Impact of Computed Tomography-to-Angiography Interval Time on Outcomes of Transarterial Embolization in Post-Traumatic Bleeding: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
- •
- Injury Severity Score (ISS), calculated according to the Abbreviated Injury Scale (AIS) 2015 update, for all included patients;
- •
- Admission and post-procedural hemodynamic parameters. At Emergency Department (ED) admission, hemodynamic parameters were recorded, including systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate (HR), and Shock Index (SI; calculated as HR(beats per minute)/SBP(mmHg));
- •
- Baseline and 24 h hemoglobin and lactate levels;
- •
- Post-embolization transfusion requirements, massive transfusion protocol (MTP) activation, and overall transfusion balance. Massive transfusion protocol (MTP) was defined as the transfusion of ≥10 units of packed red blood cells within the first 24 h after admission;
- •
- Timing intervals, including ED-to-CT and CT-to-angiography intervals;
- •
- CT and angiographic findings, classifying bleeding sites as pelvic, abdominal, thoracic, or other.
- •
- Re-interventions: re-embolization was defined as any repeated TAE for persistent or recurrent hemorrhage during the index hospitalization. Surgical rescue included damage-control laparotomy (e.g., splenectomy, bowel repair/resection), pelvic external fixation with/without preperitoneal packing (PPP), and other hemostatic procedures performed after the index TAE. Injury categories were predefined as pelvic, abdominal (solid organ or hollow viscus), thoracic, or others. For surgical rescue, multiple procedures could be performed in the same patient; therefore, totals may exceed the number of patients.
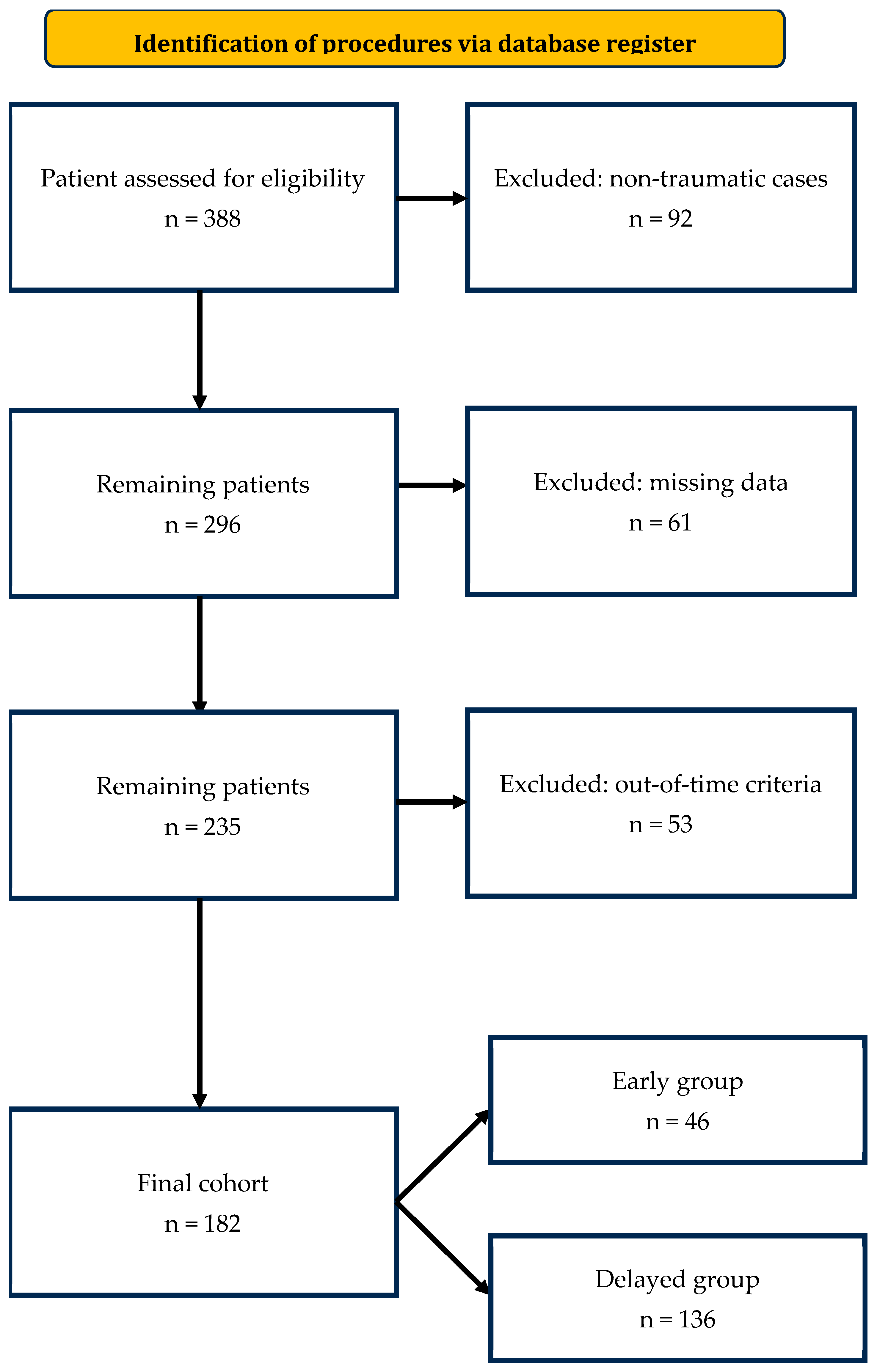
2.3. Patient Selection
2.4. Outcome Measures
2.5. Procedural Details
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
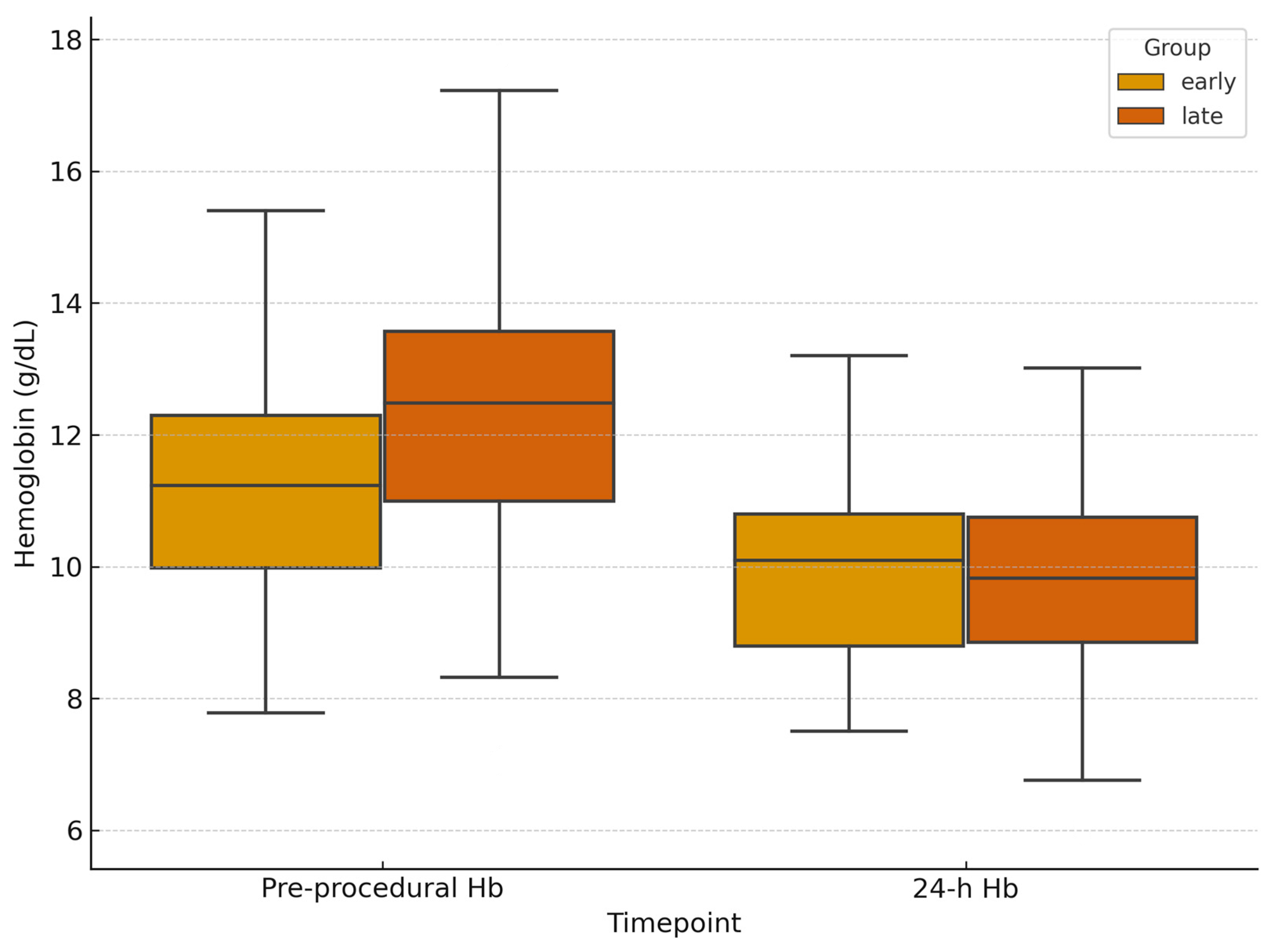
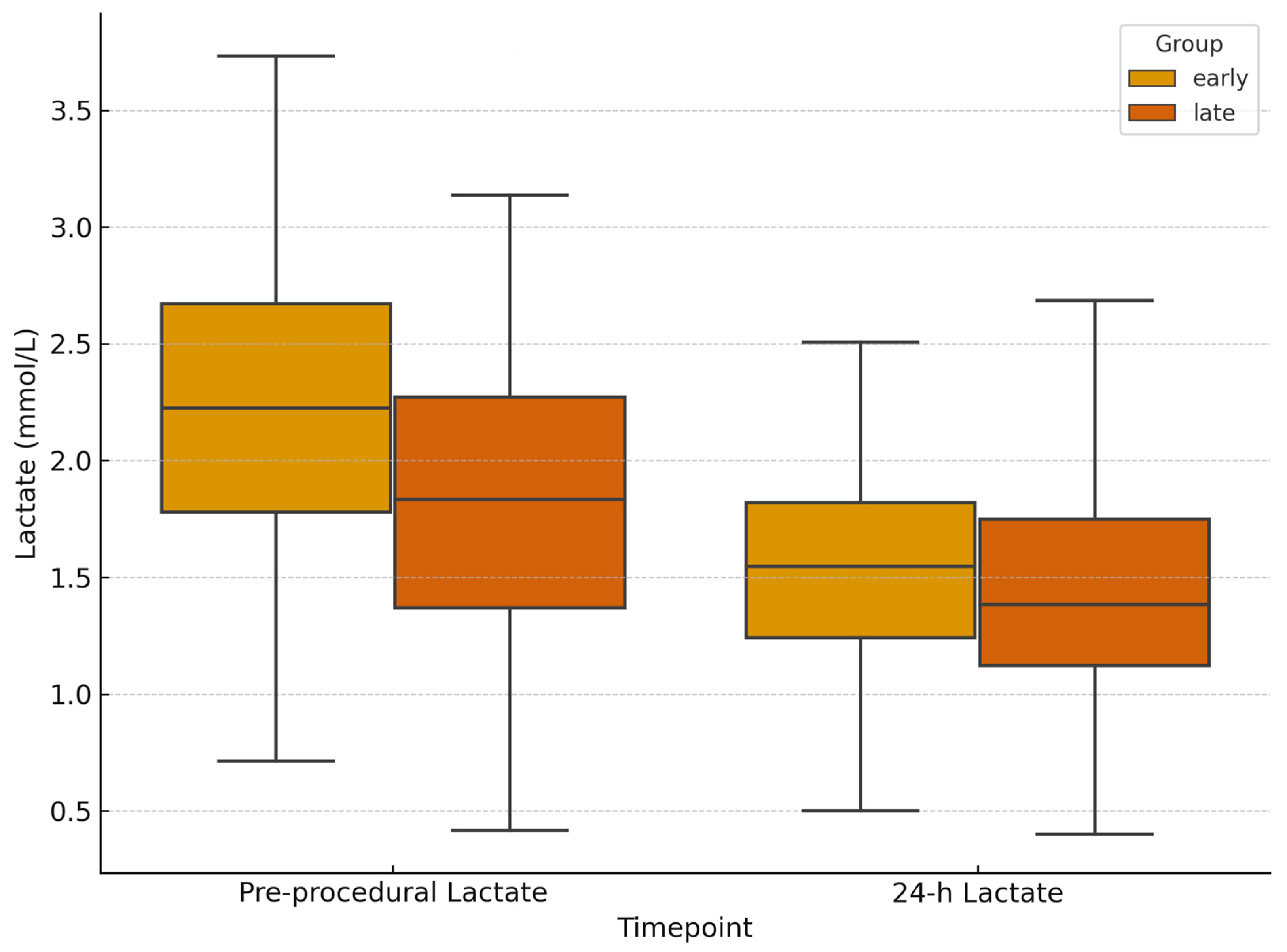
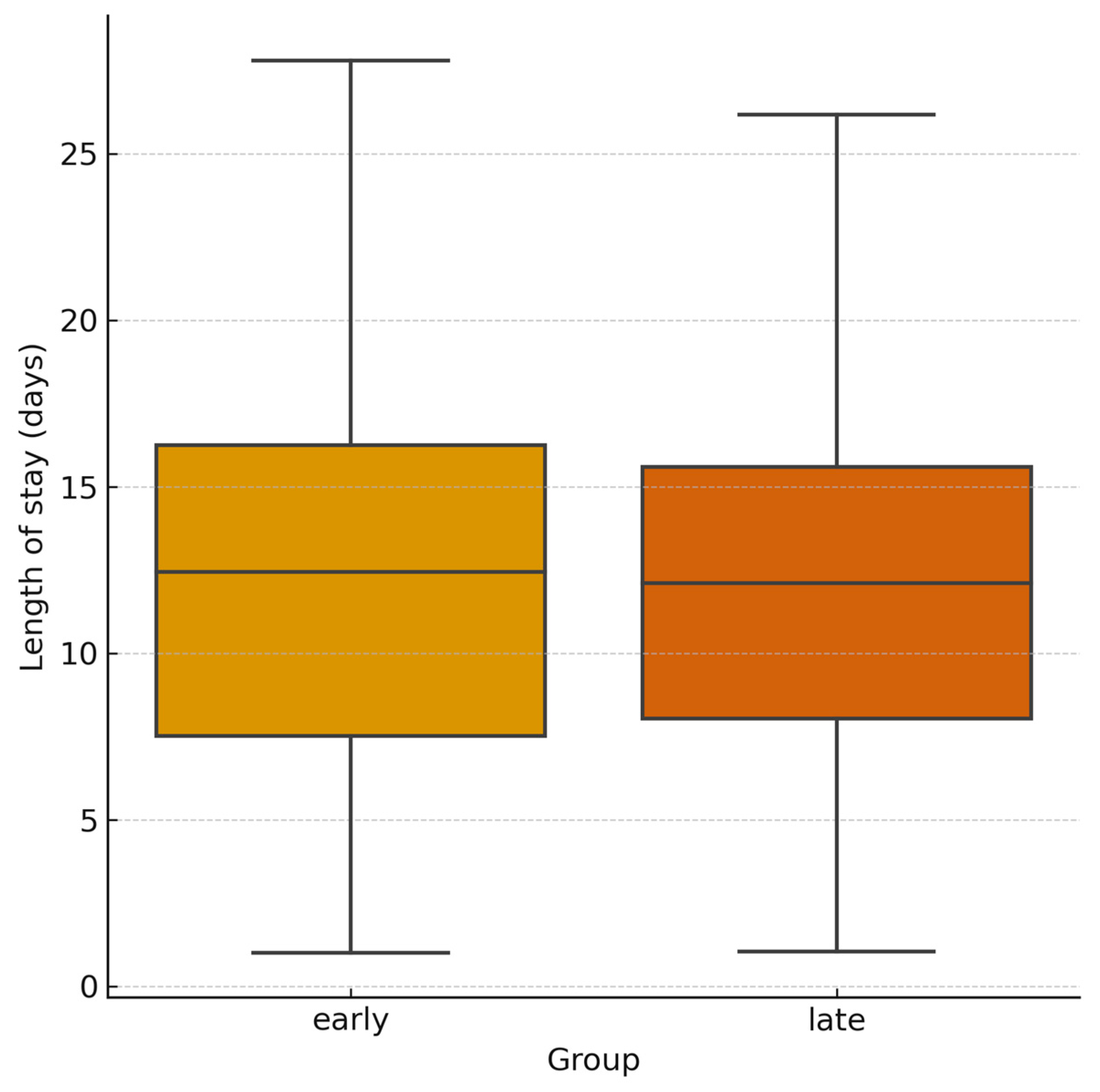
