Dysregulated miRNAs as Biomarkers and Therapeutical Targets in Neurodegenerative Diseases
Abstract
:1. Introduction
2. AD
| miRNAs | AD post-mortem CNS/AD iPSC-Derived Neurons | Validated Target | Signaling Pathway | Circulating Fluids |
|---|---|---|---|---|
| miR-7 | Up-regulated in hippocampus [25,26], entorhinal cortex, middle temporal gyrus, posterior cingulate cortex, superior frontal gyrus [26], and cortex [27]; down-regulated in grey matter [28], anterior cingulate gyrus (Brodmann area 24), motor cortex [29], and temporal cortex [30] | UCHL1 [31]; UBE2A [32] | Ubiquitin-mediated clearance of amyloid peptides mediated by ciRS-7 [32]; NF-κB-dependent regulation of APP and BACE1 protein and degradation by proteasome and lysosome through UCHL1 [31]; insulin signaling through HNRNPK–miR-7 axis [27] | Detected in peripheral blood [33] |
| miR-9 | Down-regulated in the anterior temporal cortex [34], grey matter [28], cerebellum, hippocampus, medial frontal gyrus [25], and temporal cortex [30]; up-regulated in hippocampal CA1 region [35], and temporal lobe neocortex (Brodmann area A22) [36]; used to obtain a rapid neuronal differentiation and an AD disease phenotypes detected at early time points due to rapid maturation of iPSCs [37] | BACE1 [34]; CREB [38]; OPTN [39]; CAMKK2 [40]; TGFBI, TRIM2, SIRT1 [41] | miR-9 mediates the expression of BACE1 by directly regulating CREB [38]; autophagy [39]; CAMKK2-AMPK2 pathway [40] | Down-regulated in whole blood of LOAD patients [42]; CSF decreasing with increasing of Braak stages [43]; up-regulated in exosome enriched CSF [44] |
| miR-16 | Down-regulated in white matter [28], and Braak VI hippocampus [45]; up-regulated in Braak III/IV | APP [46]; TAU1 [47] | Decreasing with the increasing of Braak stages in serum [43]; down-regulated in CSF [48] | |
| miR-29a | Down-regulated in the anterior temporal cortex [34], medial frontal gyrus [25];, and grey matter [28] | BACE1 [34] | BACE1/β-secretase expression [34] | Up-regulated in CSF [49], and cell-free CSF [50]; down-regulated in CSF [48] |
| miR-29b | Down-regulated in anterior temporal cortex [34], parietal lobe cortex [51], grey matter [28], dorsolateral prefrontal cortex (Brodmann area 9) and temporal cortex (Brodmann area 21/22) [52]; up-regulated in medial frontal gyrus [25] | BACE1 [34] | BACE1/β-secretase expression [34] | Up-regulated in CSF [49] |
| miR-32 | Down-regulated in the cerebellum, hippocampus, medial frontal gyrus [25], and white matter [28] | MECP2 [53] | Feedback loop with MeCP2 and BDNF for homeostatic regulation of MeCP2 [53] | Up-regulated in CSF [25], and in serum [30] |
| miR-34a | Up-regulated in cerebellum, hippocampus, medial frontal gyrus [25], hippocampal CA1 [54], anterior cingulate gyrus (Brodmann area 24) and motor cortex [29]; down-regulated in grey matter [28] | TREM2 [54]; SHANK3 [55] | Synaptogenesis and phagocytosis [54,55] | Down-regulated in plasma and CSF [49] |
| miR-34c | Down-regulated in white matter [28]; up-regulated in the hippocampus [56], Braak stage III/IV hippocampus [45], anterior cingulate gyrus (Brodmann area 24), and motor cortex [29] | SIRT1 [56] | Up-regulated in serum [43] | |
| miR-101 | Down-regulated in white matter [28], anterior temporal cortex [34], and parietal lobe cortex [51] | APP [57] | IL-1β-induced APP up-regulation [57] | Down-regulated in CSF [43] |
| miR-124 | Down-regulated in gray matter [28], frontal cortex [58], temporal cortex [30]; up-regulated in iPSC-derived iNEU-PSEN hippocampal neuron from the AD patient [59] | BACE1 [58,60]; PTPN1 [61,62]; APP [59] | PTPN1 signaling [61] | Down-regulated in CSF [43] |
| miR-125b | Up-regulated in hippocampal CA1 region [35,54], temporal lobe neocortex (Brodmann area A22) [36], cerebellum, hippocampus, medial frontal gyrus [25], frontal cortex (Brodmann areas 6 and 8) [63], iPSC-derived iNEU-PSEN hippocampal neuron from the AD patient [59], and APP and PS1 variants of hippocampal spheroids differentiated from iPSC (3D hippocampal structures) [64]; down-regulated in grey matter [28] | CFH [65]; DUSP6, PPP1CA; BCLW [63]; CDKN2A [66]; NR2A [67] | CFH-driven pathogenic signaling [65]; miR-125b-induced tau hyperphosphorylation [63]; astrogliosis and glial cell proliferation [66]; FMRP-associated up-regulated miRNA induces long narrow spines [67] | Down-regulated in CSF [48,49]; up-regulated in CSF [68] |
| miR-128 | Up-regulated in hippocampal CA1 [35,55], Braak III/IV and decreased in Braak VI hippocampus [45], and temporal cortex [30]; down-regulated in cerebral cortical gray matter [28], and hippocampus of LOAD patients [69] | PPARG via regulation of the NF-κB pathway [70] | NF-κB pathway [70] | Up-regulated in monocytes and lymphocytes from AD patients [71] |
| miR-132 | Up-regulated in hippocampal CA1 region [35,55], anterior cingulate gyrus (Brodmann area24) and motor cortex [29]; down-regulated in cerebellum, medial frontal gyrus [25], temporal cortex [30,72], frontal cortex [72], prefrontal cortex [73], olfactory bulb [74], hippocampus [25,72,73,74], and hippocampus and prefrontal cortex of LOAD [69] | P250GAP [75]; PTBP2 [76]; HDAC3 [77]; tau levels [72]; ITPKB [73]; SIRT1 [74]; HNRNPU [78] | FMRP-associated up-regulated miRNA increases dendritic protrusion width [67]; miR-132/ITPKB pathway [73]; CREB-regulated miRNA regulates neuronal morphogenesis [75]; HDAC3 signaling pathway [77]; hippocampal pro-neurogenic signal rescue [79] | Down-regulated in CSF [43]; up-regulated in plasma [80] |
