T Lymphocyte Subset Counts and Interferon-Gamma Production in Adults and Children with COVID-19: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
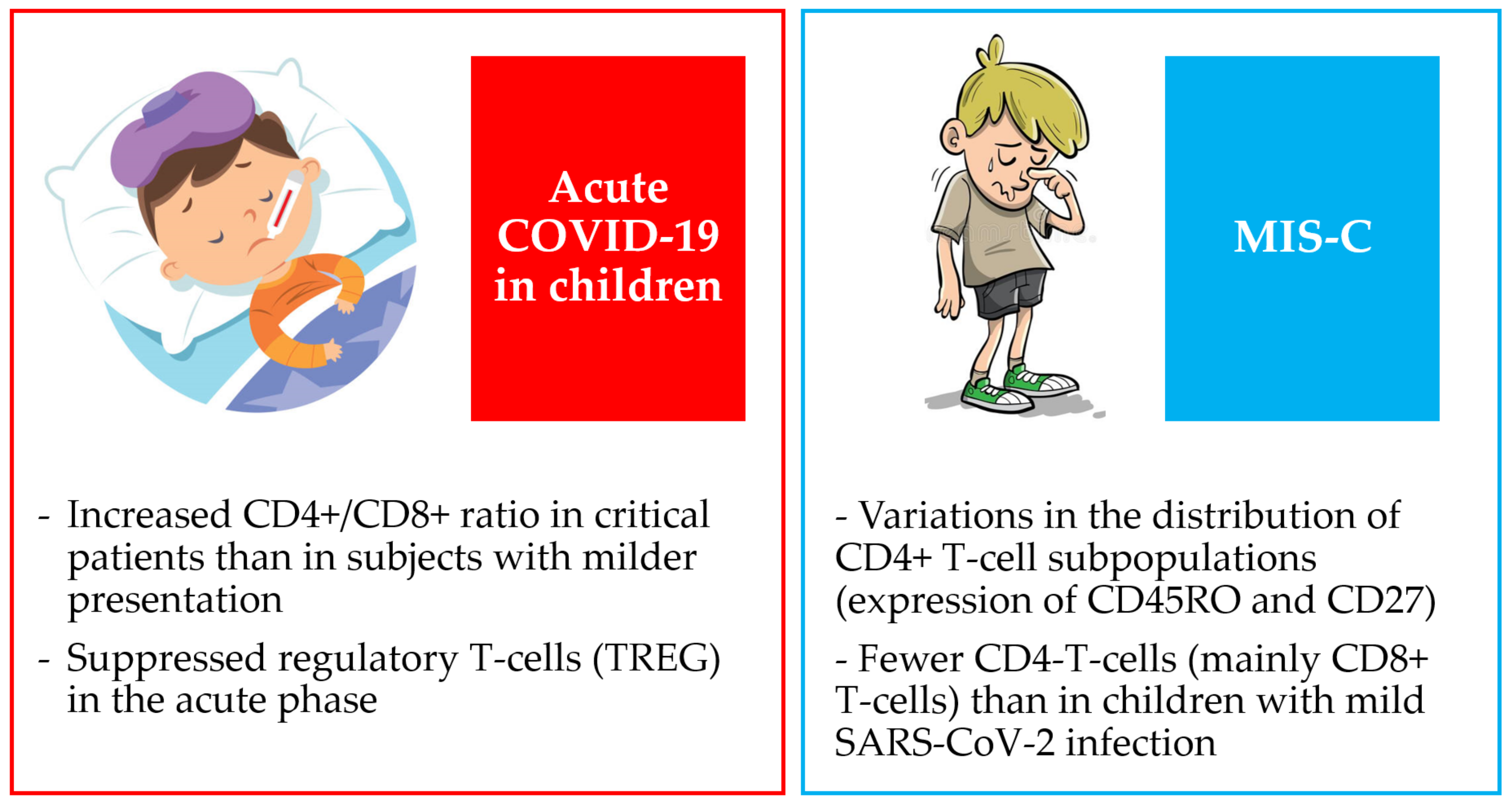
3. Differences between Children and Adults with COVID-19
4. T Lymphocyte Subset Counts: Current Knowledge and Clinical Implications
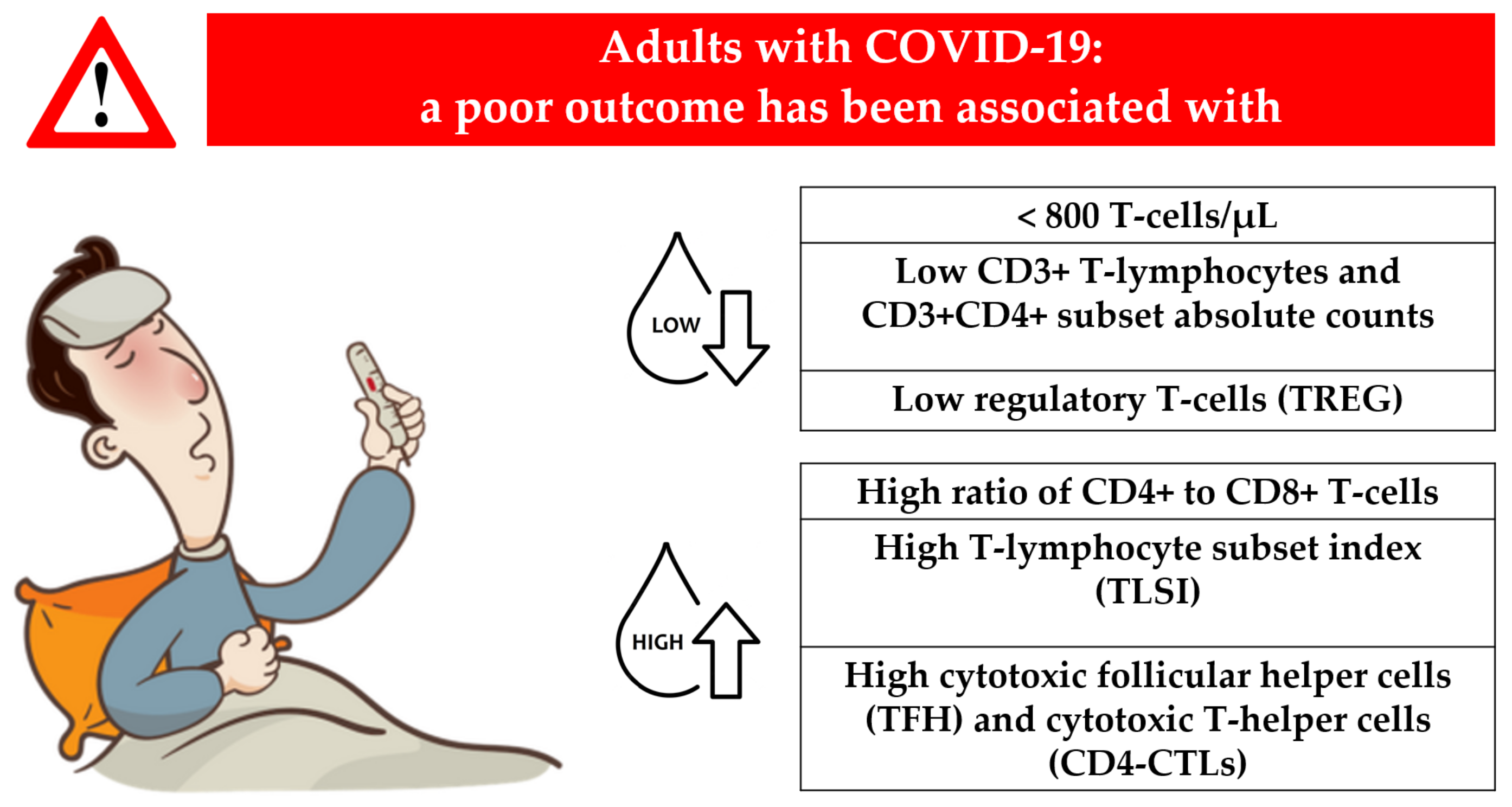
4.1. T-Lymphocyte Subset Counts in Adults
4.2. T Lymphocyte Subset Counts in Children
5. IFN-γ Production: Current Knowledge and Clinical Implications
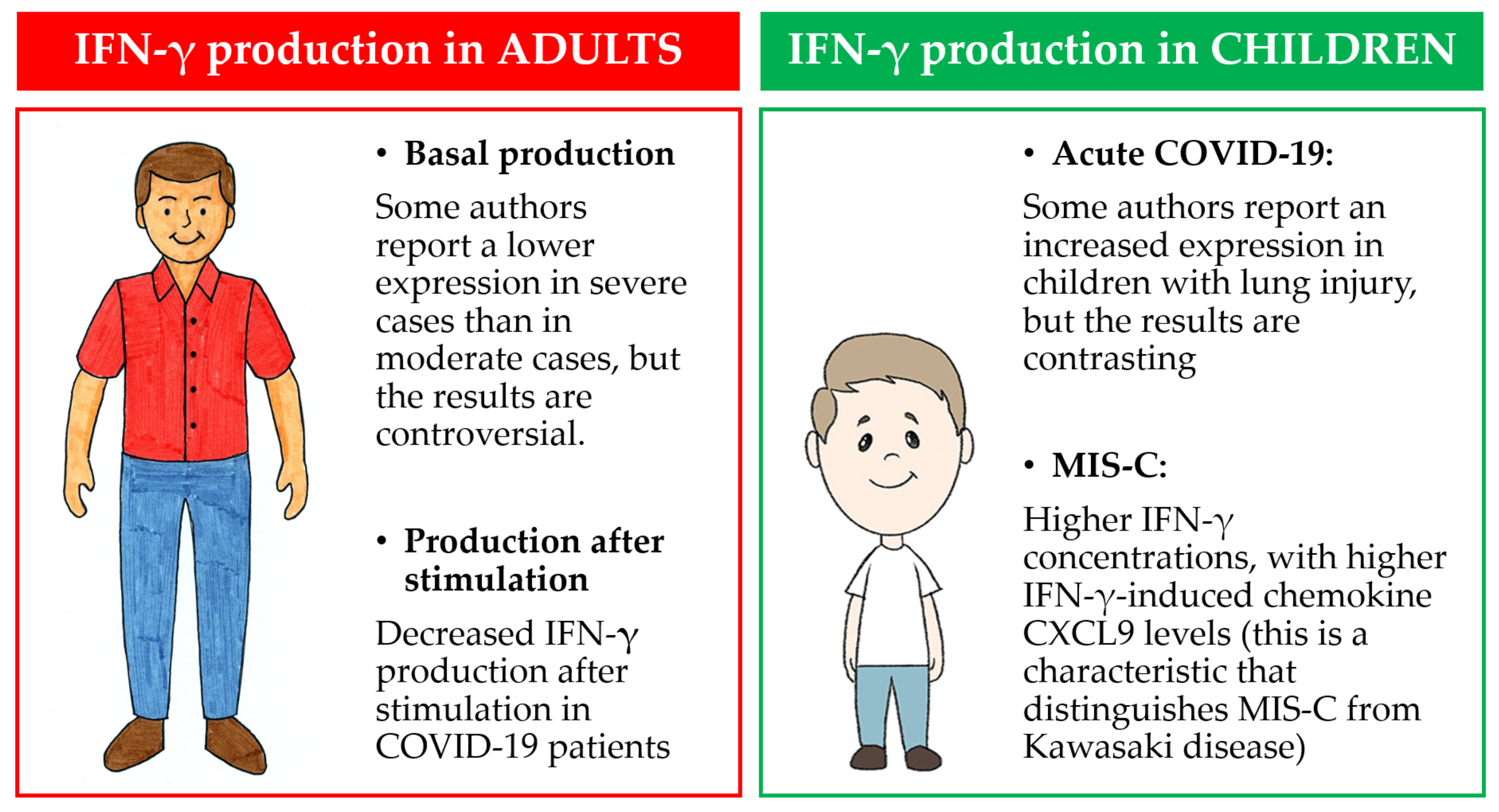
5.1. Basal IFN-γ Production in Adults
5.2. IFN-γ Production after Stimulation in Adults
5.3. IFN-γ Production in Children
6. Limitations
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Cao, X.; Tao, G.; Xie, W.; Hu, Z.; Xu, D. The lymph index: A potential hematological parameter for viral infection. Int. J. Infect. Dis. 2013, 17, 490–493. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, S.K.; Maurya, V.K.; Tripathi, A.K. Progress and Challenges Toward Generation and Maintenance of Long-Lived Memory T Lymphocyte Responses During COVID-19. Front. Immunol. 2022, 12, 2–9. [Google Scholar] [CrossRef]
- Shen, H.H.; Hou, J.; Chen, W.W.; Bai, B.K.; Wang, H.B.; Guo, T.S.; Liu, A.X.; Li, Y.L.; Zhao, M.; Mao, P.Y.; et al. Immunologic Changes during Pandemic (H1N1) 2009, China. Emerg. Infect. Dis. 2011, 17, 1053–1055. [Google Scholar] [CrossRef]
- Sharon, N.; Talnir, R.; Lavid, O.; Rubinstein, U.; Niven, M.; First, Y.; Tsivion, A.J.; Schachter, Y. Transient lymphopenia and neutropenia: Pediatric influenza A/H1N1 infection in a primary hospital in Israel. Isr. Med. Assoc. J. 2011, 13, 408–412. [Google Scholar]
- Petrova, V.N.; Sawatsky, B.; Han, A.X.; Laksono, B.M.; Walz, L.; Parker, E.; Pieper, K.; Anderson, C.A.; de Vries, R.D.; Lanzavecchia, A.; et al. Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci. Immunol. 2019, 4, eaay6125. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Thomas, K.; Sympardi, S.; Liatsos, G.D.; Pirounaki, M.; Sambatakou, H.; Marantos, T.; Karofylakis, E.; Dourakis, S.P.; Tsiodras, S.; et al. Clinical characteristics and outcomes of measles outbreak in adults: A multicenter retrospective observational study of 93 hospitalized adults in Greece. J. Clin. Virol. 2020, 131, 104608. [Google Scholar] [CrossRef]
- O’Donnell, D.R.; Carrington, D. Peripheral blood lymphopenia and neutrophilia in children with severe respiratory syncytial virus disease. Pediatr. Pulmonol. 2002, 34, 128–130. [Google Scholar] [CrossRef]
- Huang, W.; Berube, J.; McNamara, M.; Saksena, S.; Hartman, M.; Arshad, T.; Bornheimer, S.J.; O’Gorman, M. Lymphocyte Subset Counts in COVID-19 Patients: A Meta-Analysis. Cytom. A 2020, 97, 772–776. [Google Scholar] [CrossRef]
- Iannetta, M.; Buccisano, F.; Fraboni, D.; Malagnino, V.; Campogiani, L.; Teti, E.; Spalliera, I.; Rossi, B.; Di Lorenzo, A.; Palmieri, R.; et al. Baseline T-lymphocyte subset absolute counts can predict both outcome and severity in SARS-CoV-2 infected patients: A single center study. Sci. Rep. 2021, 11, 12762. [Google Scholar] [CrossRef]
