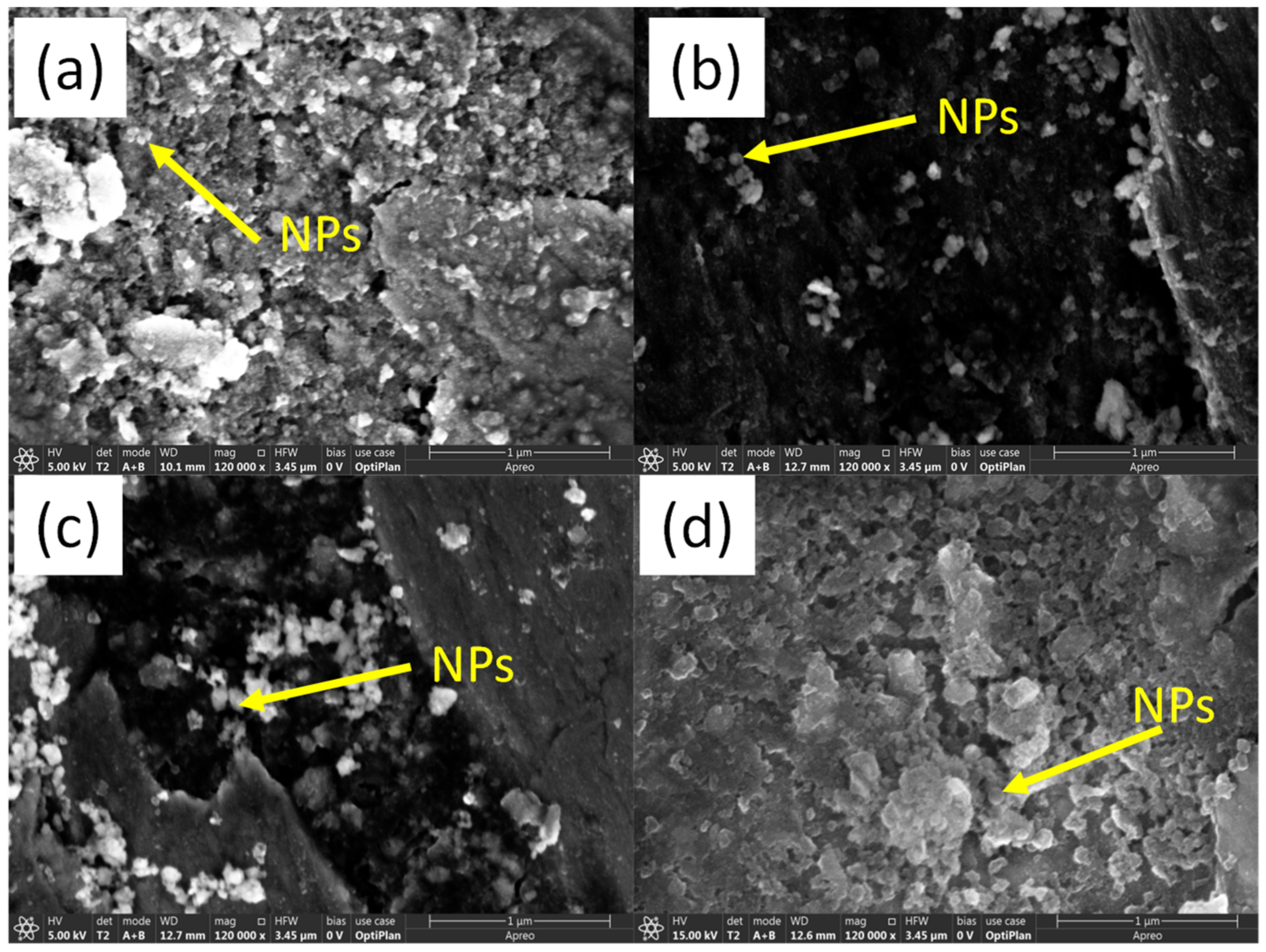
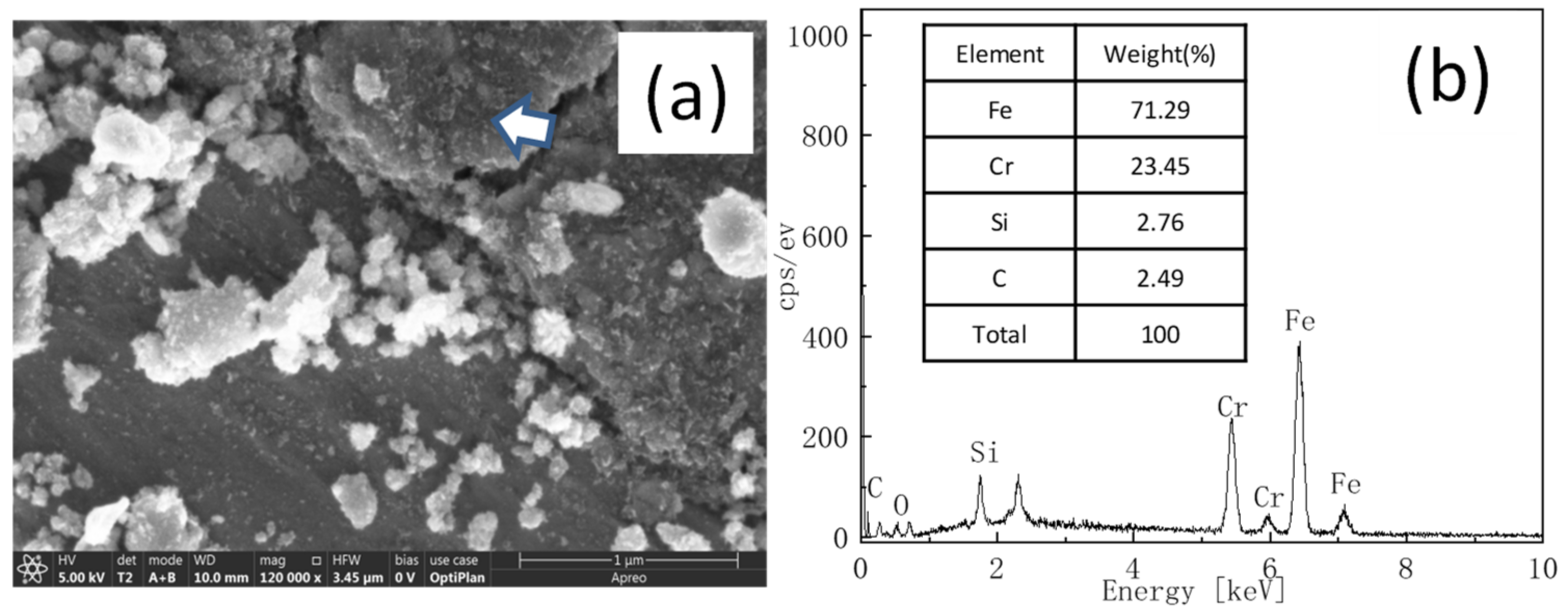
3.4. Inquiry of Lubrication Mechanisms
4. Conclusions
- The as-synthesized NIL GO@SiO2 hybrid consisted of approximately 77.9% organic components and 22.1% inorganic components, exhibiting good dispersity and stability as a WB lubricant;
- The addition of the NIL GO@SiO2 hybrid reduced the COF and AWS at all tested concentrations. Compared with the WB lubricant, the 4.0 wt% hybrid nanolubricant lowered COF and AWS by 20.7% and 36.6%, respectively;
- The tribological enhancement of the NIL GO@SiO2 hybrid can be explained by the synergistic mechanisms of micro-rolling, polishing and mending in the GO@SiO2 compound.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| NIL | nanoscale ionic liquid |
| English Symbols | |
| FSS | Ferritic Stainless Steel |
| GO | Graphene Oxide |
| TEO | Tetraethyl orthosilicate |
| WB | water-based |
| MFT | multi-functional tribometer |
| COF | coefficient of friction |
| TEM | Transmission Electron Microscope |
| XRD | X-ray diffraction |
| FTIR | Fourier Transform Infra-Red |
| TGA | Thermogravimetric analyzer |
| OM | Optical Microscope |
| AFM | Atomic Force Microscopy |
References
- Wu, H.; Zhao, J.; Luo, L.; Huang, S.; Wang, L.; Zhang, S.; Jiao, S.; Huang, H.; Jiang, Z. Performance Evaluation and Lubrication Mechanism of Water-Based Nanolubricants Containing Nano-TiO2 in Hot Steel Rolling. Lubricants 2018, 6, 57. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-Based Lubricants: Development, Properties, and Performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Tomala, A.; Karpinska, A.; Werner, W.S.M.; Olver, A.; Störi, H. Tribological properties of additives for water-based lubricants. Wear 2010, 269, 804–810. [Google Scholar] [CrossRef]
- Xie, Z.; Zhu, W. An investigation on the lubrication characteristics of floating ring bearing with consideration of multi-coupling factors. Mech. Syst. Signal Process. 2022, 162, 108086. [Google Scholar] [CrossRef]
- Khalid Shafi, W.; Charoo, M.S. NanoLubrication Systems: An Overview. Mater. Today Proc. 2018, 5, 20621–20630. [Google Scholar] [CrossRef]
- Jia, X.; Huang, J.; Li, Y.; Yang, J.; Song, H. Monodisperse Cu nanoparticles @ MoS2 nanosheets as a lubricant additive for improved tribological properties. Appl. Surf. Sci. 2019, 494, 430–439. [Google Scholar] [CrossRef]
- Darminesh, S.P.; Sidik, N.A.C.; Najafi, G.; Mamat, R.; Ken, T.L.; Asako, Y. Recent development on biodegradable nanolubricant: A review. Int. Commun. Heat Mass Transf. 2017, 86, 159–165. [Google Scholar] [CrossRef]