| miR-135a | Up-regulated in hippocampus [25], anterior cingulate gyrus and motor cortex [29]; down-regulated in gray matter [28], and frontal cortex [81] | BACE1 [82]; THBS1 [83] | CEBPD/miR135a/THBS1 axis promotes angiogenesis [83]; Rock2/Add1 signaling pathway-miRNA regulated mediates the synaptic/memory impairments [81] | Up-regulated in CSF [25], serum [43], and exosomal serum [84] |
| miR-146a | Up-regulated in hippocampal [85,86] and superior temporal lobe neocortex [36,85,86], hippocampal CA1 [54,55], Braak III/IV and decreased in Braak VI hippocampus [45]; down-regulated in temporal cortex [30] | CFH [65,85]; IRAK-1 and IRAK-2 [86,87]; SHANK3 [55]; Srsf6 [88] | Altered innate immune response and neuroinflammation through CFH modulation [65,85]; TLR/IL-1R-IRAK-NF-κB signaling causing altered innate immune response and inflammatory gene expression [86] | Down-regulated in plasma [49], CSF [45,48,49], and serum [30,89] |
| miR-195 | Down-regulated in gray matter [28], hippocampus [90], iPSC-derived astrocytes from ApoE4+/+ AD subjects compared to ApoE3+/+ normal aging iPSC-derived astrocytes [90] | BACE1 [91]; APP and BACE1 [92] | ApoE-synj1-PIP2 pathway [90] | Down-regulated in CSF [25,48,90]; up-regulated in plasma [80] |
| miR-218 | Down-regulated in gray matter [28], and temporal cortex [30]; up-regulated in dorsolateral prefrontal cortex (Brodmann area 9) and temporal cortex (Brodmann area 21/22) [52] | PTPα [93]; C3 [94] | ER-regulated tau phosphorylation [93] | Up-regulated in blood [95] |
3. PD
| miRNAs | PD post-mortem CNS/PD iPSC-Derived Neurons | Validated Target | Signaling Pathway | Circulating Fluids |
|---|---|---|---|---|
| let-7b | Up-regulated in DA neurons [113], and PD-specific iPSC-derived midbrain neurons [115]; down-regulated in amygdala [114] | HMGA2 [149] | Discriminating multiple system atrophy (an atypical parkinsonian disorder) from control [144] | |
| miR-34b | Down-regulated in putamen [150], FC, amygdala, SN, and cerebellum [151] | ADORA2A [150]; Dj1 and Parkin [151]; α-synuclein [152] | Up-regulated in serum of multiple system atrophy patients vs PD for differential diagnosis [146]; detected in CSF [140] | |
| miR-124 | Down-regulated in prefrontal cortex of the left cerebral hemisphere [107]; up-regulated in amygdala [114] | KPNB1, KPNA3, KPNA4 [107]; p62/p38 [153]; Bim [154]; C1ql3 [155]; ANXA5 [156]; EDN2 [157]; MEKK3 [158]; STAT3 [159]; NEAT1/PDE4B [160]; NEAT1 [161] | Apoptosis and Autophagy [154]; AMPK/mTOR pathway [162]; MALAT1/miR-124-3p /DAPK1 signaling cascade mediating apoptosis [163]; Calpain/cdk5 pathway [164]; Hedgehog Signaling Pathway/EDN2 [157]; STL1/NF-κB axis [165]; miR-124/KLF4 axis [166]; miR-124-3p/PTEN/AKT/mTOR pathway [167] | Reduced plasma levels in PD [136]; down-regulated in plasma [137]; up-regulated in plasma [138] |
| miR-126 | Up-regulated in DA neurons [112,113], and amygdala [114] | SP1 [168]; PLK2 [169]; LncRNA HOTAIR/RAB3IP [170]; IRS-1/PIK3R2 [171] | Insulin/IGF-1/PI3K signaling pathway [112]; GF/PI3K/AKT and ERK signaling cascades [171] | Down-regulated in CSF exosome [143], and blood [121,122] |
| miR-132 | Down-regulated in prefrontal cortex (Brodmann Area 9) [116], and in meta-analysis from different PD brain specimens [172]; up-regulated in midbrain [117] | ncRNA MIAT [173]; ULK1 [174]; Nurr1 [175]; GLRX [117] | SIRT1/P53 pathway [176] | Up-regulated in peripheral blood [147,148], and exosomes isolated from CSF [143]; down-regulated in serum samples [125] |
| miR-133b | Down-regulated in midbrain [105,106,172] | Pitx3 [105]; FAIM [177]; RhoA [178]; SNHG14 [179]; Gdnf [180] | Inhibition of cell apoptosis by regulating the ERK1/2 signaling pathway [181]; Xist/miR-133b-3p/Pitx3 axis [182] | Up-regulated in plasma [120]; down-regulated in plasma [131], and serum [142] |
| miR-144 | Up-regulated in the prefrontal cortex (Brodmann Area 9) [116], and anterior cingulate gyrus [118]; down-regulated in the prefrontal cortex of the left cerebral hemisphere [107] | KPNB1, KPNA3, and KPNA4 [107]; β-amyloid precursor protein [183] | NF-κB signaling pathway [107] | Down-regulated in serum [123]; up-regulated in CSF [124] |
| miR-148b | Down-regulated in the prefrontal cortex (Brodmann Area 9) [116], and amygdala [114] | Down-regulated in blood [146] | ||
| miR-184 | Up-regulated in DA neurons [113] and amygdala [114] | Up-regulated in exosomes; down-regulated in plasma [145] | ||
| miR-199a | Up-regulated in the amygdala [114]; down-regulated in iPSC-derived DNs from PD patients [119] | Stage-specific biomarker in serum extracellular vesicles [133] | ||
| miR-204 | Up-regulated in putamen [108]; down-regulated in amygdala [114] | SLC5A3 [184]; DYRK1A [185] | Up-regulated in CFS of Progressive Supranuclear Palsy (PSP) patients [126]; differentially expressed in plasma samples [127]; detected in CSF of patients with parkinsonian syndromes [144] | |
| miR-218 | Up-regulated in the amygdala [114], and midbrain [110]; down-regulated in the prefrontal cortex of the left cerebral hemisphere [107] | RAB6C [110,186]; LASP1 [187]; KPNB1, KPNA3, KPNA4 [107]; PRKN [188] | NF-κB signaling pathway [107] | Down-regulated after 1 h of deep brain stimulation [134,135]; up-regulated in plasma [145] |
| miR-221 | Up-regulated in putamen [108], anterior cingulate gyrus [118], and amygdala [114] | LncRNA MIAT [189]; LncRNA HOTAIR [190]; LncRNA SNHG1 [191]; DJ1 [192]; TFR2 [193]; FMR1 [194] | TGF-β1/Nrf2 axis [189]; miR-221/222/p27/mTOR pathway [191] | Up-regulated in plasma [120]; down-regulated in serum [128,129,130] |