- Ramasamy, S.; Subbian, S. Critical determinants of cytokine storm and type i interferon response in COVID-19 pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20. [Google Scholar] [CrossRef]
- Ward, J.D.; Cornaby, C.; Schmitz, J.L. Indeterminate QuantiFERON gold plus results reveal deficient interferon gamma responses in severely III COVID-19 Patients. J. Clin. Microbiol. 2021, 59, e00811-21. [Google Scholar] [CrossRef]
- Imeneo, A.; Alessio, G.; Di Lorenzo, A.; Campogiani, L.; Lodi, A.; Barreca, F.; Zordan, M.; Barchi, V.; Massa, B.; Tedde, S.; et al. In Patients with Severe COVID-19, the Profound Decrease in the Peripheral Blood T-Cell Subsets Is Correlated with an Increase of QuantiFERON-TB Gold Plus Indeterminate Rates and Reflecting a Reduced Interferon-Gamma Production. Life 2022, 12, 244. [Google Scholar] [CrossRef]
- Bellou, V.; Tzoulaki, I.; van Smeden, M.; Moons, K.G.M.; Evangelou, E.; Belbasis, L. Prognostic factors for adverse outcomes in patients with COVID-19: A field-wide systematic review and meta-analysis. Eur. Resp. J. 2022, 59, 2002964. [Google Scholar] [CrossRef]
- Auriti, C.; De Rose, D.U.; Mondì, V.; Stolfi, I.; Tzialla, C. Neonatal SARS-CoV-2 Infection: Practical Tips. Pathogens 2021, 10, 611. [Google Scholar] [CrossRef]
- Ali, A.S.; Al-Hakami, A.M.; Shati, A.A.; Asseri, A.A.; Al-Qahatani, S.M. Salient Conclusive Remarks on Epidemiology and Clinical Manifestations of Pediatric COVID-19: Narrative Review. Front. Pediatr. 2020, 8, 584694. [Google Scholar] [CrossRef]
- Badal, S.; Bajgain, K.T.; Badal, S.; Thapa, R.; Bajgain, B.B.; Santana, M.J. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: A systematic review and meta-analysis. J. Clin. Virol. 2021, 135, 104715. [Google Scholar] [CrossRef]
- Dhochak, N.; Singhal, T.; Kabra, S.K.; Lodha, R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J. Pediatr. 2020, 87, 537–546. [Google Scholar] [CrossRef]
- Howard-Jones, A.R.; Burgner, D.P.; Crawford, N.W.; Goeman, E.; Gray, P.E.; Hsu, P.; Kuek, S.; McMullan, B.J.; Tosif, S.; Wurzel, D.; et al. COVID-19 in children. II: Pathogenesis, disease spectrum and management. J. Paediatr. Child Health 2022, 58, 46–53. [Google Scholar] [CrossRef]
- De Rose, D.U.; Piersigilli, F.; Ronchetti, M.P.; Santisi, A.; Bersani, I.; Dotta, A.; Danhaive, O.; Auriti, C. Novel Coronavirus disease (COVID-19) in newborns and infants: What we know so far. Ital. J. Pediatr. 2020, 46, 56. [Google Scholar] [CrossRef]
- Devin, J.; Marano, R.; Mikhael, M.; Feaster, W.; Sanger, T.; Ehwerhemuepha, L. Epidemiology of Neonatal COVID-19 in the United States. Pediatrics 2022, 150, e2022056297. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2020, 106, 429–439. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427–2429. [Google Scholar] [CrossRef]