3.2. Laboratory and Hemodynamic Outcomes
3.3. Re-Interventions
3.4. Procedure-Related Complications
3.5. Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TAE | Transarterial Embolization |
| CT | Computed Tomography |
| NOM | Non-Operative Management |
| ISS | Injury Severity Score |
| IR | Interventional Radiology |
| MDCT | Multidetector Computed Tomography |
| WSES | World Society of Emergency Surgery |
| ED | Emergency Department |
| PACS | Picture Archiving and Communication System |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| MAP | Mean Arterial Pressure |
| HR | Heart Rate |
| SI | Shock Index |
| MTP | Massive Transfusion Protocol |
| PRBC | Packed Red Blood Cells |
| PPP | Preperitoneal Packing |
| LOS | Length of Stay |
| RTS | Revised Trauma Score |
| TRISS | Trauma and Injury Severity Score |
References
- Holcomb, J.B.; del Junco, D.J.; Fox, E.E.; Wade, C.E.; Cohen, M.J.; Schreiber, M.A.; Alarcon, L.H.; Bai, Y.; Brasel, K.J.; Bulger, E.M.; et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: Comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013, 148, 127–136. [Google Scholar] [CrossRef]
- Kauvar, D.S.; Lefering, R.; Wade, C.E. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J. Trauma 2006, 60 (Suppl. S6), S3–S11. [Google Scholar] [CrossRef]
- Moore, L.J. Blood, balloons, and blades: State of the art trauma resuscitation. Am. J. Surg. 2022, 224 Pt A, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Latif, R.K.; Clifford, S.P.; Baker, J.A.; Lenhardt, R.; Haq, M.Z.; Huang, J.; Farah, I.; Businger, J.R. Traumatic hemorrhage and chain of survival. Scand. J. Trauma Resusc. Emerg. Med. 2023, 31, 25. [Google Scholar] [CrossRef] [PubMed]
- Lopera, J.E. Embolization in trauma: Review of basic principles and techniques. Semin. Interv. Radiol. 2021, 38, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Corvino, F.; Giurazza, F.; Marra, P.; Ierardi, A.M.; Corvino, A.; Basile, A.; Galia, M.; Inzerillo, A.; Niola, R. Damage control interventional radiology in liver trauma: A comprehensive review. J. Pers. Med. 2024, 14, 365. [Google Scholar] [CrossRef]
