Growth Curves of Chinese Children with Androgen Insensitivity Syndrome: A Multicenter Registry Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Methods
2.2.1. Data Collection
2.2.2. Gene Analysis
2.2.3. Growth Curve Plotting and Data Analysis
3. Results
3.1. General Data of Chinese AIS Patients
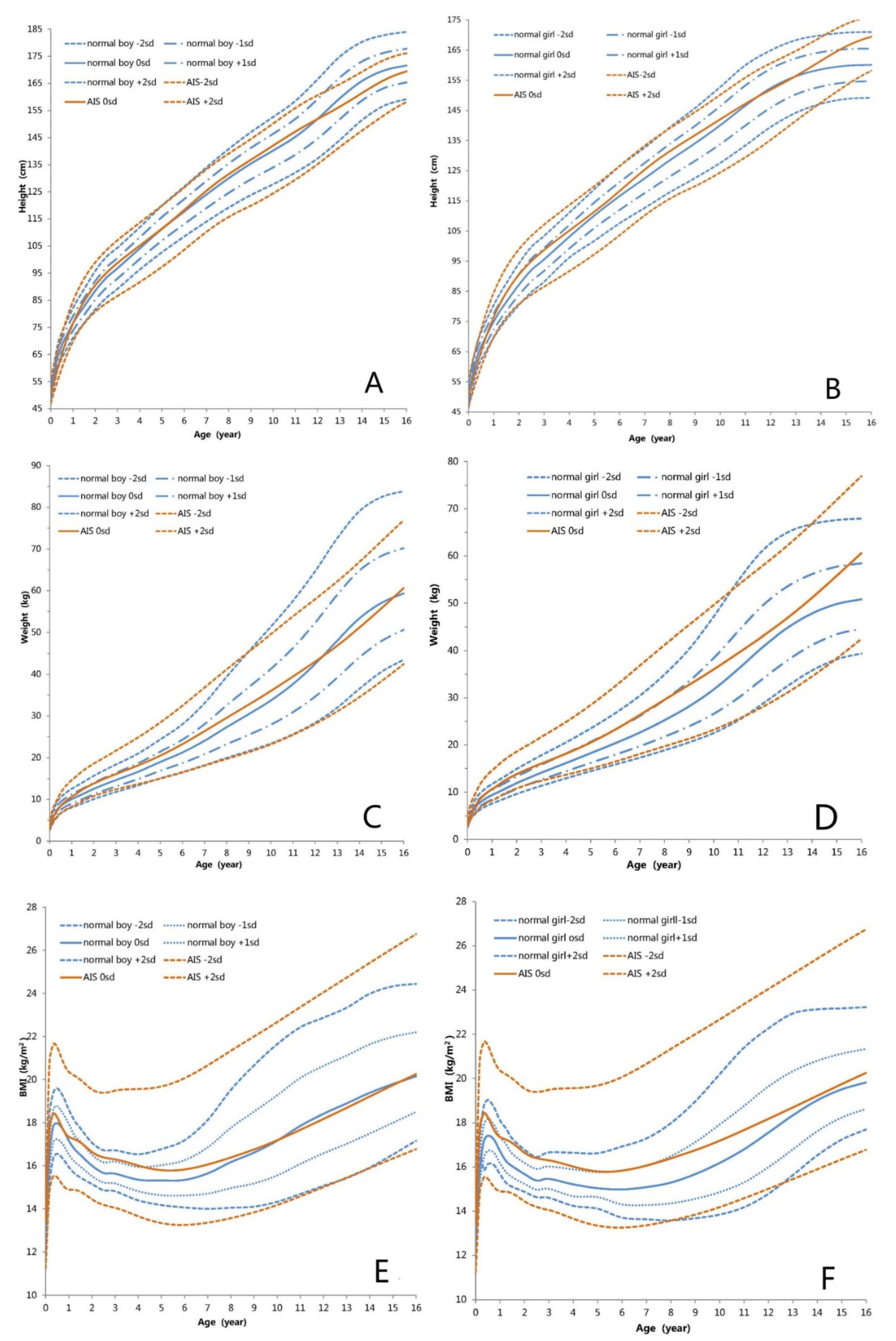
3.2. Height-for-Age Growth Curve
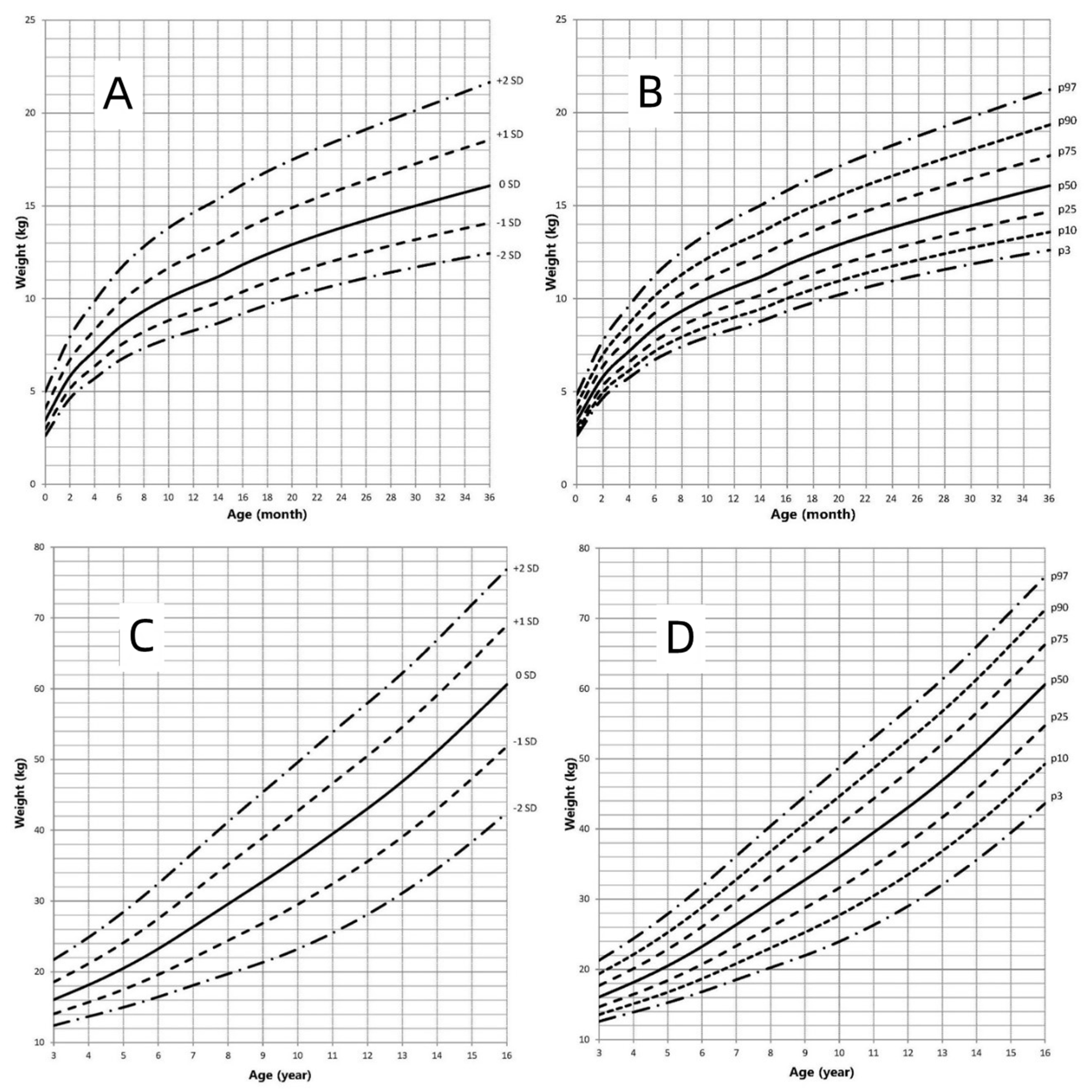
3.3. Weight-for-Age Growth Curve
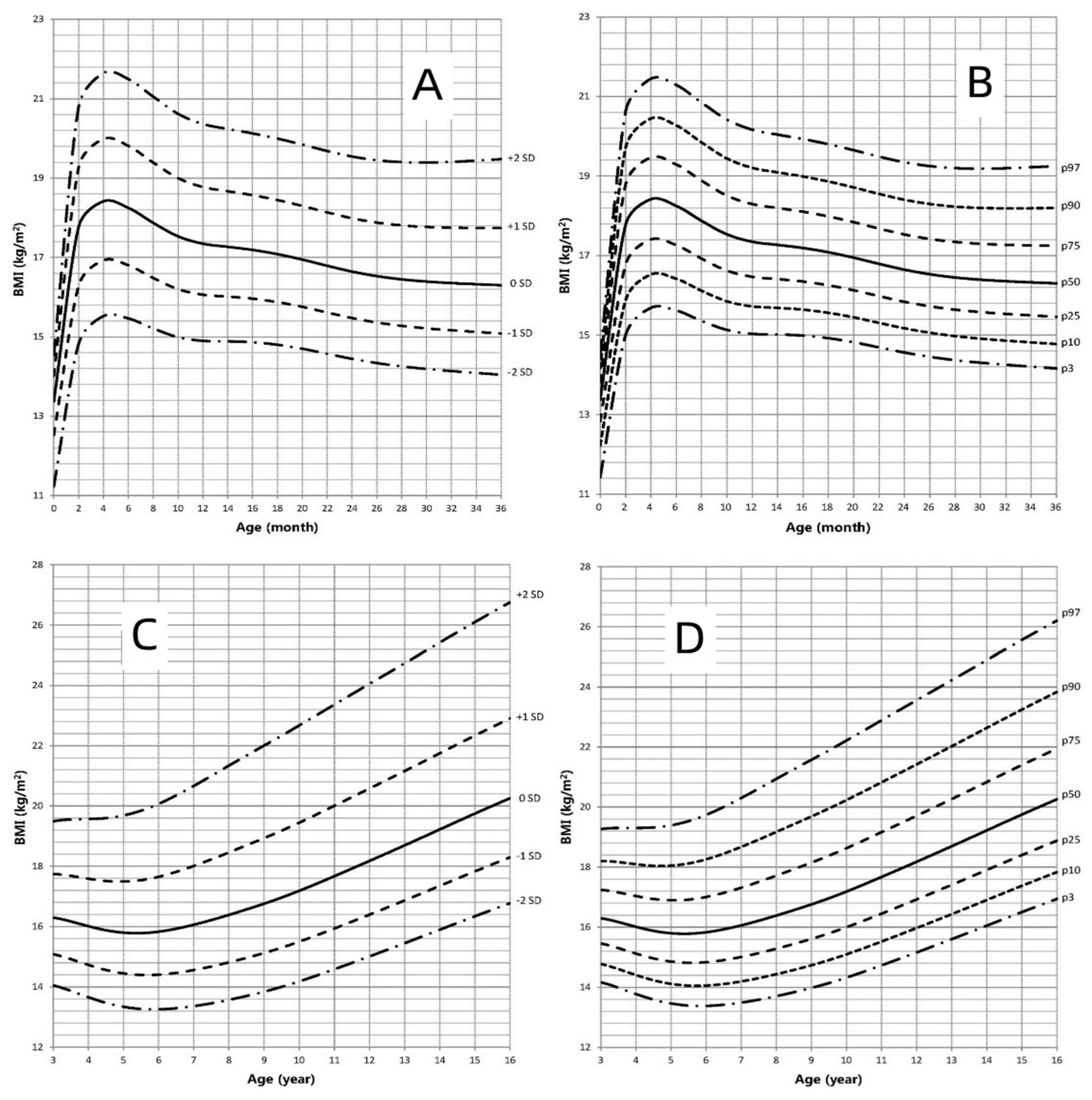
3.4. BMI-for-Age Growth Curve
3.5. Regional Differences in Chinese AIS Patients
3.6. Physical Assessment in CAIS and PAIS Patients
4. Discussion and Conclusions
5. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hughes, I.A. Disorders of sex development: A new definition and classification. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, C.; Wang, X.; Qin, M. AR mutations in 28 patients with androgen insensitivity syndrome (Prader grade 0–3). Sci. China Life Sci. 2017, 60, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Goswami, D.; Trikudanathan, S.; Creighton, S.M.; Conway, G.S. Comparison of bone mineral density and body proportions between women with complete androgen insensitivity syndrome and women with gonadal dysgenesis. Eur. J. Endocrinol. 2008, 159, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M.; Zhang, Y.N.; Du, J.; Li, W.; Tu, C.F.; Meng, L.L.; Lin, G.; Lu, G.X.; Tan, Y.Q. Phenotypic and molecular characteristics of androgen insensitivity syndrome patients. Asian J. Androl. 2018, 20, 473–478. [Google Scholar] [PubMed]
- Feldman, S.R. Androgen insensitivity syndrome (testicular feminization): A model for understanding steroid hormone receptors. J. Am. Acad. Dermatol. 1992, 27, 615–619. [Google Scholar] [CrossRef]
- Gulia, C.; Baldassarra, S.; Zangari, A.; Briganti, V.; Gigli, S.; Gaffi, M.; Signore, F.; Vallone, C.; Nucciotti, R.; Costantini, F.M.; et al. Androgen insensitivity syndrome. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3873–3887. [Google Scholar]