- Heinonen, S.; Helve, O.; Andersson, S.; Janér, C.; Süvari, L.; Kaskinen, A. Nasal expression of SARS-CoV-2 entry receptors in newborns. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 95–97. [Google Scholar] [CrossRef]
- Gorse, G.J.; Donovan, M.M.; Patel, G.B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J. Med. Virol. 2020, 92, 512–517. [Google Scholar] [CrossRef]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Herrick, K.A.; Storandt, R.J.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef]
- Pierce, C.A.; Preston-Hurlburt, P.; Dai, Y.; Aschner, C.B.; Cheshenko, N.; Galen, B.; Garforth, S.J.; Herrera, N.G.; Jangra, R.K.; Morano, N.C.; et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 2020, 12, eabd5487. [Google Scholar] [CrossRef]
- Netea, M.G.; Giamarellos-Bourboulis, E.J.; Domínguez-Andrés, J.; Curtis, N.; van Crevel, R.; van de Veerdonk, F.L.; Bonten, M. Trained Immunity: A Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef]
- De Maio, F.; Posteraro, B.; Ponziani, F.R.; Cattani, P.; Gasbarrini, A.; Sanguinetti, M. Nasopharyngeal Microbiota Profiling of SARS-CoV-2 Infected Patients. Biol. Proced. Online 2020, 22, 18. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Sehirli, A.O.; Sayiner, S.; Serakinci, N. Role of melatonin in the treatment of COVID-19; as an adjuvant through cluster differentiation 147 (CD147). Mol. Biol. Rep. 2020, 47, 8229–8233. [Google Scholar] [CrossRef]
- Kapustova, L.; Petrovicova, O.; Banovcin, P.; Antosova, M.; Bobcakova, A.; Urbancikova, I.; Rennerova, Z.; Jesenak, M. COVID-19 and the differences in physiological background between children and adults and their clinical consequences. Physiol. Res. 2021, 70, S209–S225. [Google Scholar] [CrossRef]
- Chung, E.; Chow, E.J.; Wilcox, N.C.; Burstein, R.; Brandstetter, E.; Han, P.D.; Fay, K.; Pfau, B.; Adler, A.; Lacombe, K.; et al. Comparison of Symptoms and RNA Levels in Children and Adults With SARS-CoV-2 Infection in the Community Setting. JAMA Pediatr. 2021, 175, e212025. [Google Scholar] [CrossRef]
- Buonsenso, D.; Munblit, D.; Pazukhina, E.; Ricchiuto, A.; Sinatti, D.; Zona, M.; De Matteis, A.; D’Ilario, F.; Gentili, C.; Lanni, R.; et al. Post-COVID Condition in Adults and Children Living in the Same Household in Italy: A Prospective Cohort Study Using the ISARIC Global Follow-Up Protocol. Front. Pediatr. 2022, 10, 834875. [Google Scholar] [CrossRef]
- Huang, I.; Pranata, R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J. Intensive Care 2020, 8, 36. [Google Scholar] [CrossRef]
- Delshad, M.; Tavakolinia, N.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Bagheri, N.; Bashash, D. The contributory role of lymphocyte subsets, pathophysiology of lymphopenia and its implication as prognostic and therapeutic opportunity in COVID-19. Int. Immunopharmacol. 2021, 95, 107586. [Google Scholar] [CrossRef]