| miR-338 | Down-regulated in prefrontal cortex (Brodmann Area 9) [116], and amygdala [114] | SP1 [195] | Decreased levels in plasma extracellular vesicles [139] | |
| miR-425 | Up-regulated in putamen [108]; down-regulated in SN [109] | RIPK1 [109] | miR-425-5p/TRAF5/NF-κB axis [196] | Able to discriminate PD from PSP [132] |
4. ALS
| miRNAs | ALS post-mortem CNS/ALS iPSC-Derived Neurons | Validated Target | Signaling Pathway | Circulating Fluids |
|---|---|---|---|---|
| miR-9 | Down-regulated in lumbar motor neurons [202,215,216]; dysregulated in ALS-specific iPSC-derived MN lines [217,218] | NEFL [215,216]; PRPH [218]; FoxP1 [219]; PAK4 [220] | Neuronal transcription programs, neurofilaments aggregate formation [215,216,221] | Increased in peripheral leukocytes from ALS patients [222] |
| miR-124 | Down-regulated in spinal cord [202,214] | Sox2, Sox9 [223] | Immune responses, neuroinflammation, neuronal development, synaptic plasticity, neurodegeneration [224,225,226] | Dysregulated in the CSF and leukocytes of ALS patients [222,227,228] |
| miR-133a/b | Down-regulated in spinal cord tissue [212,229], and ALS-specific iPSC-derived MN [210] | FAS, CD4, EIF2C4/AGO4, CCL2, and AQP1 [212] | Cell death, defense response, immune response, and inflammation [212] | Up-regulated in serum [230,231] |
| miR-142 | Up-regulated in spinal cord tissue [212,229] | CAMK2A [232]; Vimentin [233]; IL-6 [234]; CDKN1B, TIMP3 [235]; NRF2 [227,236,237] | Cell death, defense responses, immune responses and inflammation [212,238] | Dysregulated in CSF of ALS patients [227,238,239,240] |
| miR-146a | Dysregulated in spinal cord tissue [215,216,229] | NEFL [215,216] | Neurofilaments aggregate formation [215,216]; neuroinflammation [241] | Up-regulated in blood plasma from ALS/MND patients [242] |
| miR-155 | Up-regulated in spinal cord [212,214,229] | SHIP1 [229];SOCS1 [243];SMAD2 [244]; SMAD5 [245]; TGF-β [246] | Cell death, defense responses, immune responses, and inflammation [212] | Increased in peripheral monocytes from ALS patients [247] |
| miR-218 | Down-regulated in spinal cord tissue [212,229]; up-regulated in ALS-specific iPSC-derived MN [248] | Kcnh1 [249]; SLC1A1, SLC1A2 [248]; Tead1, SLC6A1, BCL11A, Lhx1 and FoxP2 [250] | Development, membrane excitability, NMJ synaptic connections [249] | Down-regulated in peripheral blood, CSF, serum and neuromuscular junction of ALS patients [251] |
| miR-338 | Up-regulated in spinal cord tissue [252], and motor cortex samples [209,212] | ATP5G1 [253] | Apoptosis, oligodendrocyte differentiation, maturation, mitochondrial function [254] | Up-regulated in peripheral blood, CSF, serum and neuromuscular junction of ALS patients [222,251,252,254,255] |
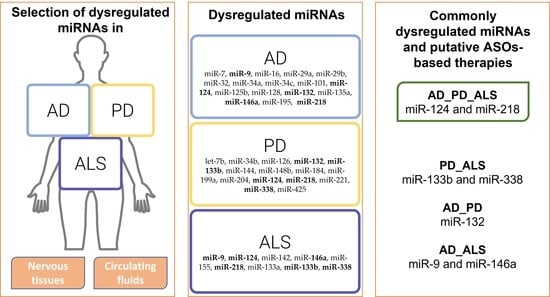
5. Common Dysregulated miRNAs in AD, PD, and ALS
5.1. Dysregulated miRNAs in AD, PD, and ALS
5.2. Dysregulated miRNAs in AD and PD
5.3. Dysregulated miRNAs in AD and ALS
5.4. Dysregulated miRNAs in PD and ALS
6. ASOs-Based miRNA Therapies
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Fändrich, M. Protein aggregation in Alzheimer’s disease: Aβ and τ and their potential roles in the pathogenesis of AD. Acta Neuropathol. 2015, 129, 163–165. [Google Scholar] [CrossRef] [Green Version]
- Gundersen, V. Protein aggregation in Parkinson’s disease. Acta Neurol. Scand. 2010, 122, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, A.M.; Groen, E.J.N.; Koppers, M.; Van Den Berg, L.H.; Pasterkamp, R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013, 125, 777–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s Disease. Subcell Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef] [Green Version]
- Shatunov, A.; Al-Chalabi, A. The genetic architecture of ALS. Neurobiol. Dis. 2020, 147, 105156. [Google Scholar] [CrossRef]
- Brotman, R.G.; Moreno-Escobar, M.C.; Joseph, J.; Pawar, G. Amyotrophic Lateral Sclerosis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mullard, A. ALS antisense drug falters in phase III. Nat. Rev. Drug Discov. 2021, 20, 883–885. [Google Scholar] [CrossRef]
- Reddy, A.P.; Ravichandran, J.; Carkaci-Salli, N. Neural regeneration therapies for Alzheime’s and Parkinson’s disease-related disorders. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1866, 165506. [Google Scholar] [CrossRef]
- Paul, S.; Vázquez, L.A.B.; Uribe, S.P.; Reyes-Pérez, P.R.; Sharma, A. Current Status of microRNA-Based Therapeutic Approaches in Neurodegenerative Disorders. Cells 2020, 9, 1698. [Google Scholar] [CrossRef]
- Maia, M.A.; Sousa, E. BACE-1 and γ-Secretase as Therapeutic Targets for Alzheimer’s Disease. Pharmaceuticals 2019, 12, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walgrave, H.; Zhou, L.; De Strooper, B.; Salta, E. The promise of microRNA-based therapies in Alzheimer’s disease: Challenges and perspectives. Mol. Neurodegener. 2021, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lu, H.C. microRNAs in Neurodegeneration: Current Findings and Potential Impacts. J. Alzheimer’s Dis. Park. 2018, 8, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.; Keon, M.; Liu, B.; Su, Z.; Saksena, N.K. Panoramic Visualization of Circulating MicroRNAs Across Neurodegenerative Diseases in Humans. Mol. Neurobiol. 2019, 56, 7380–7407. [Google Scholar] [CrossRef] [Green Version]
