Electroencephalogram Gamma Band Power Correlates with Anhedonia in a Community Sample
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Depression Scales
2.3. Electroencephalogram Data Processing
3. Results
3.1. Age, Sex, and SDS Scores
3.2. Depressive Subtypes
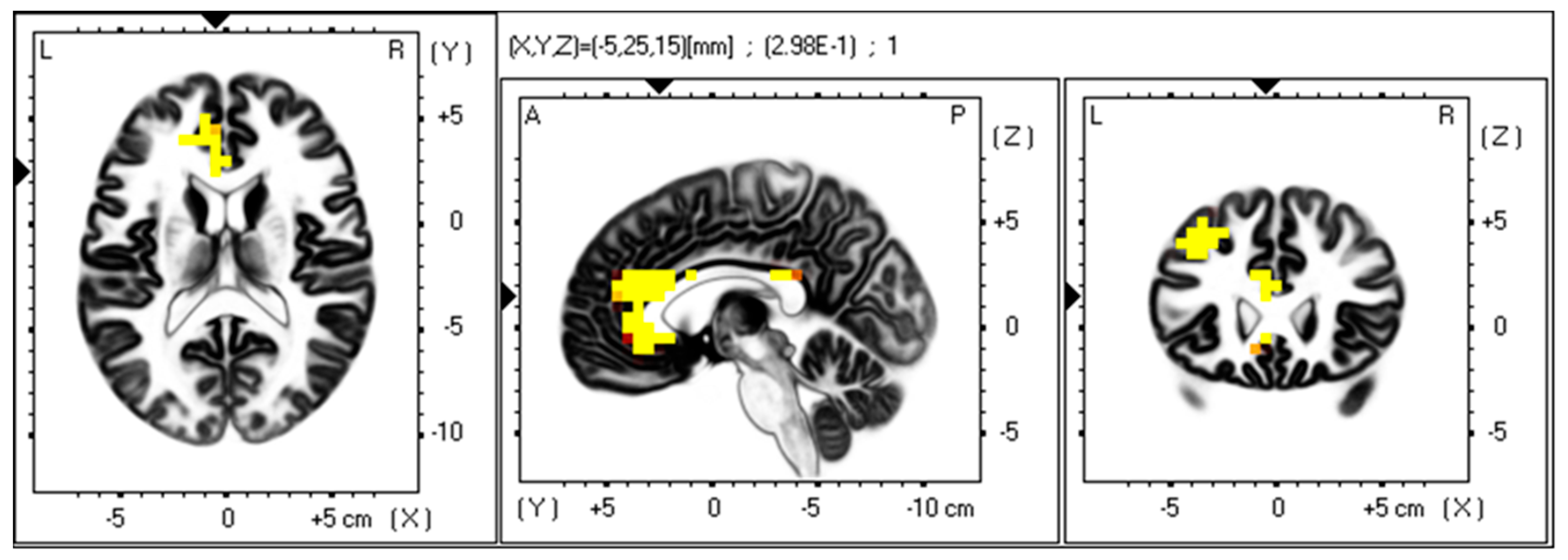
3.3. Gamma Band
4. Discussion
4.1. Anhedonic Depression Subtype and Gamma Band Power
4.2. Anterior Cingulate Cortex, Depression, Anhedonia, and Gamma Band Power
4.3. Left Posterior Cingulate and Left Middle and Superior Frontal Gyrus, Depression, and Gamma Band Power
4.4. Null Finding of Overall Depression and Other Subtypes
4.5. Eyes Open and Eyes Closed Gamma Band Power in Depression
5. Conclusions
5.1. Limitations and Future Directions
5.2. Clinical Implications and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EEG | Electroencephalogram |
| MD | Major Depression |
| DMN | Default mode network |
| SDS | Zung Self-rating Depression Scale |
| ACC | Anterior cingulate cortex |
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Khosla, A.; Khandnor, P.; Chand, T. Automated diagnosis of depression from EEG signals using traditional and deep learning approaches: A comparative analysis. Biocybern. Biomed. Eng. 2022, 42, 108–142. [Google Scholar] [CrossRef]
- Buch, A.M.; Liston, C. Dissecting diagnostic heterogeneity in depression by integrating neuroimaging and genetics. Neuropsychopharmacology 2021, 46, 156–175. [Google Scholar] [CrossRef]
- Clark, L.A.; Cuthbert, B.; Lewis-Fernández, R.; Narrow, W.E.; Reed, G.M. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol. Sci. Public Interest 2017, 18, 72–145. [Google Scholar] [CrossRef]
- Pitsillou, E.; Bresnehan, S.M.; Kagarakis, E.A.; Wijoyo, S.J.; Liang, J.; Hung, A.; Karagiannis, T.C. The cellular and molecular basis of major depressive disorder: Towards a unified model for understanding clinical depression. Mol. Biol. Rep. 2020, 47, 753–770. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatry 2017, 27, 101–111. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5-TR, 5th ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Ormel, J.; Hollon, S.D.; Kessler, R.C.; Cuijpers, P.; Monroe, S.M. More treatment but no less depression: The treatment-prevalence paradox. Clin. Psychol. Rev. 2022, 91, 102111. [Google Scholar] [CrossRef]
- Jollans, L.; Whelan, R. The clinical added value of imaging: A perspective from outcome prediction. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 423–432. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, R.J. Transdiagnostic biomarker approaches to mental health disorders: Consideration of symptom complexity, comorbidity and context. Brain Behav. Immun.-Health 2021, 16, 100303. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.; Demyttenaere, K.; Martiadis, V.; Martinotti, G. Editorial: Treatment resistant depression (TRD): Epidemiology, clinic, burden and treatment. Front. Psychiatry 2025, 16, 1588902. [Google Scholar] [CrossRef]
- Dev, A.; Roy, N.; Islam, M.K.; Biswas, C.; Ahmed, H.U.; Amin, M.A.; Sarker, F.; Vaidyanathan, R.; Mamun, K.A. Exploration of EEG-based depression biomarkers identification techniques and their applications: A systematic review. IEEE Access 2022, 10, 16756–16781. [Google Scholar] [CrossRef]
- Greco, C.; Matarazzo, O.; Cordasco, G.; Vinciarelli, A.; Callejas, Z.; Esposito, A. Discriminative power of EEG-based biomarkers in major depressive disorder: A systematic review. IEEE Access 2021, 9, 112850–112870. [Google Scholar] [CrossRef]
