Comparing Cytology Brushes for Optimal Human Nasal Epithelial Cell Collection: Implications for Airway Disease Diagnosis and Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cytology Brushes
2.3. Collection of Cells from Nasal Inferior Turbinates of Participants
2.4. Cell Count
2.5. Cell Culture
2.6. Ciliary Beat Frequency Measurements
2.7. Statistical Analysis
3. Results
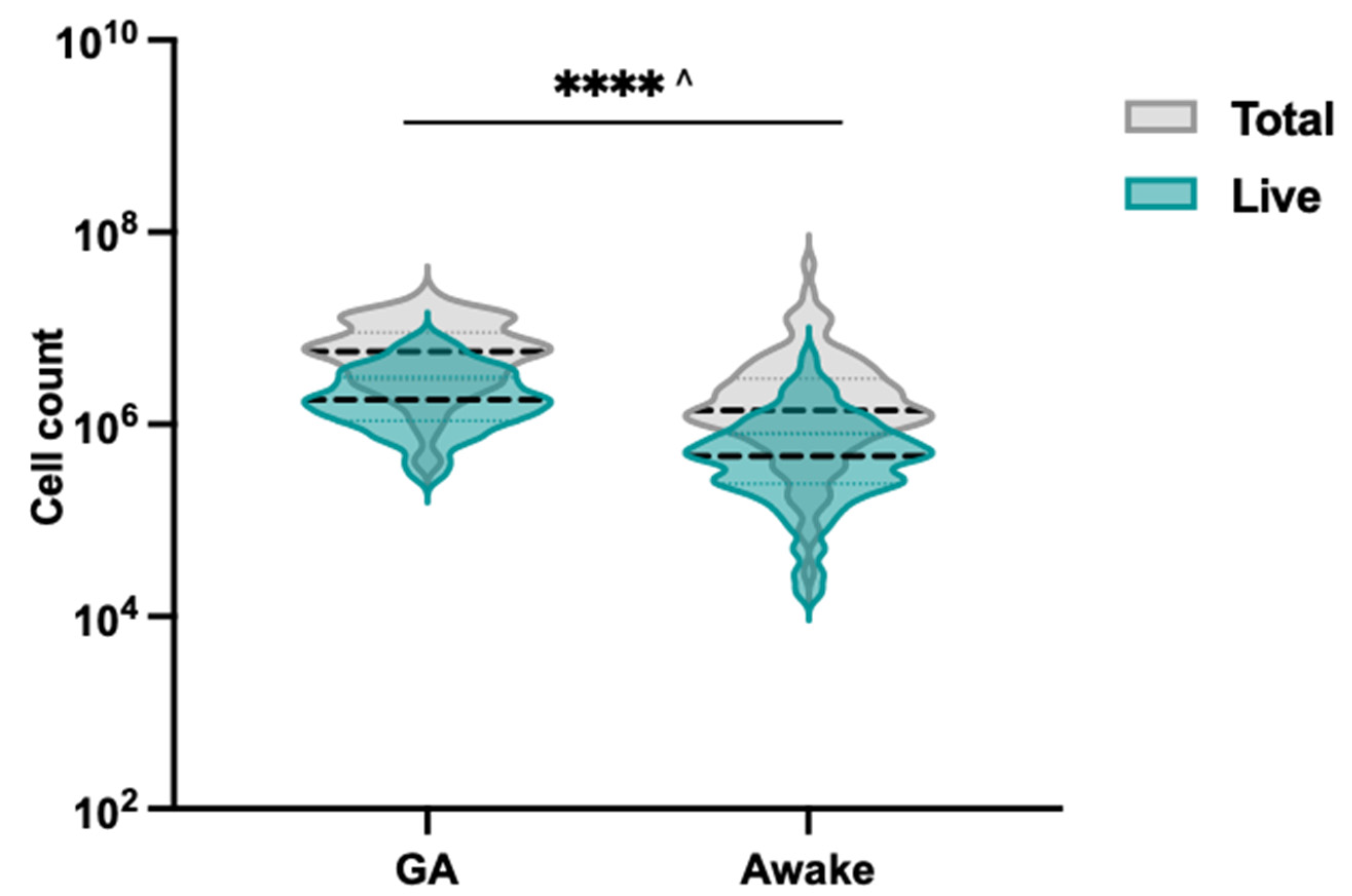
3.1. Enhanced Cell Total and Viability with Endoscan Cytology Brush Selection
3.2. Ciliary Beat Frequency Measurements Were Not Impacted by Cytology Brush Choice
3.3. Effectiveness of Endoscan Brush for Nasal Brushing in Awake Cystic Fibrosis Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, D.; Turner, S.W.; Spiteri-Cornish, D.; McInnes, N.; Scaife, A.; Danielian, P.J.; Devereux, G.; Walsh, G.M. Culture of Airway Epithelial Cells from Neonates Sampled within 48-Hours of Birth. PLoS ONE 2013, 8, e78321. [Google Scholar] [CrossRef]
- Vanders, R.L.; Hsu, A.; Gibson, P.G.; Murphy, V.E.; Wark, P.A.B. Nasal Epithelial Cells to Assess in Vitro Immune Responses to Respiratory Virus Infection in Pregnant Women with Asthma. Respir. Res. 2019, 20, 259. [Google Scholar] [CrossRef] [PubMed]
- Brewington, J.J.; Filbrandt, E.T.; LaRosa, F.J.; Moncivaiz, J.D.; Ostmann, A.J.; Strecker, L.M.; Clancy, J.P. Brushed Nasal Epithelial Cells Are a Surrogate for Bronchial Epithelial CFTR Studies. JCI Insight 2018, 3, e99385. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Daniels, L.A.; Davis, S.D.; Zariwala, M.A.; Leigh, M.W. Primary Ciliary Dyskinesia: Recent Advances in Diagnostics, Genetics, and Characterization of Clinical Disease. Am. J. Respir. Crit. Care Med. 2013, 188, 913–922. [Google Scholar] [CrossRef]
- Braun, J.J.; Boehm, N.; Metz-Favre, C.; Koscinski, I.; Teletin, M.; Debry, C. Diagnosis of Primary Ciliary Dyskinesia: When and How? Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 377–382. [Google Scholar] [CrossRef]