- Hussein, M.; Magdy, R. MicroRNAs in central nervous system disorders: Current advances in pathogenesis and treatment. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 36. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, F.; Guan, Y.; Meng, F.; Zhao, Z.; Su, Q.; Bao, W.; Wang, X.; Zhao, J.; Huo, Z.; et al. The Biogenesis of miRNAs and Their Role in the Development of Amyotrophic Lateral Sclerosis. Cells 2022, 11, 572. [Google Scholar] [CrossRef]
- Gu, S.; Jin, L.; Zhang, F.; Sarnow, P.; Kay, M.A. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Nagaraj, S.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. microRNA diagnostic panel for Alzheimer’s disease and epigenetic trade-off between neurodegeneration and cancer. Ageing Res. Rev. 2018, 49, 125–143. [Google Scholar] [CrossRef]
- Bennett, C.F.; Krainer, A.R.; Cleveland, D.W. Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu. Rev. Neurosci. 2019, 42, 385–406. [Google Scholar] [CrossRef]
- Ehrenberg, A.J.; Khatun, A.; Coomans, E.; Betts, M.J.; Capraro, F.; Thijssen, E.H.; Senkevich, K.; Bharucha, T.; Jafarpour, M.; Young, P.N.E.; et al. Relevance of biomarkers across different neurodegenerative. Alzheimer’s Res. Ther. 2020, 12, 56. [Google Scholar] [CrossRef]
- Stavljenic–Rukavina, A. Molecular Mechanisms in Alzheime’s Disease. EJIFCC 2004, 15, 100–103. [Google Scholar] [PubMed]
- Kempf, S.J.; Metaxas, A. Neurofibrillary tangles in Alzheimer’s disease: Elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen. Res. 2016, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Madadi, S.; Schwarzenbach, H.; Saidijam, M.; Mahjub, R.; Soleimani, M. Potential microRNA-related targets in clearance pathways of amyloid-β: Novel therapeutic approach for the treatment of Alzheimer’s disease. Cell Biosci. 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogswell, J.; Ward, J.; Taylor, I.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of miRNA Changes in Alzheimer’s.pdf. J. Alzheimer’s Dis. 2008, 14, 27–41. [Google Scholar] [CrossRef]
- Puthiyedth, N.; Riveros, C.; Berretta, R.; Moscato, P. Identification of Differentially Expressed Genes through Integrated Study of Alzheimer’s Disease Affected Brain Regions. PLoS ONE 2016, 11, e0152342. [Google Scholar] [CrossRef] [Green Version]
- Frutos, M.F.-D.; Galán-Chilet, I.; Goedeke, L.; Kim, B.; Pardo-Marqués, V.; Pérez-García, A.; Herrero, J.I.; Fernández-Hernando, C.; Kim, J.; Ramírez, C.M. MicroRNA 7 Impairs Insulin Signaling and Regulates Aβ Levels through Posttranscriptional Regulation of the Insulin Receptor Substrate 2, Insulin Receptor, Insulin-Degrading Enzyme, and Liver X Receptor Pathway. Mol. Cell. Biol. 2019, 39, e00170-19. [Google Scholar] [CrossRef]
- Wang, W.-X.; Huang, Q.; Hu, Y.; Stromberg, A.J.; Nelson, P.T. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: White matter versus gray matter. Acta Neuropathol. 2010, 121, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.T.; Wang, W.-X.; Janse, S.A.; Thompson, K.L. MicroRNA expression patterns in human anterior cingulate and motor cortex: A study of dementia with Lewy bodies cases and controls. Brain Res. 2017, 1678, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Hara, N.; Kikuchi, M.; Miyashita, A.; Hatsuta, H.; Saito, Y.; Kasuga, K.; Murayama, S.; Ikeuchi, T.; Kuwano, R. Serum microRNA miR-501-3p as a potential biomarker related to the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2017, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Chen, T.; Yao, Q.; Zheng, L.; Zhang, Z.; Wang, J.; Hu, Z.; Cui, H.; Han, Y.; Han, X.; et al. The circular RNA ci RS -7 promotes APP and BACE 1 degradation in an NF -κB-dependent manner. FEBS J. 2017, 284, 1096–1109. [Google Scholar] [CrossRef]
- Zhao, Y.; Alexandrov, P.N.; Jaber, V.; Lukiw, W.J. Deficiency in the Ubiquitin Conjugating Enzyme UBE2A in Alzheimer’s Disease (AD) is Linked to Deficits in a Natural Circular miRNA-7 Sponge (circRNA; ciRS-7). Genes 2016, 7, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leidinger, P.; Backes, C.; Deutscher, S.; Schmitt, K.; Mueller, S.C.; Frese, K.; Haas, J.; Ruprecht, K.; Paul, F.; Stähler, C.; et al. A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol. 2013, 14, R78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Papadopoulou, A.S.; Mandemakers, W.; Silahtaroglu, A.N.; Kauppinen, S.; Delacourte, A.; De Strooper, B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6415–6420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukiw, W.J. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. NeuroReport 2007, 18, 297–300. [Google Scholar] [CrossRef]
- Sethi, P.; Lukiw, W.J. Micro-RNA abundance and stability in human brain: Specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci. Lett. 2009, 459, 100–104. [Google Scholar] [CrossRef]
- Ishikawa, M.; Aoyama, T.; Shibata, S.; Sone, T.; Miyoshi, H.; Watanabe, H.; Nakamura, M.; Morota, S.; Uchino, H.; Yoo, A.S.; et al. miRNA-Based Rapid Differentiation of Purified Neurons from hPSCs Advancestowards Quick Screening for Neuronal Disease Phenotypes In Vitro. Cells 2020, 9, 532. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Zhao, Y.; Zhou, Y.; Liu, L.; Liu, Y.; Wang, D.; Zhang, S.; Yang, M. MiR-9 Regulates the Expression of BACE1 in Dementia Induced by Chronic Brain Hypoperfusion in Rats. Cell. Physiol. Biochem. 2017, 42, 1213–1226. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-L.; Hong, C.-G.; Yue, T.; Li, H.-M.; Duan, R.; Hu, W.-B.; Cao, J.; Wang, Z.-X.; Chen, C.-Y.; Hu, X.-K.; et al. Inhibition of miR-331-3p and miR-9-5p ameliorates Alzheimer’s disease by enhancing autophagy. Theranostics 2021, 11, 2395–2409. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, L.-H.; Xu, W.-P.; Jing, P.; Zhan, P.-Y. microRNA-9 attenuates amyloidβ-induced synaptotoxicity by targeting calcium/calmodulin-dependent protein kinase kinase. Mol. Med. Rep. 2014, 9, 1917–1922. [Google Scholar] [CrossRef]