- De Aguiar Neto, F.S.; Rosa, J.L.G. Depression biomarkers using non-invasive EEG: A review. Neurosci. Biobehav. Rev. 2019, 105, 83–93. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Watson, B.O. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl. Psychiatry 2018, 8, 177. [Google Scholar] [CrossRef]
- Coleman, S.L.; Sharpley, C.F.; Vessey, K.A.; Evans, I.D.; Williams, R.J.; Bitsika, V. Gamma oscillations as correlates of depression: Updating Fitzgerald and Watson (2018). Rev. Neurosci. 2025. [Google Scholar] [CrossRef]
- Hudson, M.R.; Jones, N.C. Deciphering the code: Identifying true gamma neural oscillations. Exp. Neurol. 2022, 357, 114205. [Google Scholar] [CrossRef]
- Ostergaard, S.D.; Jensen, S.O.; Bech, P. The heterogeneity of the depressive syndrome: When numbers get serious. Acta Psychiatr. Scand. 2011, 124, 495–496. [Google Scholar] [CrossRef]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef]
- Durisko, Z.; Mulsant, B.H.; Andrews, P.W. An adaptationist perspective on the etiology of depression. J. Affect. Disord. 2015, 172, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, C.F.; Bitsika, V. Differences in neurobiological pathways of four “clinical content” subtypes of depression. Behav. Brain Res. 2013, 256, 368–376. [Google Scholar] [CrossRef]
- Sharpley, C.F.; Bitsika, V. Validity, reliability and prevalence of four ‘clinical content’ subtypes of depression. Behav. Brain Res. 2014, 259, 9–15. [Google Scholar] [CrossRef]
- Zung, W.W.K. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef]
- Zung, W.W. From art to science. The diagnosis and treatment of depression. Arch. Gen. Psychiatry 1973, 29, 328–337. [Google Scholar] [CrossRef]
- De Jonghe, J.F.M.; Baneke, J.J. The Zung self-rating depression scale: A replication study on reliability, validity and prediction. Psychol. Rep. 1989, 64, 833–834. [Google Scholar] [CrossRef]
- Gabrys, J.B.; Peters, K. Reliability, Discriminant and Predictive Validity of the Zung Self-Rating Depression Scale. Psychol. Rep. 1985, 57, 1091–1096. [Google Scholar] [CrossRef]
- Schaefer, A.; Brown, J.; Watson, C.G.; Plemel, D.; DeMotts, J.; Howard, M.T.; Petrik, N.; Balleweg, B.J.; Anderson, D. Comparison of the validities of the Beck, Zung, and MMPI Depression scales. J. Consult. Clin. Psychol. 1985, 53, 415–418. [Google Scholar] [CrossRef]
- Chaddad, A.; Wu, Y.; Kateb, R.; Bouridane, A. Electroencephalography signal processing: A comprehensive review and analysis of methods and techniques. Sensors 2023, 23, 6434. [Google Scholar] [CrossRef] [PubMed]
- Evans, I.D.; Sharpley, C.F.; Bitsika, V.; Vessey, K.A.; Williams, R.J.; Jesulola, E.; Agnew, L.L. Differences in EEG functional connectivity in the dorsal and ventral attentional and salience networks across multiple subtypes of depression. Appl. Sci. 2025, 15, 1459. [Google Scholar] [CrossRef]
- Nottage, J.F.; Horder, J. State-of-the-art analysis of high-frequency (gamma range) electroencephalography in humans. Neuropsychobiology 2016, 72, 219–228. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef]