- Hsieh, T.-M.; Tsai, T.C.; Liang, J.-L.; Lin, C.C. Non-operative management attempted for selective high-grade blunt hepatosplenic trauma is a feasible strategy. World J. Emerg. Surg. 2014, 9, 51. [Google Scholar] [CrossRef]
- Beuran, M.; Gheju, I.; Venter, M.D.; Marian, R.; Smarandache, R. Non-operative management of splenic trauma. J. Med. Life 2012, 5, 47–58. [Google Scholar] [PubMed]
- Bou Saba, G.; Rahal, R.; Bachir, R.; El Sayed, M. Interventional angiography utilization for adult trauma patients in trauma centers across the United States: An observational study using the National Trauma Data Bank. Medicine 2022, 101, e30900. [Google Scholar] [CrossRef]
- Minici, R.; Fontana, F.; Venturini, M.; Guzzardi, G.; Piacentino, F.; Spinetta, M.; Bertucci, B.; Serra, R.; Costa, D.; Ielapi, N.; et al. A multicenter retrospective cohort study evaluating the clinical outcomes of patients with coagulopathy undergoing transcatheter arterial embolization for acute non-neurovascular bleeding. Medicina 2023, 59, 1333. [Google Scholar] [CrossRef]
- Minici, R.; Mercurio, M.; Guzzardi, G.; Venturini, M.; Fontana, F.; Brunese, L.; Guerriero, P.; Serra, R.; Piacentino, F.; Spinetta, M.; et al. Transcatheter arterial embolization for bleeding related to pelvic trauma: Comparison of technical and clinical results between hemodynamically stable and unstable patients. Tomography 2023, 9, 1660–1682. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, H.; Abdelrahman, H.; Barah, A.; Asim, M.; El-Menyar, A. Utility of angioembolization in patients with abdominal and pelvic traumatic bleeding: Descriptive observational analysis from a Level 1 trauma center. Ther. Clin. Risk Manag. 