- Lucas, J.S.; Paff, T.; Goggin, P.; Haarman, E. Diagnostic Methods in Primary Ciliary Dyskinesia. Paediatr. Respir. Rev. 2016, 18, 8–17. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Davis, S.D.; Polineni, D.; Manion, M.; Rosenfeld, M.; Dell, S.D.; Chilvers, M.A.; Ferkol, T.W.; Zariwala, M.A.; Sagel, S.D.; et al. Diagnosis of Primary Ciliary Dyskinesia: An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 197, e24–e39. [Google Scholar] [CrossRef] [PubMed]
- Keegan, D.E.; Brewington, J.J. Nasal Epithelial Cell-Based Models for Individualized Study in Cystic Fibrosis. Int. J. Mol. Sci. 2021, 22, 4448. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Kim, S.; Kim, C.H.; Yoon, J.H.; Kim, H.J. Alternative Method for Primary Nasal Epithelial Cell Culture Using Intranasal Brushing and Feasibility for the Study of Epithelial Functions in Allergic Rhinitis. Allergy Asthma Immunol. Res. 2016, 8, 69. [Google Scholar] [CrossRef]
- Hirst, R.A.; Jackson, C.L.; Coles, J.L.; Williams, G.; Rutman, A.; Goggin, P.M.; Adam, E.C.; Page, A.; Evans, H.J.; Lackie, P.M.; et al. Culture of Primary Ciliary Dyskinesia Epithelial Cells at Air-Liquid Interface Can Alter Ciliary Phenotype but Remains a Robust and Informative Diagnostic Aid. PLoS ONE 2014, 9, e89675. [Google Scholar] [CrossRef]
- Awatade, N.T.; Wong, S.L.; Hewson, C.K.; Fawcett, L.K.; Kicic, A.; Jaffe, A.; Waters, S.A. Human Primary Epithelial Cell Models: Promising Tools in the Era of Cystic Fibrosis Personalized Medicine. Front. Pharmacol. 2018, 9, 1429. [Google Scholar] [CrossRef] [PubMed]
- McDougall, C.M.; Blaylock, M.G.; Douglas, J.G.; Brooker, R.J.; Helms, P.J.; Walsh, G.M. Nasal Epithelial Cells as Surrogates for Bronchial Epithelial Cells in Airway Inflammation Studies. Am. J. Respir. Cell Mol. Biol. 2012, 39, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Awatade, N.T.; Astore, M.A.; Allan, K.M.; Carnell, M.J.; Slapetova, I.; Chen, P.C.; Setiadi, J.; Pandzic, E.; Fawcett, L.K.; et