- Wang, L.; Tieu, A.K.; Zhu, H.; Deng, G.; Cui, S.; Zhu, Q. A study of water-based lubricant with a mixture of polyphosphate and nano-TiO2 as additives for hot rolling process. Wear 2021, 477, 203895. [Google Scholar] [CrossRef]
- Thampi, A.D.; Prasanth, M.A.; Anandu, A.P.; Sneha, E.; Sasidharan, B.; Rani, S. The effect of nanoparticle additives on the tribological properties of various lubricating oils—Review. Mater. Today Proc. 2021, 47, 4919–4924. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Wang, J.; Gao, C.; Zhang, S.; Zhang, P.; Zhang, Z. Interactions of Cu nanoparticles with conventional lubricant additives on tribological performance and some physicochemical properties of an ester base oil. Tribol. Int. 2020, 141, 105941. [Google Scholar] [CrossRef]
- Chen, X.; Han, Z.; Li, X.; Lu, K. Lowering coefficient of friction in Cu alloys with stable gradient nanostructures. Sci. Adv. 2016, 2, e1601942. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, Z.; Lu, K. Enhancing wear resistance of Cu–Al alloy by controlling subsurface dynamic recrystallization. Scr. Mater. 2015, 101, 76–79. [Google Scholar] [CrossRef]
- Alves, S.M.; Mello, V.S.; Faria, E.A.; Camargo, A.P.P. Nanolubricants developed from tiny CuO nanoparticles. Tribol. Int. 2016, 100, 263–271. [Google Scholar] [CrossRef]
- Atila Dinçer, C.; Yıldız, N.; Aydoğan, N.; Çalımlı, A. A comparative study of Fe3O4 nanoparticles modified with different silane compounds. Appl. Surf. Sci. 2014, 318, 297–304. [Google Scholar] [CrossRef]
- Luo, T.; Wei, X.; Huang, X.; Huang, L.; Yang, F. Tribological properties of Al2O3 nanoparticles as lubricating oil additives. Ceram. Int. 2014, 40, 7143–7149. [Google Scholar] [CrossRef]
- He, A.; Huang, S.; Yun, J.H.; Wu, H.; Jiang, Z.; Stokes, J.; Jiao, S.; Wang, L.; Huang, H. Tribological Performance and Lubrication Mechanism of Alumina Nanoparticle Water-Based Suspensions in Ball-on-Three-Plate Testing. Tribol. Lett. 2017, 65, 40. [Google Scholar] [CrossRef]
- Ingole, S.; Charanpahari, A.; Kakade, A.; Umare, S.S.; Bhatt, D.V.; Menghani, J. Tribological behavior of nano TiO2 as an additive in base oil. Wear 2013, 301, 776–785. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.; Xia, W.; Cheng, X.; He, A.; Yun, J.H.; Wang, L.; Huang, H.; Jiao, S.; Huang, L.; et al. A study of the tribological behaviour of TiO2 nano-additive water-based lubricants. Tribol. Int. 2017, 109, 398–408. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Bao, Y.; Meng, Y. Effect of TiO2 nanoparticles on wettability and tribological performance of aqueous suspension. Wear 2017, 376, 786–791. [Google Scholar] [CrossRef]