- Hughes, I.A.; Davies, J.D.; Bunch, T.I.; Pasterski, V.; Mastroyannopoulou, K.; MacDougall, J. Androgen insensitivity syndrome. Lancet 2012, 380, 1419–1428. [Google Scholar] [CrossRef]
- Kolesinska, Z.; Ahmed, S.F.; Niedziela, M.; Bryce, J.; Molinska-Glura, M.; Rodie, M.; Jiang, J.; Sinnott, R.O.; Hughes, I.A.; Darendeliler, F.; et al. Changes over time in sex assignment for disorders of sex development. Pediatrics 2014, 134, e710–e715. [Google Scholar] [CrossRef]
- Deeb, A.; Al, S.H.; Ibukunoluwa, F.; Attia, S. Phenotype, Sex of Rearing, Gender Re-Assignment, and Response to Medical Treatment in Extended Family Members with a Novel Mutation in the SRD5A2 Gene. J. Clin. Res. Pediatric Endocrinol. 2016, 8, 236–240. [Google Scholar] [CrossRef]
- Touzon, M.S.; Garrido, N.P.; Marino, R.; Ramirez, P.; Costanzo, M.; Guercio, G.; Berensztein, E.; Rivarola, M.A.; Belgorosky, A. Androgen Insensitivity Syndrome: Clinical Phenotype and Molecular Analysis in a Single Tertiary Center Cohort. J. Clin. Res. Pediatric Endocrinol. 2019, 11, 24–33. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Jiang, J.; OuYang, H.; Wei, S.; Liang, J.; Chen, N.; Zeng, W.; Chen, L.; Xie, X. Severe forms of complete androgen insensitivity syndrome caused by a p.Q65X novel mutation in androgen receptor: Clinical manifestations, imaging findings and molecular genetics. Steroids 2019, 144, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.L.; Costa, E.; Rodrigues, A.S.; Gomes, N.L.; Faria, J.J.; Nishi, M.Y.; Arnhold, I.; Domenice, S.; Mendonca, B.B. Androgen insensitivity syndrome: A review. Arch. Endocrinol. Metab. 2018, 62, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Laurent, M.R.; Claessens, F.; Gielen, E.; Lagerquist, M.K.; Vandenput, L.; Borjesson, A.E.; Ohlsson, C. Sex steroid actions in male bone. Endocr. Rev. 2014, 35, 906–960. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Mielke, K.L.; Cosma, M.; Soares-Welch, C.; Paulo, R.; Miles, J.M.; Bowers, C.Y. Aromatase and 5alpha-reductase inhibition during an exogenous testosterone clamp unveils selective sex steroid modulation of somatostatin and growth hormone secretagogue actions in healthy older men. J. Clin. Endocrinol. Metab. 2009, 94, 973–981. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Anderson, S.M.; Iranmanesh, A.; Bowers, C.Y. Testosterone blunts feedback inhibition of growth hormone secretion by experimentally elevated insulin-like growth factor-I concentrations. J. Clin. Endocrinol. Metab. 2005, 90, 1613–1617. [Google Scholar] [CrossRef][Green Version]
- King, T.; Wat, W.; Creighton, S.M.; Conway, G.S. Bone mineral density in complete androgen insensitivity syndrome and the timing of gonadectomy. Clin. Endocrinol. 2017, 87, 136–140. [Google Scholar] [CrossRef]
- Zhao, X.; Song, Y.; Chen, S.; Wang, X.; Luo, F.; Yang, Y.; Chen, L.; Chen, R.; Chen, H.; Su, Z.; et al. Growth Pattern in Chinese Children With 5alpha-Reductase Type 2 Deficiency: A Retrospective Multicenter Study. Front. Pharmacol. 2019, 10, 173. [Google Scholar] [CrossRef]
- Li, H.; Ji, C.-Y.; Zong, X.-N.; Zhang, Y.Q. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Chin. J. Pediatrics 2009, 47, 487–492. [Google Scholar]
- Gaete, X.; Garcia, R.; Riquelme, J.; Codner, E. Age of onset of puberty in Chilean boys according to testicular volume and Tanner stage. Rev. Med. Chile 2015, 143, 297–303. [Google Scholar] [CrossRef][Green Version]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; Del, A.G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del, A.G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 10–11. [Google Scholar]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Green, P.J. Smoothing reference centile curves: The LMS method and penalized likelihood. Stat. Med. 1992, 11, 1305–1319. [Google Scholar] [CrossRef]