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S.J. Comparing Functional (PET) Images: The Assessment of Significant Change. J. Cereb. Blood Flow Metab. 1991, 11, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Keren, H.; O’Callaghan, G.; Vidal-Ribas, P.; Buzzell, G.A.; Brotman, M.A.; Leibenluft, E.; Pan, P.M.; Meffert, L.; Kaiser, A.; Wolke, S.; et al. Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. Am. J. Psychiatry 2018, 175, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Borsini, A.; Wallis, A.S.J.; Zunszain, P.; Pariante, C.M.; Kempton, M.J. Characterizing anhedonia: A systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn. Affect. Behav. Neurosci. 2020, 20, 816–841. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, E.; Nie, Z.; Deng, Z.; Gong, Q.; Ma, S.; Kang, L.; Yao, L.; Cheng, J.; Liu, Z. Exploring mechanisms of anhedonia in depression through neuroimaging and data-driven approaches. J. Affect. Disord. 2024, 363, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Roiser, J.P. Neuroscience of apathy and anhedonia: A transdiagnostic approach. Nat. Rev. Neurosci. 2018, 19, 470–484. [Google Scholar] [CrossRef]
- Hasler, G.; Drevets, W.C.; Manji, H.K.; Charney, D.S. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004, 29, 1765–1781. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Jahn, A.L.; O’Shea, J.P. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biol. Psychiatry 2005, 57, 319–327. [Google Scholar] [CrossRef]
- Der-Avakian, A.; Markou, A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012, 35, 68–77. [Google Scholar] [CrossRef]
- Sharma, A.; Wolf, D.H.; Ciric, R.; Kable, J.W.; Moore, T.M.; Vandekar, S.N.; Katchmar, N.; Daldal, A.; Ruparel, K.; Davatzikos, C.; et al. Common dimensional reward deficits across mood and psychotic disorders: A connectome-wide association study. Am. J. Psychiatry 2017, 174, 657–666. [Google Scholar] [CrossRef]
- Marco-Pallarés, J.; Münte, T.F.; Rodríguez-Fornells, A. The role of high-frequency oscillatory activity in reward processing and learning. Neurosci. Biobehav. Rev. 2015, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Doñamayor, N.; Marco-Pallarés, J.; Heldmann, M.; Schoenfeld, M.A.; Münte, T.F. Temporal dynamics of reward processing revealed by magnetoencephalography. Hum. Brain Mapp. 2011, 32, 2228–2240. [Google Scholar] [CrossRef]
- Marco-Pallares, J.; Cucurell, D.; Cunillera, T.; García, R.; Andrés-Pueyo, A.; Münte, T.F.; Rodríguez-Fornells, A. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia 2008, 46, 241–248. [Google Scholar] [CrossRef]
- HajiHosseini, A.; Rodríguez-Fornells, A.; Marco-Pallarés, J. The role of beta-gamma oscillations in unexpected rewards processing. NeuroImage 2012, 60, 1678–1685. [Google Scholar] [CrossRef]
- Sharpley, C.F.; Bitsika, V.; Christie, D.H.R. Do prostate cancer patients suffer more from depressed mood or anhedonia? Psycho-Oncol. 2013, 22, 1718–1723. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Peccoralo, L.A.; Davidson, R.J.; Cohen, J.D. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: A 128-channel EEG study. Hum. Brain Mapp. 2006, 27, 185–201. [Google Scholar] [CrossRef]
- Lai, C.-H. Fronto-limbic neuroimaging biomarkers for diagnosis and prediction of treatment responses in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110234. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, Y.; Bao, W.; Liang, K.; Cao, L.; Tang, M.; Li, H.; Hu, X.; Zhang, L.; Sun, H.; et al. Distinctive intrinsic functional connectivity alterations of anterior cingulate cortex subdivisions in major depressive disorder: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2024, 159, 105583. [Google Scholar] [CrossRef]