2021, 17, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Pane, F.; Roccatagliata, P.; Casciano, E.; Corvino, F.; Festa, P.; Ponticiello, G.; Cappabianca, S.; Romano, L.; Niola, R. Pelvic ring fractures with concomitant large hematomas: Diagnostic investigation with arteriography and eventual embolization in 157 trauma patients, with or without contrast extravasation at emergency CT. Radiol. Med. 2023, 128, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Society of Interventional Radiology. Society of Interventional Radiology position statement on endovascular intervention for trauma. J. Vasc. Interv. Radiol. 2020, 31, 363–369.e2. [Google Scholar] [CrossRef]
- Chakraverty, S.; Flood, K.; Kessel, D.; McPherson, S.; Nicholson, T.; Ray, C.E., Jr.; Robertson, I.; van Delden, O.M. CIRSE guidelines: Quality improvement guidelines for endovascular treatment of traumatic hemorrhage. Cardiovasc. Interv. Radiol. 2012, 35, 472–482. [Google Scholar] [CrossRef]
- De Simone, B.; Chouillard, E.; Podda, M.; Pararas, N.; Duarte, G.d.C.; Fugazzola, P.; Birindelli, A.; Coccolini, F.; Polistena, A.; Sibilla, M.G.; et al. The 2023 WSES guidelines on the management of trauma in elderly and frail patients. World J. Emerg. Surg. 2024, 19, 18. [Google Scholar] [CrossRef]
- Chung, R.; Chawla, A.; Shikhare, S.; Babu, S. Trends and implications of 24/7 interventional radiology in a newly opened acute hospital. CVIR Endovasc. 2018, 1, 33. [Google Scholar] [CrossRef]
- DuanMu, B.; Du, X.; Sun, J.; Dong, T.; Guan, H.; Wu, Y.; Lian, H. Timely angiography and embolization is effective emergency treatment for severe post-traumatic pelvic fractures. Sci. Rep. 2025, 15, 12530. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, C.H.; Kim, J.H.; Kwon, H.; Kim, C.W.; Kim, G.H.; Lee, C.K.; Lee, S.B.; Jang, J.H.; Kim, S.H.; et al. Relationship between door-to-embolization time and clinical outcomes after transarterial embolization in trauma patients with complex pelvic fracture. Eur. J. Trauma Emerg. Surg. 2022, 48, 1929–1938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aoki, M.; Abe, T.; Matsumoto, S.; Hagiwara, S.; Saitoh, D.; Oshima, K. Delayed embolization associated with increased mortality in pelvic fracture with hemodynamic stability at hospital arrival. World J. Emerg. Surg. 2021, 16, 21. [Google Scholar] [CrossRef]