- Gara, L.; Zou, Q. Friction and Wear Characteristics of Oil-Based ZnO Nanofluids. Tribol. Trans. 2013, 56, 236–244. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Tabassum, S.; Zia, M. Effect of capping agents: Structural, optical and biological properties of ZnO nanoparticles. Appl. Surf. Sci. 2016, 386, 319–326. [Google Scholar] [CrossRef]
- Peng, D.X.; Chen, C.H.; Kang, Y.; Chang, Y.P.; Chang, S.Y. Size effects of SiO2 nanoparticles as oil additives on tribology of lubricant. Ind. Lubr. Tribol. 2010, 62, 111–120. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sharma, T. Stability and rheological properties of nanofluids stabilized by SiO2 nanoparticles and SiO2-TiO2 nanocomposites for oilfield applications. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 171–183. [Google Scholar] [CrossRef]
- Bao, Y.; Sun, J.; Kong, L. Effects of nano-SiO2 as water-based lubricant additive on surface qualities of strips after hot rolling. Tribol. Int. 2017, 114, 257–263. [Google Scholar] [CrossRef]
- Wu, H.; Johnson, B.; Wang, L.; Dong, G.; Yang, S.; Zhang, J. High-efficiency preparation of oil-dispersible MoS2 nanosheets with superior anti-wear property in ultralow concentration. J. Nanoparticle Res. 2017, 19, 339. [Google Scholar] [CrossRef]
- Forsberg, V.; Zhang, R.; Bäckström, J.; Dahlström, C.; Andres, B.; Norgren, M.; Andersson, M.; Hummelgård, M.; Olin, H. Exfoliated MoS2 in Water without Additives. PLoS ONE 2016, 11, e0154522. [Google Scholar] [CrossRef]
- Aldana, P.U.; Vacher, B.; Le Mogne, T.; Belin, M.; Thiebaut, B.; Dassenoy, F. Action Mechanism of WS2 Nanoparticles with ZDDP Additive in Boundary Lubrication Regime. Tribol. Lett. 2014, 56, 249–258. [Google Scholar] [CrossRef]
- Loya, A.; Stair, J.L.; Ren, G. Simulation and experimental study of rheological properties of CeO2–water nanofluid. Int. Nano Lett. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Boshui, C.; Kecheng, G.; Jianhua, F.; Jiang, W.; Jiu, W.; Nan, Z. Tribological characteristics of monodispersed cerium borate nanospheres in biodegradable rapeseed oil lubricant. Appl. Surf. Sci. 2015, 353, 326–332. [Google Scholar] [CrossRef]