- Henry, B.; Cheruiyot, I.; Vikse, J.; Mutua, V.; Kipkorir, V.; Benoit, J.; Plebani, M.; Bragazzi, N.; Lippi, G. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: A meta-analysis. Acta Biomed. 2020, 91, e2020008. [Google Scholar]
- Liu, Z.; Long, W.; Tu, M.; Chen, S.; Huang, Y.; Wang, S.; Zhou, W.; Chen, D.; Zhou, L.; Wang, M.; et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Jiang, N.; Li, Z.; Yang, B.; Jin, M.; Sun, Y.; He, Y.; Liu, Y.; Wang, Y.; Si, D.; Ma, P.; et al. Peripheral Inflammatory Cytokines and Lymphocyte Subset Features of Deceased COVID-19 Patients. Biomed. Res. Int. 2021, 2021, 9101082. [Google Scholar] [CrossRef]
- Lagadinou, M.; Zareifopoulos, N.; Gkentzi, D.; Sampsonas, F.; Kostopoulou, E.; Marangos, M.; Solomou, E. Alterations in lymphocyte subsets and monocytes in patients diagnosed with SARS-CoV-2 pneumonia: A mini review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5057–5062. [Google Scholar]
- Chen, J.; Qi, T.; Liu, L.; Ling, Y.; Qian, Z.; Li, T.; Li, F.; Xu, Q.; Zhang, Y.; Xu, S.; et al. Clinical progression of patients with COVID-19 in Shangai, China. J. Infect. 2020, 80, e1–e6. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Du, R.H.; Liang, L.R.; Yang, C.Q.; Wang, W.; Cao, T.Z.; Li, M.; Guo, G.Y.; Du, J.; Zheng, C.L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2. Eur. Resp. J. 2020, 56, 2002961. [Google Scholar] [CrossRef]
- McBride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef]
- Qin, R.; He, L.; Yang, Z.; Jia, N.; Chen, R.; Xie, J.; Fu, W.; Chen, H.; Lin, X.; Huang, R.; et al. Identification of Parameters Representative of Immune Dysfunction in Patients with Severe and Fatal COVID-19 Infection: A Systematic Review and Meta-analysis. Clin. Rev. Allergy Immunol. 2023, 64, 33–65. [Google Scholar] [CrossRef]
- Meckiff, B.J.; Ramírez-Suástegui, C.; Fajardo, V.; Chee, S.J.; Kusnadi, A.; Simon, H.; Eschweiler, S.; Grifoni, A.; Pelosi, E.; Weiskopf, D.; et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4+ T Cells in COVID-19. Cell 2020, 183, 1340–1353.e16. [Google Scholar] [CrossRef]
- Sadeghi, A.; Tahmasebi, S.; Mahmood, A.; Kuznetsova, M.; Valizadeh, H.; Taghizadieh, A.; Nazemiyeh, M.; Aghebati-Maleki, L.; Jadidi-Niaragh, F.; Abbaspour-Aghdam, S.; et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J. Cell. Physiol. 2021, 236, 2829–2839. [Google Scholar] [CrossRef]
- COVID-19 Multi-omics Blood ATlas (COMBAT) Consortium. A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell 2022, 185, 916–938.e58. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef]
- Yelin, D.; Moschopoulos, C.D.; Margalit, I.; Gkrania-Klotsas, E.; Landi, F.; Stahl, J.P.; Yahav, D. ESCMID rapid guidelines for assessment and management of Long COVID. Clin. Microbiol. Infect. 2022, 28, 955–972. [Google Scholar] [CrossRef]