- Tadokoro-Cuccaro, R.; Hughes, I.A. Androgen insensitivity syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 499–503. [Google Scholar] [CrossRef]
- Lek, N.; Tadokoro-Cuccaro, R.; Whitchurch, J.B.; Mazumder, B.; Miles, H.; Prentice, P.; Bunch, T.; Zielinska, K.; Metzler, V.; Mongan, N.P.; et al. Predicting puberty in partial androgen insensitivity syndrome: Use of clinical and functional androgen receptor indices. Ebiomedicine 2018, 36, 401–409. [Google Scholar] [CrossRef]
- Yanase, T.; Fan, W.; Kyoya, K.; Min, L.; Takayanagi, R.; Kato, S.; Nawata, H. Androgens and metabolic syndrome: Lessons from androgen receptor knock out (ARKO) mice. J. Steroid Biochem. Mol. Biol. 2008, 109, 254–257. [Google Scholar] [CrossRef]
- Kosti, K.; Athanasiadis, L.; Goulis, D.G. Long-term consequences of androgen insensitivity syndrome. Maturitas 2019, 127, 51–54. [Google Scholar] [CrossRef]
- Miles, H.L.; Gidlof, S.; Nordenstrom, A.; Ong, K.K.; Hughes, I.A. The role of androgens in fetal growth: Observational study in two genetic models of disordered androgen signalling. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F435–F438. [Google Scholar] [CrossRef]
- Kirsch, S.; Weiss, B.; Zumbach, K.; Rappold, G. Molecular and evolutionary analysis of the growth-controlling region on the human Y chromosome. Hum. Genet. 2004, 114, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Melo, K.F.; Mendonca, B.B.; Billerbeck, A.E.; Costa, E.M.; Inacio, M.; Silva, F.A.; Leal, A.M.; Latronico, A.C.; Arnhold, I.J. Clinical, hormonal, behavioral, and genetic characteristics of androgen insensitivity syndrome in a Brazilian cohort: Five novel mutations in the androgen receptor gene. J. Clin. Endocrinol. Metab. 