- Shahnazar, S.; Bagheri, S.; Abd Hamid, S.B. Enhancing lubricant properties by nanoparticle additives. Int. J. Hydrog. Energy 2016, 41, 3153–3170. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Li, Z.; Zhao, X.; Liu, G.; Liu, S.; Ma, S.; Li, W.; Liu, W. Surface-Functionalized NanoMOFs in Oil for Friction and Wear Reduction and Antioxidation. Chem. Eng. J. 2021, 410, 128306. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; He, Y.; Shi, Y. Nanolubricant additives: A review. Friction 2021, 9, 891–917. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.; Cheng, X.; Xia, W.; He, A.; Yun, J.H.; Huang, S.; Wang, L.; Huang, H.; Jiao, S.; et al. Friction and wear characteristics of TiO2 nano-additive water-based lubricant on ferritic stainless steel. Tribol. Int. 2018, 117, 24–38. [Google Scholar] [CrossRef]
- Guo, J.; Barber, G.C.; Schall, D.J.; Zou, Q.; Jacob, S.B. Tribological properties of ZnO and WS2 nanofluids using different surfactants. Wear 2017, 382, 8–14. [Google Scholar] [CrossRef]
- Sonn, J.S.; Lee, J.Y.; Jo, S.H.; Yoon, I.-H.; Jung, C.-H.; Lim, J.C. Effect of surface modification of silica nanoparticles by silane coupling agent on decontamination foam stability. Ann. Nucl. Energy 2018, 114, 11–18. [Google Scholar] [CrossRef]
- Kang, T.; Jang, I.; Oh, S.-G. Surface modification of silica nanoparticles using phenyl trimethoxy silane and their dispersion stability in N-methyl-2-pyrrolidone. Colloids Surf. A Physicochem. Eng. Asp. 2016, 501, 24–31. [Google Scholar] [CrossRef]
- Man, W.; Huang, Y.; Gou, H.; Li, Y.; Zhao, J.; Shi, Y. Synthesis of novel CuO@Graphene nanocomposites for lubrication application via a convenient and economical method. Wear 2022, 498–499, 204323. [Google Scholar] [CrossRef]
- Rodriguez, R.; Herrera, R.; Archer, L.A.; Giannelis, E.P. Nanoscale Ionic Materials. Adv. Mater. 2008, 20, 4353–4358. [Google Scholar] [CrossRef]
- Rodriguez, R.; Herrera, R.; Bourlinos, A.B.; Li, R.; Amassian, A.; Archer, L.A.; Giannelis, E.P. The synthesis and properties of nanoscale ionic materials. Appl. Organomet. Chem. 2010, 24, 581–589. [Google Scholar] [CrossRef]
- Jespersen, M.L.; Mirau, P.A.; von Meerwall, E.; Vaia, R.A.; Rodriguez, R.; Giannelis, E.P. Canopy Dynamics in Nanoscale Ionic Materials. ACS Nano 2010, 4, 3735–3742. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, Y.; Wu, Y.; Qu, P.; Yang, R.; Wang, N.; Li, M. A nanoscale liquid-like graphene@Fe3O4 hybrid with excellent amphiphilicity and electronic conductivity. New J. Chem. 2014, 38, 5043–5051. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Jia, X.; Song, H. Preparation and tribological properties of core-shell Fe3O4@C microspheres. Tribol. Int. 2019, 129, 427–435. [Google Scholar] [CrossRef]