- Rodríguez-Cano, E.; Sarró, S.; Monté, G.C.; Maristany, T.; Salvador, R.; McKenna, P.J.; Pomarol-Clotet, E. Evidence for structural and functional abnormality in the subgenual anterior cingulate cortex in major depressive disorder. Psychol. Med. 2014, 44, 3263–3273. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Li, M.; Ren, Z.; Ma, P. Characterizing the subtype of anhedonia in major depressive disorder: A symptom-specific multimodal MRI study. Psychiatry Res. Neuroimaging 2021, 308, 111239. [Google Scholar] [CrossRef]
- Pilmeyer, J.; Huijbers, W.; Lamerichs, R.; Jansen, J.F.A.; Breeuwer, M.; Zinger, S. Functional MRI in major depressive disorder: A review of findings, limitations, and future prospects. J. Neuroimaging 2022, 32, 582–595. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Gong, W.; Qiu, J.; Zhou, C.; Zhang, J.; Lv, W.; Ruan, H.; Wei, D.; Cheng, K.; et al. Functional connectivity of the anterior cingulate cortex in depression and in health. Cereb. Cortex 2018, 29, 3617–3630. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H.; Hurley, R.A.; Hayman, L.A.; Taber, K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Gaspar, P.; Berger, B.; Febvret, A.; Vigny, A.; Henry, J.P. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J. Comp. Neurol. 1989, 279, 249–271. [Google Scholar] [CrossRef]
- Haber, S.N.; Kim, K.-S.; Mailly, P.; Calzavara, R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 2006, 26, 8368–8376. [Google Scholar] [CrossRef]
- Öngür, D.; Price, J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 2000, 10, 206–219. [Google Scholar] [CrossRef]
- Knutson, B.; Bhanji, J.P.; Cooney, R.E.; Atlas, L.Y.; Gotlib, I.H. Neural responses to monetary incentives in major depression. Biol. Psychiatry 2008, 63, 686–692. [Google Scholar] [CrossRef]
- Gorka, S.M.; Huggins, A.A.; Fitzgerald, D.A.; Nelson, B.D.; Phan, K.L.; Shankman, S.A. Neural response to reward anticipation in those with depression with and without panic disorder. J. Affect. Disord. 2014, 164, 50–56. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, X.; Yu, L.; Wang, D.; Liu, R.; Teng, X.; Guo, C.; Ren, J.; Zhang, C. Abnormal functional connectivity of the anterior cingulate cortex subregions mediates the association between anhedonia and sleep quality in major depressive disorder. J. Affect. Disord. 2022, 296, 400–407. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal cortex and depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Kelly, A.M.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009, 30, 625–637. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Gusnard, D.A.; Raichle, M.E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001, 2, 685–694. [Google Scholar] [CrossRef]
- Dai, Z.; Shao, J.; Zhou, H.; Chen, Z.; Zhang, S.; Wang, H.; Jiang, H.; Yao, Z.; Lu, Q. Disrupted fronto-parietal network and default-mode network gamma interactions distinguishing suicidal ideation and suicide attempt in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 113, 110475. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Y.; Lau, W.K.W.; Wei, X.; Liu, Y.; Huang, R.; Zhang, R. Hyperconnectivity between the posterior cingulate and middle frontal and temporal gyrus in depression: Based on functional connectivity meta-analyses. Brain Imaging Behav. 2022, 16, 1538–1551. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Yan, D.; Chen, S.; Liu, Y.; Hao, X.; Ou, W.; Huang, Z.; Su, F.; He, F.; et al. Alterations in patients with first-episode depression in the eyes-open and eyes-closed conditions: A resting-state EEG study. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1019–1029. [Google Scholar] [CrossRef]