al. Molecular Dynamics and Theratyping in Airway and Gut Organoids Reveal R352Q-CFTR Conductance Defect. Am. J. Respir. Cell Mol. Biol. 2022, 67, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Hewson, C.K.; Capraro, A.; Wong, S.L.; Pandzic, E.; Zhong, L.; Fernando, B.S.M.; Awatade, N.T.; Hart-Smith, G.; Whan, R.M.; Thomas, S.R.; et al. Novel Antioxidant Therapy with the Immediate Precursor to Glutathione, γ-Glutamylcysteine (GGC), Ameliorates LPS-Induced Cellular Stress in In Vitro 3D-Differentiated Airway Model from Primary Cystic Fibrosis Human Bronchial Cells. Antioxidants 2020, 9, 1204. [Google Scholar] [CrossRef]
- Komalla, V.; Allam, V.S.R.R.; Kwok, P.C.L.; Sheikholeslami, B.; Owen, L.; Jaffe, A.; Waters, S.A.; Mohammad, S.; Oliver, B.G.; Chen, H.; et al. A Phospholipid-Based Formulation for the Treatment of Airway Inflammation in Chronic Respiratory Diseases. Eur. J. Pharm. Biopharm. 2020, 157, 47–58. [Google Scholar] [CrossRef]
- Allan, K.M.; Astore, M.A.; Fawcett, L.K.; Wong, S.L.; Chen, P.C.; Griffith, R.; Jaffe, A.; Kuyucak, S.; Waters, S.A. S945L-CFTR Molecular Dynamics, Functional Characterization and Tezacaftor/Ivacaftor Efficacy in Vivo and in Vitro in Matched Pediatric Patient-Derived Cell Models. Front. Pediatr. 2022, 10, 2058. [Google Scholar] [CrossRef]
- Daley, P.; Castriciano, S.; Chernesky, M.; Smieja, M. Comparison of Flocked and Rayon Swabs for Collection of Respiratory Epithelial Cells from Uninfected Volunteers and Symptomatic Patients. J. Clin. Microbiol. 2006, 44, 2265. [Google Scholar] [CrossRef]
- de Courcey, F.; Zholos, A.V.; Atherton-Watson, H.; Williams, M.T.S.; Canning, P.; Danahay, H.L.; Elborn, J.S.; Ennis, M. Development of Primary Human Nasal Epithelial Cell Cultures for the Study of Cystic Fibrosis Pathophysiology. Am. J. Physiol. Cell Physiol. 2012, 303, 1173–1179. [Google Scholar] [CrossRef]