2003, 88, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.L.; Rodrigues, A.D.S.; Machado, A.Z.; Nishi, M.Y.; Cunha, F.S.; Silva, R.B.; Costa, E.M.F.; Mendonca, B.B.; Domenice, S. Partial androgen insensitivity syndrome due to somatic mosaicism of the androgen receptor. J. Pediatric Endocrinol. Metab. 2018, 31, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Hughes, I.A.; Werner, R.; Bunch, T.; Hiort, O. Androgen insensitivity syndrome. Semin. Reprod. Med. 2012, 30, 432–442. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Cheng, A.; Hughes, I.A. Assessment of the gonadotrophin-gonadal axis in androgen insensitivity syndrome. Arch. Dis. Child. 1999, 80, 324–329. [Google Scholar] [CrossRef]
- Boyar, R.M.; Moore, R.J.; Rosner, W.; Aiman, J.; Chipman, J.; Madden, J.D.; Marks, J.F.; Griffin, J.E. Studies of gonadotropin-gonadal dynamics in patients with androgen insensitivity. J. Clin. Endocrinol. Metab. 1978, 47, 1116–1122. [Google Scholar] [CrossRef]
- Hughes, I.A.; Deeb, A. Androgen resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 577–598. [Google Scholar] [CrossRef]
- Papadimitriou, D.T.; Linglart, A.; Morel, Y.; Chaussain, J.L. Puberty in subjects with complete androgen insensitivity syndrome. Horm. Res. 2006, 65, 126–131. [Google Scholar] [CrossRef]
- Bermudez, D.L.V.J.; Fernandez-Cancio, M.; Bernal, S.; Audi, L. Complete androgen insensitivity syndrome associated with male gender identity or female precocious puberty in the same family. Sex. Dev. 2015, 9, 75–79. [Google Scholar] [CrossRef]
- Shang, L.; Li, H.; Jiang, X. Comparison of constitution indexes of Han male youth recruited from different areas. Chin. J. Public Health 2009, 25, 157–160. [Google Scholar]
- Zhang, X.Y. Geographical distribution of the height level of Han Chinese children and adolescents in China. Chin. J. Sch. Health 2003, 24, 390–391. [Google Scholar]
- Lin, W.S.; Hu, C.K. A study of environmental differences in children’s growth and development in China. Acta Anthropol. Sin. 1990, 9, 152–159. [Google Scholar]