- Robertson, C.V.; Skein, M.; Wingfield, G.; Hunter, J.R.; Miller, T.D.; Hartmann, T.E. Acute electroencephalography responses during incremental exercise in those with mental illness. Front. Psychiatry 2023, 13, 1049700. [Google Scholar] [CrossRef]
- Zeng, Y.; Lao, J.; Wu, Z.; Lin, G.; Wang, Q.; Yang, M.; Zhang, S.; Xu, D.; Zhang, M.; Liang, S.; et al. Altered resting-state brain oscillation and the associated cognitive impairments in late-life depression with different depressive severity: An EEG power spectrum and functional connectivity study. J. Affect. Disord. 2024, 348, 124–134. [Google Scholar] [CrossRef]
- Hong, S.Y.; Park, Y.M.; Park, E.J. Non-suicidal self-injury and quantified electroencephalogram in adolescents and young adults with depression. Clin. Psychopharmacol. Neurosci. 2024, 22, 151–158. [Google Scholar] [CrossRef]
- Krepel, N.; Benschop, L.; Baeken, C.; Sack, A.T.; Arns, M. An EEG signature of suicidal behavior in female patients with major depressive disorder? A non-replication. Biol. Psychol. 2021, 161, 108058. [Google Scholar] [CrossRef]
- Arikan, M.K.; Gunver, M.G.; Tarhan, N.; Metin, B. High-gamma: A biological marker for suicide attempt in patients with depression. J. Affect. Disord. 2019, 254, 1–6. [Google Scholar] [CrossRef]
- Van der Vinne, N.; Vollebregt, M.A.; van Putten, M.J.A.M.; Arns, M. Frontal alpha asymmetry as a diagnostic marker in depression: Fact or fiction? A meta-analysis. NeuroImage Clin. 2017, 16, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, S.; Li, M.; Su, F.; Chen, S.; Ke, Y.; Ming, D. Altered gamma oscillations and beta–gamma coupling in drug-naive first-episode major depressive disorder: Association with sleep and cognitive disturbance. J. Affect. Disord. 2022, 316, 99–108. [Google Scholar] [CrossRef]
- Mirabella, G. The power of null findings. J. Neurophysiol. 2024, 132, 1085–1086. [Google Scholar] [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. Neurosci. 2019, 12, 521. [Google Scholar] [CrossRef]
- Barry, R.J.; De Blasio, F.M. EEG differences between eyes-closed and eyes-open resting remain in healthy ageing. Biol. Psychol. 2017, 129, 293–304. [Google Scholar] [CrossRef]
- Petro, N.M.; Ott, L.R.; Penhale, S.H.; Rempe, M.P.; Embury, C.M.; Picci, G.; Wang, Y.-P.; Stephen, J.M.; Calhoun, V.D.; Wilson, T.W. Eyes-closed versus eyes-open differences in spontaneous neural dynamics during development. NeuroImage 2022, 258, 119337. [Google Scholar] [CrossRef] [PubMed]
- Siegle, G.J.; Condray, R.; Thase, M.E.; Keshavan, M.; Steinhauer, S.R. Sustained gamma-band EEG following negative words in depression and schizophrenia. Int. J. Psychophysiol. 2010, 75, 107–118. [Google Scholar] [CrossRef]
- Strelets, V.B.; Garakh, Z.V.; Novototskii-Vlasov, V.Y. Comparative study of the gamma rhythm in normal conditions, during examination stress, and in patients with first depressive episode. Neurosci. Behav. Physiol. 2007, 37, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Kim, B.S.; Seo, B.A.; Lee, S.T.; Jung, K.H.; Chu, K.; Lee, S.K.; Jeon, D. Gamma oscillation in functional brain networks is involved in the spontaneous remission of depressive behavior induced by chronic restraint stress in mice. BMC Neurosci. 2016, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.T.; Burkhouse, K.L.; Kinney, K.L.; Phan, K.L. The roles of early-life adversity and rumination in neural response to emotional faces amongst anxious and depressed adults. Psychol. Med. 2019, 49, 2267–2278. [Google Scholar] [CrossRef]