- Stokes, A.B.; Kieninger, E.; Schögler, A.; Kopf, B.S.; Casaulta, C.; Geiser, T.; Regamey, N.; Alves, M.P. Comparison of Three Different Brushing Techniques to Isolate and Culture Primary Nasal Epithelial Cells from Human Subjects. Exp. Lung Res. 2014, 40, 327–332. [Google Scholar] [CrossRef]
- Allan, K.M.; Wong, S.L.; Fawcett, L.K.; Capraro, A.; Jaffe, A.; Herbert, C.; Pandzic, E.; Waters, S.A. Collection, Expansion, and Differentiation of Primary Human Nasal Epithelial Cell Models for Quantification of Cilia Beat Frequency. JoVE 2021, 177, e63090. [Google Scholar] [CrossRef]
- Mou, H.; Vinarsky, V.; Tata, P.R.; Brazauskas, K.; Choi, S.H.; Crooke, A.K.; Zhang, B.; Solomon, G.M.; Turner, B.; Bihler, H.; et al. Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 2016, 19, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Awatade, N.T.; Wong, S.L.; Capraro, A.; Pandzic, E.; Slapetova, I.; Zhong, L.; Turgutoglu, N.; Fawcett, L.K.; Whan, R.M.; Jaffe, A.; et al. Significant Functional Differences in Differentiated Conditionally Reprogrammed (CRC)- and Feeder-Free Dual SMAD Inhibited-Expanded Human Nasal Epithelial Cells. J. Cyst. Fibros. 2021, 20, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Nikolaizik, W.; Hahn, J.; Bauck, M.; Weber, S. Comparison of Ciliary Beat Frequencies at Different Temperatures in Young Adults. ERJ Open Res. 2020, 6, 00477-2020. [Google Scholar] [CrossRef]
- Hussain, R.; Hugosson, S.; Roomans, G.M. Isolation and Culture of Primary Human Nasal Epithelial Cells from Anesthetized Nasal Epithelia. Acta Oto-Laryngol. 2014, 134, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Kempeneers, C.; Seaton, C.; Garcia Espinosa, B.; Chilvers, M.A. Ciliary Functional Analysis: Beating a Path towards Standardization. Pediatr. Pulmonol. 2019, 54, 1627–1638. [Google Scholar] [CrossRef]
- Meskini, M.; Siadat, S.D.; Seifi, S.; Movafagh, A.; Sheikhpour, M. An Overview on the Upper and Lower Airway Microbiome in Cystic Fibrosis Patients. Tanaffos 2021, 20, 86. [Google Scholar]