- Cui, H.L.; Guo, X.H. Investigation and analysis on the influencing factors of overweight and obesity in children and their parents’ related knowledge and attitudes. Chronic Pathematol. J. 2013, 14, 288–290. [Google Scholar]
- Qian, Y.Y.; Xu, C.Q.; Lu, L.Q. Analysis of family influencing factors of children’s physical development. Chin. J. Public Health 2009, 25, 947–949. [Google Scholar]


| Region | n | Age (Year) | THt (cm) | BWt (kg) | BL (cm) | HtSDS | WtSDS | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|
| South | 39 | 4.08 (1.16, 8.98) | 169.64 ± 3.70 * | 3.40 (3.15, 3.50) | 50 (49, 51) | 0.25 ± 1.36 | 0.24 ± 1.21 | 17.45 ± 2.92 |
| North | 79 | 1.85 (0.92, 5.08) * | 173.00 (170.00, 174.63) | 3.40 (3.20, 3.82) | 50 (50, 50) | 0.64 ± 1.34 | 0.82 ± 1.23 * | 17.21 (16.33, 18.90) |
| Total | 118 | 2.17 (1.00, 6.19) | 172.50 (171.00, 176.00) | 3.40 (3.20, 3.76) | 50 (50, 50) | 0.51 ± 1.35 | 0.62 ± 1.25 | 17.28 (16.30, 18.93) |
| t/Z | −2.123 | −2.804 | −0.247 | −0.144 | −1.499 | −2.426 | −0.781 | |
| p | 0.034 | 0.005 | 0.805 | 0.885 | 0.137 | 0.017 | 0.436 | |
| Groups | n | Age (Year) | THt (cm) | BWt (kg) | BL (cm) | HtSDS | WtSDS | BMI (kg/m2) | |
|---|---|---|---|---|---|---|---|---|---|
| PAIS | 0–36 months | 42 | 1.08 ± 0.72 | 173.50 (171.50, 176.00) | 3.46 ± 0.51 | 50.00 (50.00, 50.00) | 0.93 ± 1.45 | 0.30 ± 1.17 | 17.16 (16.33, 18.95) |
| 3–16 years | 34 | 7.13 (4.94, 11.94) | 171.00 ± 3.46 | 3.40 ± 0.52 | 50.00 (48.25, 50.25) | 0.60 ± 1.12 | 0.80 ± 1.22 | 17.57 (16.43, 20.28) | |
| Total | 76 | 2.25 (1.00, 6.56) | 173.00 (170.00, 174.63) | 3.45 ± 0.51 | 50.00 (49.00, 51.00) | 0.66 ± 1.32 | 0.65 ± 1.18 | 17.37 (16.35, 19.35) | |
| CAIS | 0–36 months | 25 | 1.00 ± 0.74 | 176.00 ± 3.54 * | 3.30 (3.20, 3.50) | 50.00 (50.00, 51.00) | 0.72 ± 1.49 | 0.59 ± 1.29 | 17.00 (16.08, 17.99) |
| 3–16 years | 17 | 7.42 (4.37, 8.75) | 173.00 (172.25, 176.50) | 3.40 (3.15, 3.80) | 50.00 (50.00, 51.75) | −0.1 ± 1.16 | 0.65 ± 1.31 | 17.29 (15.02, 19.21) | |