- Nejad, A.B.; Fossati, P.; Lemogne, C. Self-referential processing, rumination, and cortical midline structures in major depression. Front. Hum. Neurosci. 2013, 7, 666. [Google Scholar] [CrossRef]
- Braem, S.; King, J.A.; Korb, F.M.; Krebs, R.M.; Notebaert, W.; Egner, T. The Role of Anterior Cingulate Cortex in the Affective Evaluation of Conflict. J. Cogn. Neurosci. 2017, 29, 137–149. [Google Scholar] [CrossRef]
- Lombardo, C.; Bruno, A.; Turiaco, F.; Imbesi, M.; Arena, F.; Capillo, A.; Pandolfo, G.; Silvestri, M.C.; Muscatello, M.R.A.; Mento, C. The predictivity role of affective temperaments in mood alteration. J. Affect. Disord. Rep. 2024, 17, 100819. [Google Scholar] [CrossRef]
- Luca, A.; Luca, M.; Kasper, S.; Pecorino, B.; Zohar, J.; Souery, D.; Montgomery, S.; Ferentinos, P.; Rujescu, D.; Messina, A.; et al. Anhedonia is associated with a specific depression profile and poor antidepressant response. Int. J. Neuropsychopharmacol. 2024, 27, pyae055. [Google Scholar] [CrossRef]
- Murty, D.V.P.S.; Manikandan, K.; Kumar, W.S.; Ramesh, R.G.; Purokayastha, S.; Javali, M.; Rao, N.P.; Ray, S. Gamma oscillations weaken with age in healthy elderly in human EEG. NeuroImage 2020, 215, 116826. [Google Scholar] [CrossRef]
- Geiser, C.; Keller, B.T.; Lockhart, G.; Eid, M.; Cole, D.A.; Koch, T. Distinguishing state variability from trait change in longitudinal data: The role of measurement (non)invariance in latent state-trait analyses. Behav. Res. Methods 2015, 47, 172–203. [Google Scholar] [CrossRef]
- Salinsky, M.C.; Oken, B.S.; Morehead, L. Test-retest reliability in EEG frequency analysis. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 382–392. [Google Scholar] [CrossRef]
- Tozzi, L.; Zhang, X.; Pines, A.; Olmsted, A.M.; Zhai, E.S.; Anene, E.T.; Chesnut, M.; Holt-Gosselin, B.; Chang, S.; Stetz, P.C.; et al. Personalized brain circuit scores identify clinically distinct biotypes in depression and anxiety. Nat. Med. 2024, 30, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Ritsner, M.S. Anhedonia: A Comprehensive Handbook Volume I; Springer: Dordrecht, The Netherlands, 2014; Volume 1, pp. 19–54. [Google Scholar]
- Di Nicola, M.; De Risio, L.; Battaglia, C.; Camardese, G.; Tedeschi, D.; Mazza, M.; Martinotti, G.; Pozzi, G.; Niolu, C.; Di Giannantonio, M.; et al. Reduced hedonic capacity in euthymic bipolar subjects: A trait-like feature? J. Affect. Disord. 2013, 147, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sugaya, N.; Siegle, G.J.; Kumano, H.; Shimada, H.; Machado, S.; Murillo-Rodriguez, E.; Rocha, N.B.; Nardi, A.E.; Takamura, M.; et al. Altered gamma-band activity as a potential biomarker for the recurrence of major depressive disorder. Front. Psychiatry 2018, 9, 691. [Google Scholar] [CrossRef] [PubMed]
| Subtype | Depressed Mood | Anhedonia | Cognitive | Somatic |
|---|---|---|---|---|
| Profile | Worthlessness, withdrawal from adverse environments | Withdrawal from reward-seeking, negative bias | Executive functioning deficits, negative emotions | Disrupted appetite, metabolism, sleep, and energy levels |