- Odak, I.; Barros-Martins, J.; Bošnjak, B.; Stahl, K.; David, S.; Wiesner, O.; Busch, M.; Hoeper, M.M.; Pink, I.; Welte, T.; et al. Reappearance of effector T cells is associated with recovery from COVID-19. EBioMedicine 2020, 57, 102885. [Google Scholar] [CrossRef]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Zhu, F.; Ang, J.Y. COVID-19 Infection in Children: Diagnosis and Management. Curr. Infect. Dis. Rep. 2022, 24, 51–62. [Google Scholar] [CrossRef]
- Ji, S.Q.; Zhang, M.; Zhang, Y.; Xia, K.; Chen, Y.; Chu, Q.; Wei, Y.C.; Zhou, F.L.; Bu, B.T.; Tu, H.L.; et al. Characteristics of immune and inflammatory responses among different age groups of pediatric patients with COVID-19 in China. World J. Ped. 2021, 17, 375–384. [Google Scholar] [CrossRef]
- Lu, W.; Yang, L.; Li, X.; Sun, M.; Zhang, A.; Qi, S.; Chen, Z.; Zhang, L.; Li, J.; Xiong, H. Early immune responses and prognostic factors in children with COVID-19: A single-center retrospective analysis. BMC Pediatr. 2021, 21, 181. [Google Scholar] [CrossRef]
- Li, H.; Chen, K.; Liu, M.; Xu, H.; Xu, Q. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J. Infect. 2020, 81, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021, 38, 51–57. [Google Scholar] [PubMed]
- Lazova, S.; Dimitrova, Y.; Hristova, D.; Tzotcheva, I.; Velikova, T. Cellular, Antibody and Cytokine Pathways in Children with Acute SARS-CoV-2 Infection and MIS-C-Can We Match the Puzzle? Antibodies 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Panaro, S.; Cattalini, M. The Spectrum of Manifestations of Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV2) Infection in Children: What We Can Learn From Multisystem Inflammatory Syndrome in Children (MIS-C). Front. Med. 2021, 8, 747190. [Google Scholar] [CrossRef]
- Jia, R.; Wang, X.; Liu, P.; Liang, X.; Ge, Y.; Tian, H.; Chang, H.; Zhou, H.; Zeng, M.; Xu, J. Mild Cytokine Elevation, Moderate CD4+ T Cell Response and Abundant Antibody Production in Children with COVID-19. Virol. Sin. 2020, 35, 734–743. [Google Scholar] [CrossRef]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020, 183, 968–981.e7. [Google Scholar] [CrossRef]
- Moreews, M.; Le Gouge, K.; Khaldi-Plassart, S.; Pescarmona, R.; Mathieu, A.L.; Malcus, C.; Djebali, S.; Bellomo, A.; Dauwalder, O.; Perret, M.; et al. Polyclonal expansion of TCR Vbeta 21.3+ CD4+ and CD8+ T cells is a hallmark of Multisystem Inflammatory Syndrome in Children. Sci. Immunol. 2021, 6, eabh1516. [Google Scholar] [CrossRef]
- Caldarale, F.; Giacomelli, M.; Garrafa, E.; Tamassia, N.; Morreale, A.; Poli, P.; Timpano, S.; Baresi, G.; Zunica, F.; Cattalini, M.; et al. Plasmacytoid Dendritic Cells Depletion and Elevation of IFN-γ Dependent Chemokines CXCL9 and CXCL10 in Children With Multisystem Inflammatory Syndrome. Front. Immunol. 2021, 12, 654587. [Google Scholar] [CrossRef]
- Tian, X.; Bai, Z.; Cao, Y.; Liu, H.; Liu, D.; Liu, W.; Li, J. Evaluation of Clinical and Immune Responses in Recovered Children with Mild COVID-19. Viruses 2022, 14, 85. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Murphy, K.M.; Ouyang, W.; Farrar, J.D.; Yang, J.; Ranganath, S.; Asnagli, H.; Afkarian, M.; Murphy, T.L. Signaling and transcription in T helper development. Annu. Rev. Immunol. 2000, 18, 451–494. [Google Scholar] [CrossRef] [PubMed]