- Min, C.; He, Z.; Song, H.; Liang, H.; Liu, D.; Dong, C.; Jia, W. Fluorinated graphene oxide nanosheet: A highly efficient water-based lubricated additive. Tribol. Int. 2019, 140, 105867. [Google Scholar] [CrossRef]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Fernandes, N.; Dallas, P.; Rodriguez, R.; Bourlinos, A.B.; Georgakilas, V.; Giannelis, E.P. Fullerol ionic fluids. Nanoscale 2010, 2, 1653–1656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Li, P.; Zhang, Z.; Wang, X.; Tang, J.; Liu, H.; Shao, Q.; Ding, T.; Umar, A.; Guo, Z. Solvent-free graphene liquids: Promising candidates for lubricants without the base oil. J. Colloid Interface Sci. 2019, 542, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Sun, J.; Bao, Y. Preparation, characterization and tribological mechanism of nanofluids. RSC Adv. 2017, 7, 12599–12609. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Turkson, R.F.; Peng, Z.; Chen, X. Enhancing the thermophysical properties and tribological behaviour of engine oils using nano-lubricant additives. RSC Adv. 2016, 6, 77913–77924. [Google Scholar]
- Azman, N.F.; Samion, S. Dispersion Stability and Lubrication Mechanism of Nanolubricants: A Review. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 393–414. [Google Scholar] [CrossRef]
- Ranga Babu, J.A.; Kumar, K.K.; Srinivasa Rao, S. State-of-art review on hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 77, 551–565. [Google Scholar] [CrossRef]
- Hao, L.; Wang, Z.; Zhang, G.; Zhao, Y.; Duan, Q.; Wang, Z.; Chen, Y.; Li, T. Tribological evaluation and lubrication mechanisms of nanoparticles enhanced lubricants in cold rolling. Mech. Ind. 2020, 21, 108–113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, L.; Hao, W.; Li, P.; Liu, G.; Li, H.; Aljabri, A.; Xie, Z. Friction and Wear Properties of a Nanoscale Ionic Liquid-like GO@SiO2 Hybrid as a Water-Based Lubricant Additive. Lubricants 2022, 10, 125. https://doi.org/10.3390/lubricants10060125
Hao L, Hao W, Li P, Liu G, Li H, Aljabri A, Xie Z. Friction and Wear Properties of a Nanoscale Ionic Liquid-like GO@SiO2 Hybrid as a Water-Based Lubricant Additive. Lubricants. 2022; 10(6):125. https://doi.org/10.3390/lubricants10060125
Chicago/Turabian StyleHao, Liang, Wendi Hao, Peipei Li, Guangming Liu, Huaying Li, Abdulrahman Aljabri, and Zhongliang Xie. 2022. "Friction and Wear Properties of a Nanoscale Ionic Liquid-like GO@SiO2 Hybrid as a Water-Based Lubricant Additive" Lubricants 10, no. 6: 125. https://doi.org/10.3390/lubricants10060125
APA StyleHao, L., Hao, W., Li, P., Liu, G., Li, H., Aljabri, A., & Xie, Z. (2022). Friction and Wear Properties of a Nanoscale Ionic Liquid-like GO@SiO2 Hybrid as a Water-Based Lubricant Additive. Lubricants, 10(6), 125. https://doi.org/10.3390/lubricants10060125