| SDS items | 1. I feel downhearted and blue 3. I have crying spells or feel like it 14. I feel hopeful about the future 15. I am more irritable than usual 17. I feel that I am useful and needed 19. I feel that others would be better off if I were dead | 5. I eat as much as I used to 6. I still enjoy sex 18. My life is pretty full 20. I still enjoy doing the things I used to | 11. My mind is as clear as it used to be 12. I find it easy to do the things I used to do 16. I find it easy to make decisions | 4. I have trouble sleeping at night 7. I notice that I am losing weight 8. I have trouble with constipation 9. My heart beats faster than usual 10. I get tired for no reason 13. I am restless and can’t keep still |
| n | Age (Mean) | SD | Range (Years) | Total SDS (Mean) | SD | Range (Total Score) | |
|---|---|---|---|---|---|---|---|
| Total | 100 | 32.53 | 14.125 | 18–75 | 36.7 | 11.256 | 21–66 |
| Male | 44 | 33.57 | 13.961 | 18–68 | 36.18 | 10.31 | 23–61 |
| Female | 56 | 31.71 | 14.326 | 18–75 | 37.11 | 12.024 | 21–66 |
| Sex | Total SDS | Mood | Anhedonia | Cognitive | Somatic | |
|---|---|---|---|---|---|---|
| Age | −0.065 | 0.055 | 0.077 | 0.104 | 0.136 | −0.015 |
| Sex | 0.041 | 0.059 | 0.012 | −0.007 | 0.054 | |
| Total | 0.921 ** | 0.846 ** | 0.925 ** | 0.874 ** | ||
| Mood | 0.678 ** | 0.831 ** | 0.748 ** | |||
| Anhedonia | 0.789 ** | 0.626 ** | ||||
| Cognitive | 0.736 ** |
| Subtype | Depressed Mood | Anhedonia | Cognitive | Somatic |
|---|---|---|---|---|
| Questions | 1, 3, 14, 15, 17, 19 | 5, 6, 18, 20 | 11, 12, 16 | 4, 7, 8, 9, 10, 13 |
| Mean | 10.42 | 7.4 | 6.55 | 9.71 |
| SD | 3.92 | 2.71 | 2.43 | 3.07 |
| Max r-Value | Two-Tailed p-Value | Brain Structure | Brodmann Area | |
|---|---|---|---|---|
| Total SDS | 0.225 | 0.19 | Anterior Cingulate | 24 |
| Depressed Mood | 0.175 | 0.44 | Anterior Cingulate | 24 |
| Anhedonia | 0.298 | 0.032 | Anterior Cingulate | 24 |
| Cognitive | 0.219 | 0.208 | Anterior Cingulate | 24 |
| Somatic | 0.164 | 0.521 | Anterior Cingulate | 24 |
| Max r-Value | Two-Tailed p-Value | Brain Structure | Brodmann Area | |
|---|---|---|---|---|
| Total SDS | 0.089 | 0.516 | Middle Frontal Gyrus | 6 |
| Depressed Mood | 0.106 | 0.415 | Inferior Frontal Gyrus | 9 |
| Anhedonia | 0.090 | 0.83 | Precentral Gyrus | 4 |
| Cognitive | 0.128 | 0.872 | Inferior Frontal Gyrus | 9 |
| Somatic | 0.137 | 0.414 | Middle Frontal Gyrus | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coleman, S.L.; Evans, I.D.; Sharpley, C.F.; Bitsika, V.; Odierna, G.L.; Vessey, K.A. Electroencephalogram Gamma Band Power Correlates with Anhedonia in a Community Sample. J. Pers. Med. 2025, 15, 536. https://doi.org/10.3390/jpm15110536
Coleman SL, Evans ID, Sharpley CF, Bitsika V, Odierna GL, Vessey KA. Electroencephalogram Gamma Band Power Correlates with Anhedonia in a Community Sample. Journal of Personalized Medicine. 2025; 15(11):536. https://doi.org/10.3390/jpm15110536
Chicago/Turabian StyleColeman, Sarah L., Ian D. Evans, Christopher F. Sharpley, Vicki Bitsika, G. Lorenzo Odierna, and Kirstan A. Vessey. 2025. "Electroencephalogram Gamma Band Power Correlates with Anhedonia in a Community Sample" Journal of Personalized Medicine 15, no. 11: 536. https://doi.org/10.3390/jpm15110536
APA StyleColeman, S. L., Evans, I. D., Sharpley, C. F., Bitsika, V., Odierna, G. L., & Vessey, K. A. (2025). Electroencephalogram Gamma Band Power Correlates with Anhedonia in a Community Sample. Journal of Personalized Medicine, 15(11), 536. https://doi.org/10.3390/jpm15110536