| Total | 42 | 2.10 (0.93, 6.20) | 174.75 (169.90, 175.13) | 3.35 (3.20, 3.50) | 50.00 (50.00, 51.00) | 0.43 ± 1.37 | 0.61 ± 1.29 | 17.15 (15.88, 18.42) | |
| 0–36 months | 0.487 | 2.558 | 0.709 | 0.927 | −0.204 | 0.278 | -0.318 | ||
| t/Z | 3–16 years | −1.099 | 0.107 | 0.577 | −0.209 | −1.206 | −0.561 | −0.909 | |
| Total | −0.481 | −1.040 | −0.769 | −0.901 | 0.902 | 0.171 | −0.925 | ||
| p | 0–36 months | 0.627 | 0.011 | 0.479 | 0.955 | 0.839 | 0.782 | 0.251 | |
| 3–16 years | 0.272 | 0.109 | 0.584 | 0.263 | 0.234 | 0.578 | 0.363 | ||
| Total | 0.631 | 0.298 | 0.442 | 0.368 | 0.369 | 0.864 | 0.355 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Su, Z.; Chen, S.; Wang, X.; Yang, Y.; Chen, L.; Liang, L.; Liu, G.; Wang, Y.; Song, Y.; et al. Growth Curves of Chinese Children with Androgen Insensitivity Syndrome: A Multicenter Registry Study. J. Pers. Med. 2022, 12, 771. https://doi.org/10.3390/jpm12050771
Zhao X, Su Z, Chen S, Wang X, Yang Y, Chen L, Liang L, Liu G, Wang Y, Song Y, et al. Growth Curves of Chinese Children with Androgen Insensitivity Syndrome: A Multicenter Registry Study. Journal of Personalized Medicine. 2022; 12(5):771. https://doi.org/10.3390/jpm12050771
Chicago/Turabian StyleZhao, Xiu, Zhe Su, Shaoke Chen, Xiumin Wang, Yu Yang, Linqi Chen, Li Liang, Geli Liu, Yi Wang, Yanning Song, and et al. 2022. "Growth Curves of Chinese Children with Androgen Insensitivity Syndrome: A Multicenter Registry Study" Journal of Personalized Medicine 12, no. 5: 771. https://doi.org/10.3390/jpm12050771
APA StyleZhao, X., Su, Z., Chen, S., Wang, X., Yang, Y., Chen, L., Liang, L., Liu, G., Wang, Y., Song, Y., Fan, L., Ren, X., & Gong, C. (2022). Growth Curves of Chinese Children with Androgen Insensitivity Syndrome: A Multicenter Registry Study. Journal of Personalized Medicine, 12(5), 771. https://doi.org/10.3390/jpm12050771





