Feasibility of Ultrasound-Guided, Peripherally Inserted Central Catheter Placement at the Bedside in a Communicable-Disease Isolation Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
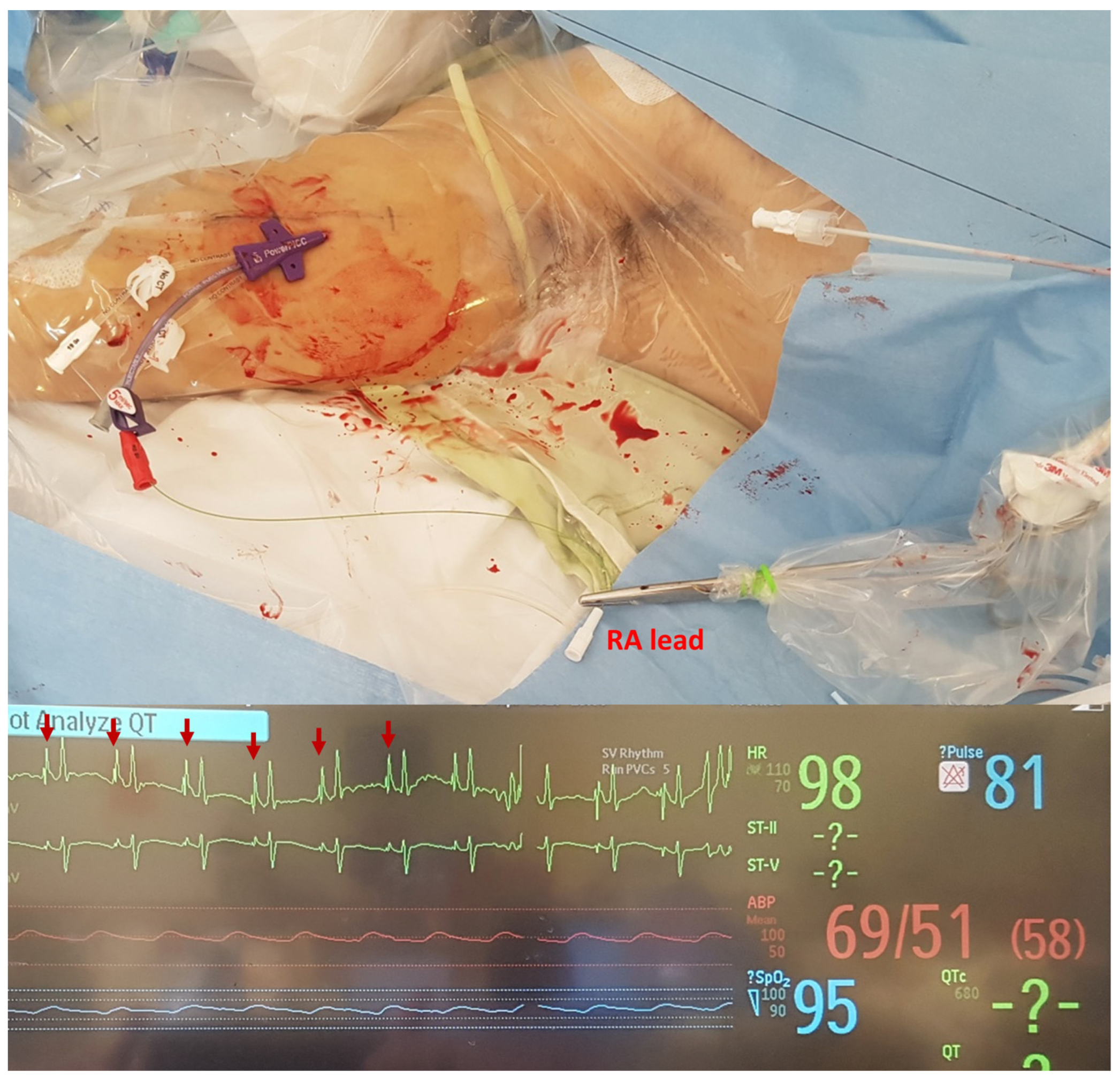
2.2. Procedure
2.3. Data Collection, Endpoints and Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Procedural Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campagna, S.; Gonella, S.; Berchialla, P.; Morano, G.; Rigo, C.; Zerla, P.A.; Fuzzi, R.; Corona, G.; Storto, S.; Dimonte, V. Can peripherally inserted central catheters be safely placed in patients with cancer receiving chemotherapy? A retrospective study of almost 400,000 catheter-days. Oncologist 2019, 24, e953–e959. [Google Scholar] [CrossRef]
- Govindan, S.; Snyder, A.; Flanders, S.A.; Chopra, V. Peripherally Inserted Central Catheters (PICCs) in the ICU: A Retrospective Study of Adult Medical Patients in 52 Hospitals. Crit. Care Med. 2018, 46, e1136. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.; Bihari, S.; Holt, A.; Bersten, A.; Kulkarni, H. Rate of catheter-related bloodstream infections between tunneled central venous catheters versus peripherally inserted central catheters in adult home parenteral nutrition: A meta-analysis. J. Parenter. Enter. Nutr. 2019, 43, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Son, S.M.; Lee, S.H.; Kim, J.H.; Kim, H.; Kim, J.Y.; Kim, J.I.; Moon, I.S. Outcomes of bedside peripherally inserted central catheter placement: A retrospective study at a single institution. Acute Crit. Care 2020, 35, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Chung, C.R.; Gil, E.; Park, C.M.; Suh, G.Y.; Ryu, J.A. Safety and feasibility of ultrasound-guided placement of peripherally inserted central catheter performed by neurointensivist in neurosurgery intensive care unit. PLoS ONE 2019, 14, e0217641. [Google Scholar] [CrossRef]
- Pittiruti, M.; Pinelli, F. Recommendations for the use of vascular access in the COVID-19 patients: An Italian perspective. Crit. Care 2020, 24, 269. [Google Scholar] [CrossRef]
- Fan, E.M.P.; Nguyen, N.H.L.; Ang, S.Y.; Aloweni, F.; Goh, H.Q.I.; Quek, L.T.; Ayre, T.C.; Pourghaderi, A.R.; Lam, S.W.; Ong, E.H.M. Impact of COVID-19 on acute isolation bed capacity and nursing workforce requirements: A retrospective review. J. Nurs. Manag. 2021, 29, 1220–1227. [Google Scholar] [CrossRef]
- Lilly, C.M.; Oropello, J.M.; Pastores, S.M.; Coopersmith, C.M.; Khan, R.A.; Sessler, C.; Christman, J.W. Workforce, workload, and burnout in critical care organizations: Survey results and research agenda. Crit. Care Med. 2020, 48, 1565. [Google Scholar] [CrossRef]
- Gebhard, R.E.; Szmuk, P.; Pivalizza, E.G.; Melnikov, V.; Vogt, C.; Warters, R.D. The accuracy of electrocardiogram-controlled central line placement. Anesth. Analg. 2007, 104, 65–70. [Google Scholar] [CrossRef]
- Lee, J.-H.; Bahk, J.-H.; Ryu, H.-G.; Jung, C.-W.; Jeon, Y. Comparison of the bedside central venous catheter placement techniques: Landmark vs electrocardiogram guidance. Br. J. Anaesth. 2009, 102, 662–666. [Google Scholar] [CrossRef]