- Gadotti, A.C.; de Castro Deus, M.; Telles, J.P.; Wind, R.; Goes, M.; Ossoski, R.G.C.; de Padua, A.M.; de Noronha, L.; Moreno-Amaral, A.; Baena, C.P.; et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020, 289, 198171. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Totura, A.L.; Baric, R.S. SARS coronavirus pathogenesis: Host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2012, 2, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Morales-Nebreda, L.; Markov, N.S.; Swaminathan, S.; Querrey, M.; Guzman, E.R.; Abbott, D.A.; Donnelly, H.K.; Donayre, A.; Goldberg, I.A.; et al. NU SCRIPT Study Investigators. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 2021, 590, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Lin, Z.; Niu, J.; Xu, Y.; Qin, L.; Ding, J.; Zhou, L. Clinical efficacy and adverse events of baricitinib treatment for coronavirus disease-2019 (COVID-19): A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 1523–1534. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet 2022, 400, 359–368. [Google Scholar] [CrossRef]
- Ni, L.; Cheng, M.L.; Feng, Y.; Zhao, H.; Liu, J.; Ye, F.; Ye, Q.; Zhu, G.; Li, X.; Wang, P.; et al. Impaired Cellular Immunity to SARS-CoV-2 in Severe COVID-19 Patients. Front. Immunol. 2021, 12, 603563. [Google Scholar] [CrossRef]
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Fard, S.N.; Alonzi, T.; Castilletti, C.; Palmieri, F.; Gualano, G.; Vittozzi, P.; et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin. Microbiol. Infect. 2021, 27, 286.e7–286.e13. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Tincati, C.; Cannizzo, E.S.; Giacomelli, M.; Badolato, R.; d’Arminio Monforte, A.; Marchetti, G. Heightened Circulating Interferon-Inducible Chemokines, and Activated Pro-Cytolytic Th1-Cell Phenotype Features COVID-19 Aggravation in the Second Week of Illness. Front. Immunol. 2020, 11, 580987. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, H.; Lu, X.X.; Xiao, H.; Ren, J.; Zhang, F.R.; Liu, Z.S. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: A single center’s observational study. World J. Ped. 2020, 16, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Shafiek, H.K.; El Lateef, H.M.A.; Boraey, N.F.; Nashat, M.; Abd-Elrehim, G.A.B.; Abouzeid, H.; Hafez, S.F.M.; Shehata, H.; Elhewala, A.A.; Abdel-Aziz, A.; et al. Cytokine profile in Egyptian children and adolescents with COVID-19 pneumonia: A multicenter study. Pediatr. Pulmonol. 2021, 56, 3924–3933. [Google Scholar] [CrossRef]
- Tovo, P.A.; Garazzino, S.; Daprà, V.; Pruccoli, G.; Calvi, C.; Mignone, F.; Alliaudi, C.; Denina, M.; Scolfaro, C.; Zoppo, M.; et al. COVID-19 in children: Expressions of type I/II/III interferons, TRIM28, SETDB1, and endogenous retroviruses in mild and severe cases. Int. J. Mol. Sci. 2021, 22, 7481. [Google Scholar] [CrossRef]