- O’Connell, K.M.; Kolnik, S.; Arif, K.; Qiu, Q.; Jones, S.; Ingraham, C.; Rivara, F.; Vavilala, M.S.; Maier, R.; Bulger, E.M. Balloons up: Shorter time to angioembolization is associated with reduced mortality in patients with shock and complex pelvic fractures. Trauma Surg. Acute Care Open 2021, 6, e000663. [Google Scholar] [CrossRef]
- Jarvis, S.; Orlando, A.; Blondeau, B.; Banton, K.; Reynolds, C.; Berg, G.M.; Patel, N.; Kelly, M.; Carrick, M.; Bar-Or, D. Variability in the timeliness of interventional radiology availability for angioembolization of hemodynamically unstable pelvic fractures: A prospective survey among U.S. level I trauma centers. Patient Saf. Surg. 2019, 13, 23. [Google Scholar] [CrossRef]
- Matsushima, K.; Piccinini, A.; Schellenberg, M.; Cheng, V.; Heindel, P.; Strumwasser, A.; Benjamin, E.; Inaba, K.; Demetriades, D. Effect of door-to-angio time on mortality in pelvic fracture: Every hour of delay counts. J. Trauma Acute Care Surg. 2018, 85, 110–116. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. CIRSE Quality Assurance Document and Standards for classification of complications: The CIRSE classification system. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Maeshima, K.; Funabiki, T.; Eastridge, B.J.; Cestero, R.F.; Sasaki, J. Immediate Angiography and Decreased In-Hospital Mortality of Adult Trauma Patients: A Nationwide Study. Cardiovasc. Interv. Radiol. 2024, 47, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, M.; Döll, S.; Kramer, A.; Weidhase, L.; Hartwig, T.; Petros, S.; Gries, A. Elevated admission lactates levels in the emergency department are associated with increased 30-day mortality in non-trauma critically ill patients. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 82. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, E.; Hardcastle, T.C.; Barle, H.; Muckart, D.J.J. Lactates clearance predicts outcome after major trauma. Afr. J. Emerg. Med. 2014, 4, 123–129. [Google Scholar] [CrossRef]