- Smith, B.; Neuharth, R.M.; Hendrix, M.A.; McDonnall, D.; Michaels, A.D. Intravenous electrocardiographic guidance for placement of peripherally inserted central catheters. J. Electrocardiol. 2010, 43, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Peck, K.R. Early diagnosis and rapid isolation: Response to COVID-19 outbreak in Korea. Clin. Microbiol. Infect. 2020, 26, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Brat, G.A.; Hersey, S.; Chhabra, K.; Gupta, A.; Scott, J. Protecting surgical teams during the COVID-19 outbreak: A narrative review and clinical considerations. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Jha, A.K.; Kulkarni, S.G. Evolution of COVID-19 management in critical care: Review and perspective from a hospital in the United Kingdom. Acute Crit. Care 2021, 36, 1–14. [Google Scholar] [CrossRef]
- Wax, R.S.; Christian, M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can. J. Anesth./J. Can. D’anesthésie 2020, 67, 568–576. [Google Scholar] [CrossRef]
- Miller, S.L.; Clements, N.; Elliott, S.A.; Subhash, S.S.; Eagan, A.; Radonovich, L.J. Implementing a negative-pressure isolation ward for a surge in airborne infectious patients. Am. J. Infect. Control 2017, 45, 652–659. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeong, H.W. Rapid expansion of temporary, reliable airborne-infection isolation rooms with negative air machines for critical COVID-19 patients. Am. J. Infect. Control 2020, 48, 822–824. [Google Scholar] [CrossRef]
- Liew, M.F.; Siow, W.T.; Yau, Y.W.; See, K.C. Safe patient transport for COVID-19. Crit. Care 2020, 24, 1–3. [Google Scholar] [CrossRef]
- Parmentier-Decrucq, E.; Poissy, J.; Favory, R.; Nseir, S.; Onimus, T.; Guerry, M.-J.; Durocher, A.; Mathieu, D. Adverse events during intrahospital transport of critically ill patients: Incidence and risk factors. Ann. Intensive Care 2013, 3, 10. [Google Scholar] [CrossRef]
- Bianchi, S.; Savinelli, C.; Paolucci, E.; Pelagatti, L.; Sibona, E.; Fersini, N.; Buggea, M.; Tozzi, C.; Allescia, G.; Paolini, D. Point-of-care ultrasound (PoCUS) in the early diagnosis of novel coronavirus 2019 disease (COVID-19) in a first-level emergency department during a SARS-CoV-2 outbreak in Italy: A real-life analysis. Intern. Emerg. Med. 2022, 17, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.J.; Bechara, R.; Islam, S. Point-of-care ultrasound in the intensive care unit. Clin. Chest Med. 2018, 39, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Liebo, M.J.; Israel, R.L.; Lillie, E.O.; Smith, M.R.; Rubenson, D.S.; Topol, E.J. Is pocket mobile echocardiography the next-generation stethoscope? A cross-sectional comparison of rapidly acquired images with standard transthoracic echocardiography. Ann. Intern. Med. 2011, 155, 33–38. [Google Scholar] [CrossRef]
- Mancusi, C.; Carlino, M.V.; Sforza, A. Point-of-care ultrasound with pocket-size devices in emergency department. Echocardiography 2019, 36, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Schweickert, W.D.; Herlitz, J.; Pohlman, A.S.; Gehlbach, B.K.; Hall, J.B.; Kress, J.P. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit. Care Med. 2009, 37, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Paquet, F.; Boucher, L.M.; Valenti, D.; Lindsay, R. Impact of arm selection on the incidence of PICC complications: Results of a randomized controlled trial. J. Vasc. Access 2017, 18, 408–414. [Google Scholar] [CrossRef]