- Hoste, L.; Roels, L.; Naesens, L.; Bosteels, V.; Vanhee, S.; Dupont, S.; Bosteels, C.; Browaeys, R.; Vandamme, N.; Verstaen, K.; et al. TIM3+ TRBV11-2 T cells and IFNγ signature in patrolling monocytes and CD16+ NK cells delineate MIS-C. J. Exp. Med. 2022, 219, e20211381. [Google Scholar] [CrossRef]
- Carter, M.J.; Fish, M.; Jennings, A.; Doores, K.J.; Wellman, P.; Seow, J.; Acors, S.; Graham, C.; Timms, E.; Kenny, J.; et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020, 26, 1701–1707. [Google Scholar] [CrossRef]
- Esteve-Sole, A.; Anton, J.; Pino-Ramirez, R.M.; Sanchez-Manubens, J.; Fumadó, V.; Fortuny, C.; Rios-Barnes, M.; Sanchez-de-Toledo, J.; Girona-Alarcón, M.; Mosquera, J.M.; et al. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease. J. Clin. Investig. 2021, 131, e144554. [Google Scholar] [CrossRef]
- Diorio, C.; Shraim, R.; Vella, L.A.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Canna, S.W.; Henrickson, S.E.; McNerney, K.O.; Balamuth, F.; et al. Proteomic profiling of MIS-C patients indicates heterogeneity relating to interferon gamma dysregulation and vascular endothelial dysfunction. Nat. Commun. 2021, 12, 7222. [Google Scholar] [CrossRef]
- Rodriguez-Smith, J.J.; Verweyen, E.L.; Clay, G.M.; Esteban, Y.M.; de Loizaga, S.R.; Baker, E.J.; Do, T.; Dhakal, S.; Lang, S.M.; Grom, A.A.; et al. Inflammatory biomarkers in COVID-19-associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: A cohort study. Lancet Rheumatol. 2021, 3, e574–e584. [Google Scholar] [CrossRef]
- Pierce, C.A.; Sy, S.; Galen, B.; Goldstein, D.Y.; Orner, E.; Keller, M.J.; Herold, K.C.; Herold, B.C. Natural mucosal barriers and COVID-19 in children. JCI Insight 2021, 6, e148694. [Google Scholar] [CrossRef] [PubMed]
- Lapp, S.A.; Abrams, J.; Lu, A.T.; Hussaini, L.; Kao, C.M.; Hunstad, D.A.; Rosenberg, R.B.; Zafferani, M.J.; Ede, K.C.; Ballan, W.; et al. Serologic and Cytokine Signatures in Children with Multisystem Inflammatory Syndrome and Coronavirus Disease 2019. Open Forum Infect. Dis. 2022, 9, ofac070. [Google Scholar] [CrossRef] [PubMed]
- De Cevins, C.; Luka, M.; Smith, N.; Meynier, S.; Magérus, A.; Carbone, F.; García-Paredes, V.; Barnabei, L.; Batignes, M.; Boullé, A.; et al. Clinical and Translational Article A monocyte/dendritic cell molecular signature of SARS-CoV-2-related multisystem inflammatory syndrome in children with severe myocarditis A monocyte/dendritic cell molecular signature of SARS-CoV-2-related multisyste. Med 2021, 2, 1072–1092. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tang, K.; Levin, M.; Irfan, O.; Morris, S.K.; Wilson, K.; Klein, J.D.; Bhutta, Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020, 20, e276–e288. [Google Scholar] [CrossRef]