- Schonrock, N.; Humphreys, D.; Preiss, T.; Götz, J. Target Gene Repression Mediated by miRNAs miR-181c and miR-9 Both of Which Are Down-regulated by Amyloid-β. J. Mol. Neurosci. 2011, 46, 324–335. [Google Scholar] [CrossRef]
- Souza, V.C.; Morais, J.G.S.; Henriques, A.D.; Machado-Silva, W.; Perez, D.V.; Brito, C.J.; Camargos, E.F.; Moraes, C.F.; Nóbrega, O.T. Whole-Blood Levels of MicroRNA-9 Are Decreased in Patients with Late-Onset Alzheimer Disease. Am. J. Alzheimer’s Dis. Other Dementiasr. 2020, 35, 153331752091157. [Google Scholar] [CrossRef] [PubMed]
- Burgos, K.; Malenica, I.; Metpally, R.; Courtright, A.; Rakela, B.; Beach, T.; Shill, H.; Adler, C.; Sabbagh, M.; Villa, S.; et al. Profiles of Extracellular miRNA in Cerebrospinal Fluid and Serum from Patients with Alzheimer’s and Parkinson’s Diseases Correlate with Disease Status and Features of Pathology. PLoS ONE 2014, 9, e94839. [Google Scholar] [CrossRef]
- Riancho, J.; Vázquez-Higuera, J.L.; Pozueta, A.; Lage, C.; Kazimierczak, M.; Bravo, M.; Calero, M.; Gonalezález, A.; Rodríguez, E.; Lleó, A.; et al. MicroRNA Profile in Patients with Alzheimer’s Disease: Analysis of miR-9-5p and miR-598 in Raw and Exosome Enriched Cerebrospinal Fluid Samples. J. Alzheimer’s Dis. 2017, 57, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Kuiperij, H.B.; Claassen, J.A.; Küsters, B.; Verbeek, M.M. MicroRNAs in Alzheimer’s disease: Differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol. Aging 2014, 35, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, C.; Zhu, J.; Shu, P.; Yin, B.; Gong, Y.; Qiang, B.; Yuan, J.; Peng, X. MicroRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer’s-associated pathogenesis in SAMP8 mice. Neurobiol. Aging 2012, 33, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Hébert, S.S.; Papadopoulou, A.S.; Smith, P.; Galas, M.-C.; Planel, E.; Silahtaroglu, A.N.; Sergeant, N.; Buée, L.; De Strooper, B. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum. Mol. Genet. 2010, 19, 3959–3969. [Google Scholar] [CrossRef] [Green Version]
- Lusardi, T.A.; Phillips, J.I.; Wiedrick, J.T.; Harrington, C.A.; Lind, B.; Lapidus, J.A.; Quinn, J.F.; Saugstad, J.A. MicroRNAs in Human Cerebrospinal Fluid as Biomarkers for Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 55, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. MicroRNAs in Plasma and Cerebrospinal Fluid as Potential Markers for Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 39, 253–259. [Google Scholar] [CrossRef]
- Müller, M.; Jäkel, L.; Bruinsma, I.B.; Claassen, J.A.; Kuiperij, B.; Verbeek, M.M. MicroRNA-29a Is a Candidate Biomarker for Alzheimer’s Disease in Cell-Free Cerebrospinal Fluid. Mol. Neurobiol. 2016, 53, 2894–2899. [Google Scholar] [CrossRef] [Green Version]
- Nunez-Iglesias, J.; Liu, C.-C.; Morgan, T.E.; Finch, C.E.; Zhou, X.J. Joint Genome-Wide Profiling of miRNA and mRNA Expression in Alzheimer’s Disease Cortex Reveals Altered miRNA Regulation. PLoS ONE 2010, 5, e8898. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, A.; Chiricosta, L.; Boccardi, V.; Mecocci, P.; Bramanti, P.; Mazzon, E. MicroRNAs Modulate the Pathogenesis of Alzheimer’s Disease: An In Silico Analysis in the Human Brain. Genes 2020, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.M.; Lioy, D.T.; Ma, L.; Impey, S.; Mandel, G.; Goodman, R.H. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat. Neurosci. 2007, 10, 1513–1514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bhattacharjee, S.; Jones, B.M.; Dua, P.; Alexandrov, P.N.; Hill, J.M.; Lukiw, W.J. Regulation of TREM2 expression by an NF-кB-sensitive miRNA-34a. NeuroReport 2013, 24, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaber, V.; Zhao, Y.; Lukiw, W.J. Alterations in micro RNA-messenger RNA (miRNA-mRNA) Coupled Signaling Networks in Sporadic Alzheimer’s Disease (AD) Hippocampal CA1. J. Alzheimer’s Dis. Park. 2017, 7, 312. [Google Scholar] [CrossRef]
- Zovoilis, A.; Agbemenyah, H.Y.; Agís-Balboa, R.C.; Stilling, R.; Edbauer, D.; Rao, P.; Farinelli, L.; Delalle, I.; Schmitt, A.; Falkai, P.; et al. microRNA-34c is a novel target to treat dementias. EMBO J. 2011, 30, 4299–4308. [Google Scholar] [CrossRef]
- Vilardo, E.; Barbato, C.; Ciotti, M.; Cogoni, C.; Ruberti, F. MicroRNA-101 Regulates Amyloid Precursor Protein Expression in Hippocampal Neurons. J. Biol. Chem. 2010, 285, 18344–18351. [Google Scholar] [CrossRef] [Green Version]
- An, F.; Gong, G.; Wang, Y.; Bian, M.; Yu, L.; Wei, C. MiR-124 acts as a target for Alzheimer’s disease by regulating BACE1. Oncotarget 2017, 8, 114065–114071. [Google Scholar] [CrossRef] [Green Version]
- Garcia, G.; Pinto, S.; Cunha, M.; Fernandes, A.; Koistinaho, J.; Brites, D. Neuronal dynamics and mirna signaling differ between sh-sy5y appswe and psen1 mutant ipsc-derived ad models upon modulation with mir-124 mimic and inhibitor. Cells 2021, 10, 2424. [Google Scholar] [CrossRef]