- Raidt, J.; Wallmeier, J.; Hjeij, R.; Onnebrink, J.G.; Pennekamp, P.; Loges, N.T.; Olbrich, H.; Häffner, K.; Dougherty, G.W.; Omran, H.; et al. Ciliary Beat Pattern and Frequency in Genetic Variants of Primary Ciliary Dyskinesia. Eur. Respir. J. 2014, 44, 1579–1588. [Google Scholar] [CrossRef]

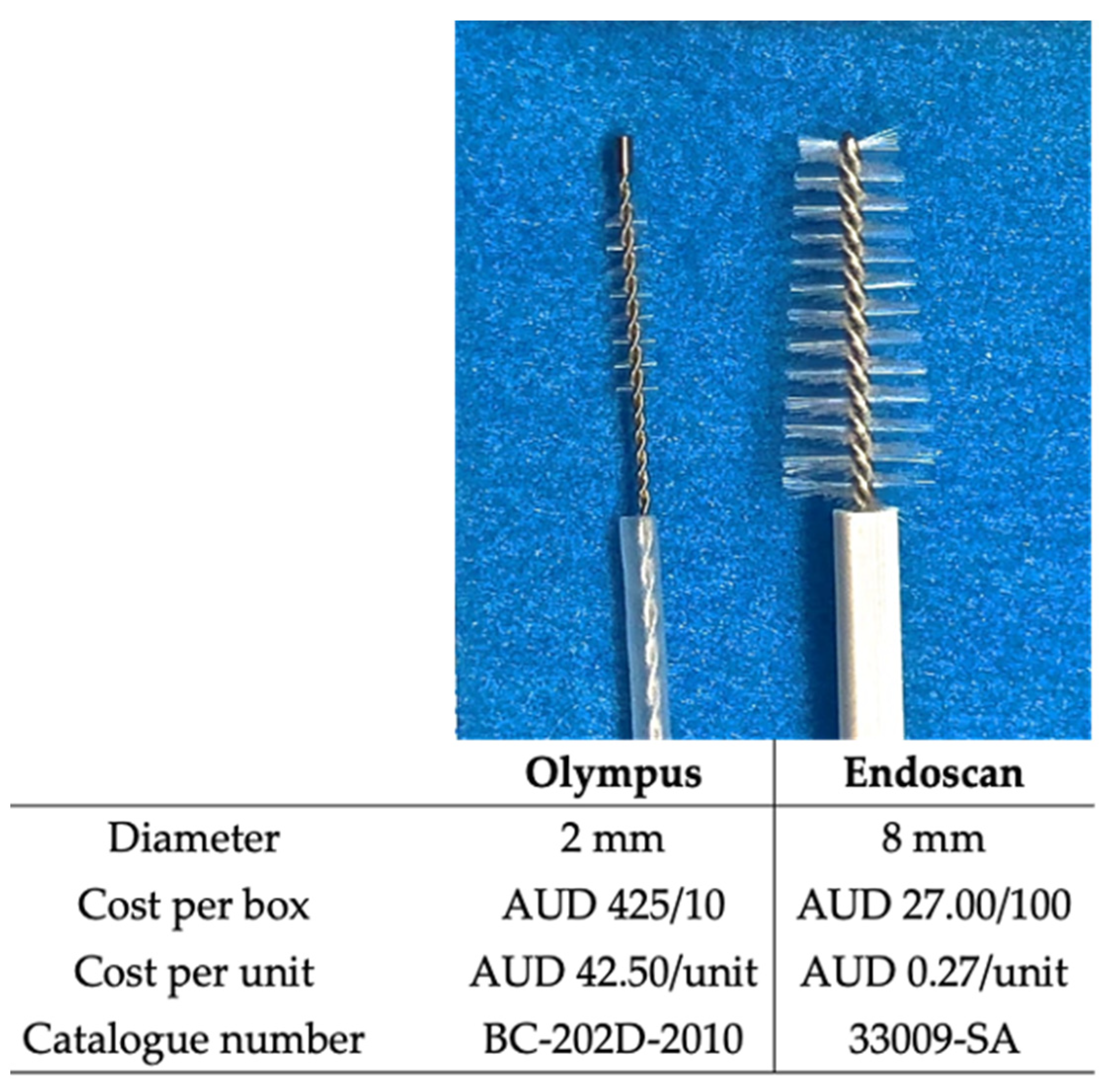


| Participants | |
|---|---|
| n | 13 |
| Male (%) | 85 |
| Median Age (years) | 3.8 |
| Age Range (years) | 1.0–14.2 |
| Sedation state | GA |
| GA | Awake | |
|---|---|---|
| n | 52 | 93 |
| Male (%) | 51 | 46 |
| Median Age (years) | 4.0 | 15.5 |
| Age Range (years) | 0.6–17.8 | 5.7–58.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawcett, L.K.; Turgutoglu, N.; Allan, K.M.; Belessis, Y.; Widger, J.; Jaffe, A.; Waters, S.A. Comparing Cytology Brushes for Optimal Human Nasal Epithelial Cell Collection: Implications for Airway Disease Diagnosis and Research. J. Pers. Med. 2023, 13, 864. https://doi.org/10.3390/jpm13050864
Fawcett LK, Turgutoglu N, Allan KM, Belessis Y, Widger J, Jaffe A, Waters SA. Comparing Cytology Brushes for Optimal Human Nasal Epithelial Cell Collection: Implications for Airway Disease Diagnosis and Research. Journal of Personalized Medicine. 2023; 13(5):864. https://doi.org/10.3390/jpm13050864
Chicago/Turabian StyleFawcett, Laura K., Nihan Turgutoglu, Katelin M. Allan, Yvonne Belessis, John Widger, Adam Jaffe, and Shafagh A. Waters. 2023. "Comparing Cytology Brushes for Optimal Human Nasal Epithelial Cell Collection: Implications for Airway Disease Diagnosis and Research" Journal of Personalized Medicine 13, no. 5: 864. https://doi.org/10.3390/jpm13050864
APA StyleFawcett, L. K., Turgutoglu, N., Allan, K. M., Belessis, Y., Widger, J., Jaffe, A., & Waters, S. A. (2023). Comparing Cytology Brushes for Optimal Human Nasal Epithelial Cell Collection: Implications for Airway Disease Diagnosis and Research. Journal of Personalized Medicine, 13(5), 864. https://doi.org/10.3390/jpm13050864