- De Rose, D.U.; Pugnaloni, F.; Calì, M.; Ronci, S.; Caoci, S.; Maddaloni, C.; Martini, L.; Santisi, A.; Dotta, A.; Auriti, C. Multisystem Inflammatory Syndrome in Neonates Born to Mothers with SARS-CoV-2 Infection (MIS-N) and in Neonates and Infants Younger Than 6 Months with Acquired COVID-19 (MIS-C): A Systematic Review. Viruses 2022, 14, 750. [Google Scholar] [CrossRef]
- Goenka, A.; Halliday, A.; Gregorova, M.; Milodowski, E.; Thomas, A.; Williamson, M.K.; Baum, H.; Oliver, E.; Long, A.E.; Knezevic, L.; et al. Young infants exhibit robust functional antibody responses and restrained IFN-γ production to SARS-CoV-2. Cell. Rep. Med. 2021, 2, 100327. [Google Scholar] [CrossRef]
- Chou, J.; Platt, C.D.; Habiballah, S.; Nguyen, A.A.; Elkins, M.; Weeks, S.; Peters, Z.; Day-Lewis, M.; Novak, T.; Armant, M.; et al. Taking on COVID-19 Together Study Investigators. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C). J. Allergy Clin. Immunol. 2021, 148, 732–738.e1. [Google Scholar] [CrossRef]
| MIS-C Clinical Findings | |
|---|---|
| Age | More frequent in older children (>8 years old) |
| Sex | More frequent in males |
| Fever | Yes (about 3–5 days) |
| Cardiovascular involvement | Myocardial dysfunction, coronary artery dilation/aneurysms, arrhythmias |
| Respiratory involvement | Tachypnea, acute respiratory failure requiring noninvasive or invasive ventilation |
| Gastrointestinal involvement | Abdominal pain, vomiting, diarrhea |
| Neurological involvement | Headache, lethargy, confusion, irritability |
| Skin involvement | Rash, red or swollen lips, strawberry tongue |
| Renal involvement | Acute kidney injury |
| Other clinical findings |
|
| Laboratory anomalies |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rose, D.U.; Pace, P.G.; Ceccherini-Silberstein, F.; Dotta, A.; Andreoni, M.; Sarmati, L.; Iannetta, M. T Lymphocyte Subset Counts and Interferon-Gamma Production in Adults and Children with COVID-19: A Narrative Review. J. Pers. Med. 2023, 13, 755. https://doi.org/10.3390/jpm13050755
De Rose DU, Pace PG, Ceccherini-Silberstein F, Dotta A, Andreoni M, Sarmati L, Iannetta M. T Lymphocyte Subset Counts and Interferon-Gamma Production in Adults and Children with COVID-19: A Narrative Review. Journal of Personalized Medicine. 2023; 13(5):755. https://doi.org/10.3390/jpm13050755
Chicago/Turabian StyleDe Rose, Domenico Umberto, Pier Giorgio Pace, Francesca Ceccherini-Silberstein, Andrea Dotta, Massimo Andreoni, Loredana Sarmati, and Marco Iannetta. 2023. "T Lymphocyte Subset Counts and Interferon-Gamma Production in Adults and Children with COVID-19: A Narrative Review" Journal of Personalized Medicine 13, no. 5: 755. https://doi.org/10.3390/jpm13050755
APA StyleDe Rose, D. U., Pace, P. G., Ceccherini-Silberstein, F., Dotta, A., Andreoni, M., Sarmati, L., & Iannetta, M. (2023). T Lymphocyte Subset Counts and Interferon-Gamma Production in Adults and Children with COVID-19: A Narrative Review. Journal of Personalized Medicine, 13(5), 755. https://doi.org/10.3390/jpm13050755