- Du, X.; Huo, X.; Yang, Y.; Hu, Z.; Botchway, B.O.; Jiang, Y.; Fang, M. miR-124 downregulates BACE 1 and alters autophagy in APP/PS1 transgenic mice. Toxicol. Lett. 2017, 280, 195–205. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Huang, H.-Z.; Wang, Z.-H.; Hou, T.-Y.; Yang, X.; Pang, P.; Wei, N.; Zhou, Y.; Dupras, M.-J.; et al. A Novel MicroRNA-124/PTPN1 Signal Pathway Mediates Synaptic and Memory Deficits in Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 395–405. [Google Scholar] [CrossRef]
- Hou, T.; Zhou, Y.; Zhu, L.; Wang, X.; Pang, P.; Wang, D.; Liuyang, Z.; Man, H.; Lu, Y.; Liu, D. Correcting abnormalities in miR-124/PTPN1 signaling rescues tau pathology in Alzheimer’s disease. J. Neurochem. 2020, 154, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Banzhaf-Strathmann, J.; Benito, E.; May, S.; Arzberger, T.; Tahirovic, S.; Kretzschmar, H.; Fischer, A.; Edbauer, D. Micro RNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J. 2014, 33, 1667–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomeshchik, Y.; Klementieva, O.; Gil, J.; Martinsson, I.; Hansen, M.G.; de Vries, T.; Sancho-Balsells, A.; Russ, K.; Savchenko, E.; Collin, A.; et al. Human iPSC-Derived Hippocampal Spheroids: An Innovative Tool for Stratifying Alzheimer Disease Patient-Specific Cellular Phenotypes and Developing Therapies. Stem Cell Rep. 2020, 15, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Alexandrov, P.N. Regulation of Complement Factor H (CFH) by Multiple miRNAs in Alzheimer’s Disease (AD) Brain. Mol. Neurobiol. 2012, 46, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Pogue, A.; Cui, J.; Li, Y.; Zhao, Y.; Culicchia, F.; Lukiw, W. Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci. Lett. 2010, 476, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Edbauer, D.; Neilson, J.R.; Foster, K.A.; Wang, C.-F.; Seeburg, D.P.; Batterton, M.N.; Tada, T.; Dolan, B.M.; Sharp, P.A.; Sheng, M. Regulation of Synaptic Structure and Function by FMRP-Associated MicroRNAs miR-125b and miR-132. Neuron 2010, 65, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Dangla-Valls, A.; Molinuevo, J.L.; Altirriba, J.; Sanchez-Valle, R.; Alcolea, D.; Fortea, J.; Rami, L.; Balasa, M.; Muñoz-García, C.; Ezquerra, M.; et al. CSF microRNA Profiling in Alzheimer’s Disease: A Screening and Validation Study. Mol. Neurobiol. 2016, 54, 6647–6654. [Google Scholar] [CrossRef]
- Lau, P.; Bossers, K.; Janky, R.; Salta, E.; Frigerio, C.S.; Barbash, S.; Rothman, R.; Sierksma, A.S.R.; Thathiah, A.; Greenberg, D.; et al. Alteration of the micro RNA network during the progression of Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 1613–1634. [Google Scholar] [CrossRef] [Green Version]
- Geng, L.; Zhang, T.; Liu, W.; Chen, Y. Inhibition of miR-128 Abates Aβ-Mediated Cytotoxicity by Targeting PPAR-γ via NF-κB Inactivation in Primary Mouse Cortical Neurons and Neuro2a Cells. Yonsei Med. J. 2018, 59, 1096–1106. [Google Scholar] [CrossRef]
- Tiribuzi, R.; Crispoltoni, L.; Porcellati, S.; Di Lullo, M.; Florenzano, F.; Pirro, M.; Bagaglia, F.; Kawarai, T.; Zampolini, M.; Orlacchio, A.; et al. miR128 up-regulation correlates with impaired amyloid β(1-42) degradation in monocytes from patients with sporadic Alzheimer’s disease. Neurobiol. Aging 2014, 35, 345–356. [Google Scholar] [CrossRef]
- Smith, P.Y.; Hernandez-Rapp, J.; Jolivette, F.; Lecours, C.; Bisht, K.; Goupil, C.; Dorval, V.; Parsi, S.; Morin, F.; Planel, E.; et al. miR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum. Mol. Genet. 2015, 24, 6721–6735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salta, E.; Sierksma, A.; Vanden Eynden, E.; De Strooper, B. miR-132 loss de-represses ITPKB and aggravates amyloid and TAU pathology in Alzheimer’s brain. EMBO Mol. Med. 2016, 8, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Hadar, A.; Milanesi, E.; Walczak, M.; Puzianowska-Kuznicka, M.; Kuznicki, J.; Squassina, A.; Niola, P.; Chillotti, C.; Attems, J.; Gozes, I.; et al. SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s Disease. Sci. Rep. 2018, 8, 8465. [Google Scholar] [CrossRef]
- Vo, N.; Klein, M.E.; Varlamova, O.; Keller, D.M.; Yamamoto, T.; Goodman, R.H.; Impey, S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 16426–16431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.Y.; Delay, C.; Girard, J.; Papon, M.-A.; Planel, E.; Sergeant, N.; Buée, L.; Hébert, S.S. MicroRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy. Hum. Mol. Genet. 2011, 20, 4016–4024. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Meng, X.; El Fatimy, R.; Sun, B.; Mai, D.; Zhang, J.; Arora, R.; Zeng, A.; Xu, P.; Qu, S.; et al. Environmental enrichment prevents Aβ oligomer-induced synaptic dysfunction through mirna-132 and hdac3 signaling pathways. Neurobiol. Dis. 2019, 134, 104617. [Google Scholar] [CrossRef]
- Qu, J.; Xiong, X.; Hujie, G.; Ren, J.; Yan, L.; Ma, L. MicroRNA-132-3p alleviates neuron apoptosis and impairments of learning and memory abilities in Alzheimer’s disease by downregulation of HNRNPU stabilized BACE1. Cell Cycle 2021, 20, 2309–2320. [Google Scholar] [CrossRef]
- Walgrave, H.; Balusu, S.; Snoeck, S.; Eynden, E.V.; Craessaerts, K.; Thrupp, N.; Wolfs, L.; Horré, K.; Fourne, Y.; Ronisz, A.; et al. Restoring miR-132 expression rescues adult hippocampal neurogenesis and memory deficits in Alzheimer’s disease. Cell Stem Cell 2021, 28, 1805–1821.e8. [Google Scholar] [CrossRef]