- Bou Chebl, R.; El Khuri, C.; Shami, A.; Rajha, E.; Faris, N.; Bachir, R.; Abou Dagher, G. Serum lactates is an independent predictor of hospital mortality in critically ill patients in the emergency department: A retrospective study. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 69. [Google Scholar] [CrossRef]
- Sadrzadeh, S.M.; Talebzadeh, V.; Mousavi, S.M.; Kakhki, B.R.; Moradi, E.V.; Disfani, H.F. Prognostic value of lactates levels in trauma patients’ outcomes in emergency department. Bull. Emerg. Trauma 2025, 13, 32–36. [Google Scholar] [CrossRef]
- Green, C.S.M.; Bulger, E.M.; Kwan, S.W. Outcomes and complications of angioembolization for hepatic trauma: A systematic review of the literature. J. Trauma Acute Care Surg. 2016, 80, 529–537. [Google Scholar] [CrossRef]
- Segalini, E.; Morello, A.; Leati, G.; Di Saverio, S.; Aseni, P. Primary angioembolization in liver trauma: Major hepatic necrosis as a severe complication of a minimally invasive treatment—A narrative review. Updates Surg. 2022, 74, 1511–1519. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernández-Mondéjar, E.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Nardi, G.; et al. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Crit. Care 2013, 17, R76. [Google Scholar] [CrossRef]
- Ryan, A.G.; Slijepčević, B.; Cannavale, A.; Krokidis, M.; Chun, J.Y.; de Baere, T.; Dezman, R.; Duvnjak, S.; Ruffino, M.A.; Urbano, J.; et al. Developing a clinical service in interventional radiology: Results from the 2024 CIRSE clinical practice survey. Cardiovasc. Interv. Radiol. 2024, 47, 1795–1800. [Google Scholar] [CrossRef]
- Clements, W.; Giurazza, F. A commentary discussing the overlooked domains of complications, adverse events, and quality improvement in interventional radiology. Cardiovasc. Interv. Radiol. 2024, 47, 1694–1695. [Google Scholar] [CrossRef]