- Hayıroğlu, M.İ. Telemedicine: Current concepts and future perceptions. Anatol. J. Cardiol. 2019, 22 (Suppl. S2), 21–22. [Google Scholar] [CrossRef]
- Liem, T.K.; Yanit, K.E.; Moseley, S.E.; Landry, G.J.; DeLoughery, T.G.; Rumwell, C.A.; Mitchell, E.L.; Moneta, G.L. Peripherally inserted central catheter usage patterns and associated symptomatic upper extremity venous thrombosis. J. Vasc. Surg. 2012, 55, 761–767. [Google Scholar] [CrossRef]
- Chopra, V.; Anand, S.; Hickner, A.; Buist, M.; Rogers, M.A.; Saint, S.; Flanders, S.A. Risk of venous thromboembolism associated with peripherally inserted central catheters: A systematic review and meta-analysis. Lancet 2013, 382, 311–325. [Google Scholar] [CrossRef]
| Characteristics | (n = 74) |
|---|---|
| Sex (Male, %) | 40 (54.1) |
| Age (Years, Median (IQR)) | 69 (59–75) |
| Body Mass Index (kg/m2, Median (IQR)) | 24.96 (23.0–28.34) |
| Length of Hospital Stay (Days, Median (IQR)) | 26 (16–67) |
| Catheter Indwelling Time (Days, Median (IQR)) | 14 (9–22) |
| Access Side (Right, %) | 61 (82.4) |
| Underlying Diseases | |
| Hypertension | 39 |
| Diabetes | 35 |
| Chronic Lung Disease | 14 |
| Chronic Heart Disease | 12 |
| Cancer | 7 |
| PICC Catheter Type | |
| PowerPICC™ | 45 |
| Turbo-Ject® | 29 |
| Access Vein | |
| Basilic | 57 (77.0) |
| Brachial | 9 (12.1) |
| Cephalic | 8 (10.8) |
| Interval Between CDIU Admission and PICC Insertion (Hours) | |
| <24 | 31 (41.9) |
| 24–48 | 18 (24.3) |
| ≥48 | 25 (33.8) |
| Confirmation of Catheter-Tip Location | |
| Electrocardiography | 52 (70.3) |
| Chest radiography | 21 (28.4) |
| Not Confirmed Immediately After the Procedure | 1 |
| Oxygen Therapy during the Procedure | |
| Room Air | 3 |
| Nasal Cannula | 8 |
| Mask with Reservoir | 2 |
| High-Flow Nasal Cannula | 34 |
| Mechanical Ventilation | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, K.W.; Wi, W.; Choi, M.S.; Gil, E.; Park, C.-M.; Yoo, K. Feasibility of Ultrasound-Guided, Peripherally Inserted Central Catheter Placement at the Bedside in a Communicable-Disease Isolation Unit. J. Pers. Med. 2023, 13, 863. https://doi.org/10.3390/jpm13050863
Yoon KW, Wi W, Choi MS, Gil E, Park C-M, Yoo K. Feasibility of Ultrasound-Guided, Peripherally Inserted Central Catheter Placement at the Bedside in a Communicable-Disease Isolation Unit. Journal of Personalized Medicine. 2023; 13(5):863. https://doi.org/10.3390/jpm13050863
Chicago/Turabian StyleYoon, Kyoung Won, Wongook Wi, Moon Suk Choi, Eunmi Gil, Chi-Min Park, and Keesang Yoo. 2023. "Feasibility of Ultrasound-Guided, Peripherally Inserted Central Catheter Placement at the Bedside in a Communicable-Disease Isolation Unit" Journal of Personalized Medicine 13, no. 5: 863. https://doi.org/10.3390/jpm13050863
APA StyleYoon, K. W., Wi, W., Choi, M. S., Gil, E., Park, C.-M., & Yoo, K. (2023). Feasibility of Ultrasound-Guided, Peripherally Inserted Central Catheter Placement at the Bedside in a Communicable-Disease Isolation Unit. Journal of Personalized Medicine, 13(5), 863. https://doi.org/10.3390/jpm13050863