- Guévremont, D.; Tsui, H.; Knight, R.; Fowler, C.J.; Masters, C.L.; Martins, R.N.; Abraham, W.C.; Tate, W.P.; Cutfield, N.J.; Williams, J.M. Plasma microRNA vary in association with the progression of Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12251. [Google Scholar] [CrossRef]
- Zheng, K.; Hu, F.; Zhou, Y.; Zhang, J.; Zheng, J.; Lai, C.; Xiong, W.; Cui, K.; Hu, Y.-Z.; Han, Z.-T.; et al. miR-135a-5p mediates memory and synaptic impairments via the Rock2/Adducin1 signaling pathway in a mouse model of Alzheimer’s disease. Nat. Commun. 2021, 12, 1903. [Google Scholar] [CrossRef]
- Liu, C.-G.; Wang, J.-L.; Li, L.; Xue, L.-X.; Zhang, Y.-Q.; Wang, P.-C. MicroRNA-135a and -200b, potential Biomarkers for Alzheimer’s disease, regulate β secretase and amyloid precursor protein. Brain Res. 2014, 1583, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-Y.; Chu, Y.-Y.; Narumiya, S.; Chi, J.-Y.; Furuyashiki, T.; Aoki, T.; Wang, S.-M.; Chang, W.-C.; Wang, J.-M. The CCAAT/enhancer-binding protein delta/miR135a/thrombospondin 1 axis mediates PGE2-induced angiogenesis in Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1356–1368. [Google Scholar] [CrossRef]
- Yang, T.T.; Liu, C.G.; Gao, S.C.; Zhang, Y.; Wang, P.C. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. 2018, 31, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Zhao, Y.; Cui, J.G. An NF-κB-sensitive Micro RNA-146a-mediated Inflammatory Circuit in Alzheimer Disease and in Stressed Human Brain Cells. J. Biol. Chem. 2008, 283, 31315–31322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.G.; Li, Y.Y.; Zhao, Y.; Bhattacharjee, S.; Lukiw, W.J. Differential Regulation of Interleukin-1 Receptor-associated Kinase-1 (IRAK-1) and IRAK-2 by MicroRNA-146a and NF-κB in Stressed Human Astroglial Cells and in Alzheimer Disease. J. Biol. Chem. 2010, 285, 38951–38960. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Cui, J.G.; Dua, P.; Pogue, A.I.; Bhattacharjee, S.; Lukiw, W.J. Differential expression of miRNA-146a-regulated inflammatory genes in human primary neural, astroglial and microglial cells. Neurosci. Lett. 2011, 499, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Mai, H.; Fan, W.; Wang, Y.; Cai, Y.; Li, X.; Chen, F.; Chen, X.; Yang, J.; Tang, P.; Chen, H.; et al. Intranasal Administration of miR-146a Agomir Rescued the Pathological Process and Cognitive Impairment in an AD Mouse Model. Mol. Ther.–Nucleic Acids 2019, 18, 681–695. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Li, J.; Huang, L.; Chen, X.; Li, D.; Wang, T.; Hu, C.; Xu, J.; Zhang, C.; Zen, K.; et al. Serum MicroRNA Profiles Serve as Novel Biomarkers for the Diagnosis of Alzheimer’s Disease. Dis. Markers 2015, 2015, 625659. [Google Scholar] [CrossRef]
- Cao, J.; Huang, M.; Guo, L.; Zhu, L.; Hou, J.; Zhang, L.; Pero, A.; Ng, S.; El Gaamouch, F.; Elder, G.; et al. MicroRNA-195 rescues ApoE4-induced cognitive deficits and lysosomal defects in Alzheimer’s disease pathogenesis. Mol. Psychiatry 2020, 26, 4687–4701. [Google Scholar] [CrossRef]
- Zhu, H.-C.; Wang, L.-M.; Wang, M.; Song, B.; Tan, S.; Teng, J.-F.; Duan, D.-X. MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting BACE1. Brain Res. Bull. 2012, 88, 596–601. [Google Scholar] [CrossRef]
- Ai, J.; Sun, L.-H.; Che, H.; Zhang, R.; Zhang, T.-Z.; Wu, W.-C.; Su, X.-L.; Chen, X.; Yang, G.; Li, K.; et al. MicroRNA-195 Protects Against Dementia Induced by Chronic Brain Hypoperfusion via Its Anti-Amyloidogenic Effect in Rats. J. Neurosci. 2013, 33, 3989–4001. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, F.; Liu, D.; Huang, H.; Wei, N.; Tan, L.; Chen, J.; Man, H.; Gong, C.; Lu, Y.; et al. Opposite effects of two estrogen receptors on tau phosphorylation through disparate effects on the miR-218/ PTPA pathway. Aging Cell 2015, 14, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Fu, C.L.; Liang, L.; Yang, B.; Shen, W.; Wang, Q.W.; Chen, Y.; Chen, Y.F.; Liu, Y.N.; Zhu, L.; et al. miR-218-2 regulates cognitive functions in the hippocampus through complement component 3-dependent modulation of synaptic vesicle release. Proc. Natl. Acad. Sci. USA 2021, 118, e2021770118. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Z.Y.; The Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing; Thalamuthu, A.; Cheng, L.; Fowler, C.; Masters, C.L.; Sachdev, P.; Mather, K.A. Differential blood miRNA expression in brain amyloid imaging-defined Alzheimer’s disease and controls. Alzheimer’s Res. Ther. 2020, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Snow, W.M.; Albensi, B.C. Neuronal gene targets of NF-κB and their dysregulation in alzheimer’s disease. Front. Mol. Neurosci. 2016, 9, 118. [Google Scholar] [CrossRef]
- Kern, F.; Fehlmann, T.; Solomon, J.; Schwed, L.; Grammes, N.; Backes, C.; Van Keuren-Jensen, K.; Craig, D.W.; Meese, E.; Keller, A. miEAA 2.0: Integrating multi-species microRNA enrichment analysis and workflow management systems. Nucleic Acids Res. 2020, 48, W521–W528. [Google Scholar] [CrossRef]
- Serpente, M.; Bonsi, R.; Scarpini, E.; Galimberti, D. Innate Immune System and Inflammation in Alzheimer’s Disease: From Pathogenesis to Treatment. Neuroimmunomodulation 2014, 21, 79–87. [Google Scholar] [CrossRef]
- Kinney, J.W.; BeMiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of parkinson’s disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef] [Green Version]
- La Cognata, V.; Morello, G.; D’Agata, V.; Cavallaro, S. Copy number variability in Parkinson’s disease: Assembling the puzzle through a systems biology approach. Hum. Genet. 2017, 136, 13–37. [Google Scholar] [CrossRef] [Green Version]