| Parameter | Early (n = 46) | 95% CI | Delayed (n = 136) | 95% CI | p-Value |
|---|---|---|---|---|---|
| Injury Severity Score (ISS) | 24 ± 8 | 21.7–26.3 | 18 ± 6 | 17.0–19.0 | <0.0001 |
| Mean arterial pressure (mmHg) | 80 ± 13 | 76.1–83.9 | 83 ± 14 | 80.6–85.4 | 0.18 |
| Systolic BP (mmHg) | 110 ± 18 | — | 113 ± 20 | — | 0.34 |
| Diastolic BP (mmHg) | 68 ± 12 | — | 70 ± 11 | — | 0.32 |
| Heart rate (bpm) | 98 ± 18 | 92.7–103.3 | 95 ± 17 | 92.1–97.9 | 0.32 |
| Shock Index (HR/SBP) | 0.92 ± 0.20 | 0.86–0.98 | 0.85 ± 0.17 | 0.82–0.88 | 0.10 |
| Variable | Early Group (≤1 h) | Delayed Group (>1 h) | p-Value | 95% CI |
|---|---|---|---|---|
| CT-to-angiography time (min, median [IQR]) | 58 [45–60] | 158 [110–212.5] | <0.001 | −111 to −86 min |
| ED-to-CT time (min, median [IQR]) | 60.5 [44.8–85.3] | 62.0 [46.0–102.0] | 0.26 | −9.5 to 6.5 min |
| Age (years, median [IQR]) | 51 [30.5–67] | 58 [36.5–75.5] | - | - |
| Male sex (%) | 73.9% | 66.9% | - | - |
| Baseline hemoglobin (g/dL, median [IQR]) | 11.7 [9.68–13.17] | 12.3 [10.23–13.58] | 0.13 | −1.38 to 0.18 |
| Baseline lactates (mmol/L, median [IQR]) | 2.2 [1.48–3.13] | 1.9 [1.3–2.65] | 0.46 | −0.18 to 0.78 |
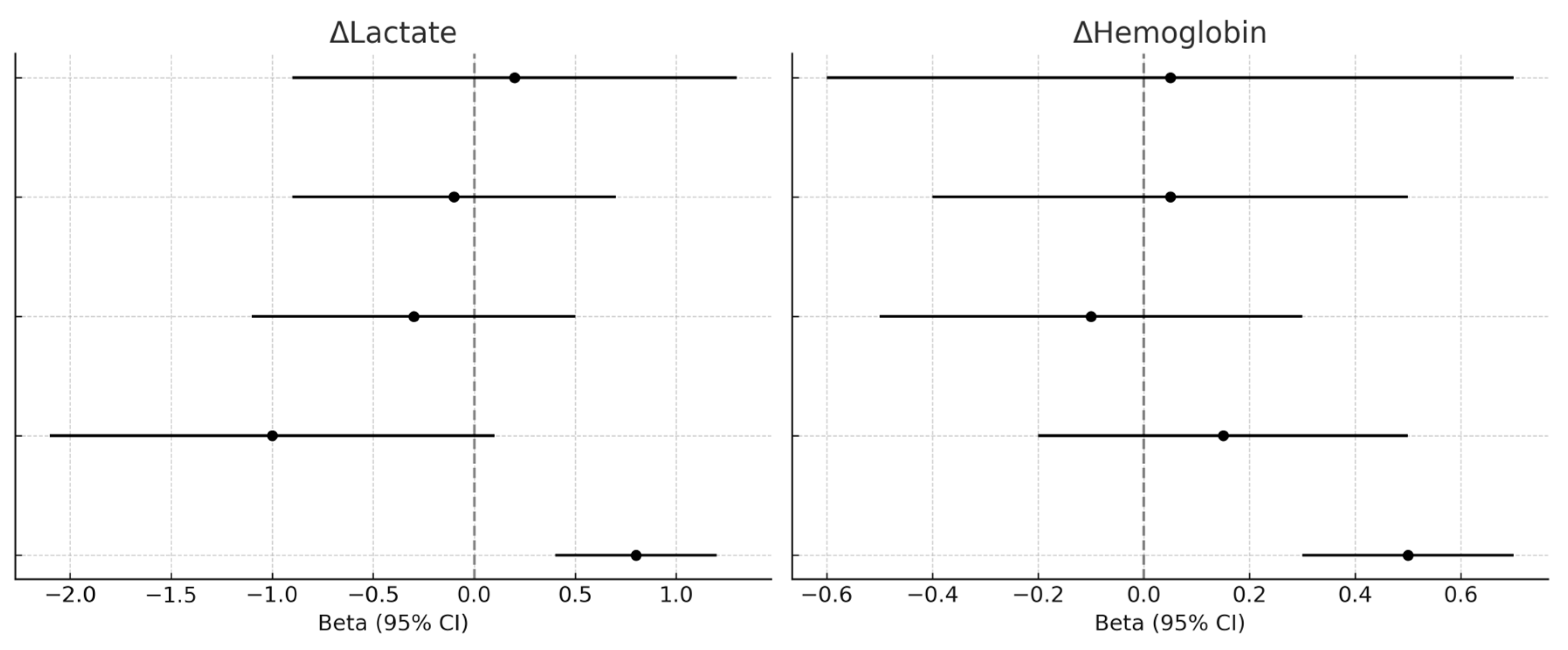
| ΔHemoglobin at 24 h (g/dL) | −0.95 (95% CI −1.42 to −0.48) | −1.60 (95% CI −1.94 to −1.26) | 0.12 | −0.12 to 1.42 |
| ΔLactates at 24 h (mmol/L) | −0.7 (95% CI −0.95 to −0.45) | −0.5 (95% CI −0.62 to −0.38) | 0.79 | −0.19 to 0.59 |
| PRBC transfusion post-procedure (units, median [IQR]) | 1 [0–2] | 1 [0–2] | - | - |
| Total PRBC transfused (units) | 69 | 158 | n.a. | - |
| Massive transfusion protocol activation | 0 | 0 | - | - |
| Technical success (%) | 100% | 100% | - | - |
| Re-embolization (%) | 8.7% | 1.5% | 0.036 | OR 1.10–12.45 |
| Surgical rescue (%) | 13.0% | 3.7% | 0.033 | OR 1.13–9.85 |
| In-hospital mortality (%) | 2.9% | 2.5% | 0.40 | OR 0.25–9.12 |
| Length of hospital stay (days, median [IQR]) | 12 [6–25] | 11 [6–20] | 0.89 | −2.5 to 3.5 |
| Minor complications (CIRSE Grade 1–2, %) | 10.9% | 8.1% | 0.56 | OR 0.32–2.98 |
| Major complications (CIRSE Grade 3–4, %) | 2.2% | 2.9% | 1.00 | OR 0.05–6.52 |
| Procedure | Early (n = 46) | Delayed (n = 136) |
|---|---|---|
| Preperitoneal pelvic packing (PPP) | 3 (6.5%) | 2 (1.5%) |
| Pelvic fixation/SI screws/C-clamp | 3 (6.5%) | 1 (0.7%) |
| Damage-control laparotomy + temporary abdominal closure | 2 (4.3%) | 1 (0.7%) |
| Splenectomy | 1 (2.2%) | 2 (1.5%) |
| Bowel repair/resection | 1 (2.2%) | 1 (0.7%) |
| Hepatorrhaphy/hepatic packing | 1 (2.2%) | 0 (0.0%) |
| Nephrectomy/renorrhaphy | 0 (0.0%) | 1 (0.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrubba, C.; Giurazza, F.; Corvino, F.; Capozzoli, F.; Niola, R. Impact of Computed Tomography-to-Angiography Interval Time on Outcomes of Transarterial Embolization in Post-Traumatic Bleeding: A Retrospective Observational Study. J. Pers. Med. 2025, 15, 528. https://doi.org/10.3390/jpm15110528
Carrubba C, Giurazza F, Corvino F, Capozzoli F, Niola R. Impact of Computed Tomography-to-Angiography Interval Time on Outcomes of Transarterial Embolization in Post-Traumatic Bleeding: A Retrospective Observational Study. Journal of Personalized Medicine. 2025; 15(11):528. https://doi.org/10.3390/jpm15110528
Chicago/Turabian StyleCarrubba, Claudio, Francesco Giurazza, Fabio Corvino, Federico Capozzoli, and Raffaella Niola. 2025. "Impact of Computed Tomography-to-Angiography Interval Time on Outcomes of Transarterial Embolization in Post-Traumatic Bleeding: A Retrospective Observational Study" Journal of Personalized Medicine 15, no. 11: 528. https://doi.org/10.3390/jpm15110528
APA StyleCarrubba, C., Giurazza, F., Corvino, F., Capozzoli, F., & Niola, R. (2025). Impact of Computed Tomography-to-Angiography Interval Time on Outcomes of Transarterial Embolization in Post-Traumatic Bleeding: A Retrospective Observational Study. Journal of Personalized Medicine, 15(11), 528. https://doi.org/10.3390/jpm15110528