- La Cognata, V.; Morello, G.; Cavallaro, S. Omics Data and Their Integrative Analysis to Support Stratified Medicine in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 4820. [Google Scholar] [CrossRef] [PubMed]
- La Cognata, V.; Morello, G.; Gentile, G.; D’Agata, V.; Criscuolo, C.; Cavalcanti, F.; Cavallaro, S. A customized high-resolution array-comparative genomic hybridization to explore copy number variations in Parkinson’s disease. Neurogenetics 2016, 17, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, G.; La Cognata, V.; Cavallaro, S. The contribution of CNVs to the most common aging-related neurodegenerative diseases. Aging Clin. Exp. Res. 2020, 33, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Inoue, K.; Ishii, J.; Vanti, W.B.; Voronov, S.V.; Murchison, E.; Hannon, G.; Abeliovich, A. A MicroRNA Feedback Circuit in Midbrain Dopamine Neurons. Science 2007, 317, 1220–1224. [Google Scholar] [CrossRef] [Green Version]
- Schlaudraff, F.; Gründemann, J.; Fauler, M.; Dragicevic, E.; Hardy, J.; Liss, B. Orchestrated increase of dopamine and PARK mRNAs but not miR-133b in dopamine neurons in Parkinson’s disease. Neurobiol. Aging 2014, 35, 2302–2315. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.; Li, L.; Liu, X.; Tian, B.; Cheng, Y. Down regulation of miR -218, miR -124, and miR -144 relates to Parkinson’s disease via activating NF-κB signaling. Kaohsiung J. Med Sci. 2020, 36, 786–792. [Google Scholar] [CrossRef]
- Nair, V.D.; Ge, Y. Alterations of miRNAs reveal a dysregulated molecular regulatory network in Parkinson’s disease striatum. Neurosci. Lett. 2016, 629, 99–104. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Zhang, Y.-F.; Wang, H.; Ren, R.-J.; Cui, H.-L.; Huang, W.-Y.; Cheng, Q.; Chen, H.-Z.; Wang, G. miR-425 deficiency promotes necroptosis and dopaminergic neurodegeneration in Parkinson’s disease. Cell Death Dis. 2019, 10, 589. [Google Scholar] [CrossRef] [Green Version]
- Gomes, L.C.; Galhoz, A.; Jain, G.; Roser, A.; Maass, F.; Carboni, E.; Barski, E.; Lenz, C.; Lohmann, K.; Klein, C.; et al. Multi-omic landscaping of human midbrains identifies disease-relevant molecular targets and pathways in advanced-stage Parkinson’s disease. Clin. Transl. Med. 2022, 12, e692. [Google Scholar] [CrossRef]
- Nicolas, A.; Kenna, K.P.; Renton, A.E.; Ticozzi, N.; Faghri, F.; Chia, R.; Dominov, J.A.; Kenna, B.J.; Nalls, M.A.; Keagle, P.; et al. Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron 2018, 97, 1268–1283.e6. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Lee, Y.; McKenna, N.D.; Yi, M.; Simunovic, F.; Wang, Y.; Kong, B.; Rooney, R.J.; Seo, H.; Stephens, R.M.; et al. miR-126 contributes to Parkinson’s disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiol. Aging 2014, 35, 1712–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, C.E.; Wang, Y.; Kong, B.; Woo, T.-U.W.; Iyer, L.K.; Sonntag, K.C. Midbrain dopamine neurons in Parkinson’s disease exhibit a dysregulated miRNA and target-gene network. Brain Res. 2015, 1618, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantano, L.; Friedländer, M.R.; Escaramís, G.; Lizano, E.; Pallarès-Albanell, J.; Ferrer, I.; Estivill, X.; Martí, E. Specific small-RNA signatures in the amygdala at premotor and motor stages of Parkinson’s disease revealed by deep sequencing analysis. Bioinformatics 2016, 32, 673–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, M.; Sommer, A.; Plötz, S.; Farrell, M.; Winner, B.; Grosch, J.; Winkler, J.; Riemenschneider, M.J. Sporadic Parkinson’s disease derived neuronal cells show disease-specific mRNA and small RNA signatures with abundant deregulation of piRNAs. Acta Neuropathol. Commun. 2018, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Hoss, A.G.; Labadorf, A.; Beach, T.G.; Latourelle, J.C.; Myers, R.H. microRNA Profiles in Parkinson’s Disease Prefrontal Cortex. Front. Aging Neurosci. 2016, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Huang, M.; Chen, L. Mechanism of miR-132-3p Promoting Neuroinflammation and Dopaminergic Neurodegeneration in Parkinson’s Disease. Eneuro 2022, 9, ENEURO.0393-21.2021. [Google Scholar] [CrossRef]
- Tatura, R.; Kraus, T.; Giese, A.; Arzberger, T.; Buchholz, M.; Höglinger, G.; Müller, U. Parkinson’s disease: SNCA-, PARK2-, and LRRK2- targeting microRNAs elevated in cingulate gyrus. Park. Relat. Disord. 2016, 33, 115–121. [Google Scholar] [CrossRef]
- Tolosa, E.; Botta-Orfila, T.; Morató, X.; Calatayud, C.; Ferrer-Lorente, R.; Martí, M.-J.; Fernández, M.; Gaig, C.; Raya, A.; Consiglio, A.; et al. MicroRNA alterations in iPSC-derived dopaminergic neurons from Parkinson disease patients. Neurobiol. Aging 2018, 69, 283–291. [Google Scholar] [CrossRef]
- Chen, Q.; Deng, N.; Lu, K.; Liao, Q.; Long, X.; Gou, D.; Bi, F.; Zhou, J. Elevated plasma miR-133b and miR-221-3p as biomarkers for early Parkinson’s disease. Sci. Rep. 2021, 11, 15268. [Google Scholar] [CrossRef]
- Martins, M.; Rosa, A.; Guedes, L.C.; Fonseca, B.V.; Gotovac, K.; Violante, S.; Mestre, T.; Coelho, M.; Rosa, M.M.; Martin, E.R.; et al. Convergence of miRNA Expression Profiling, α-Synuclein Interacton and GWAS in Parkinson’s Disease. PLoS ONE 2011, 6, e25443. [Google Scholar] [CrossRef]
- Chi, J.; Xie, Q.; Jia, J.; Liu, X.; Sun, J.; Deng, Y.; Yi, L. Integrated Analysis and Identification of Novel Biomarkers in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Zago, E.; Molin, A.D.; Dimitri, G.M.; Xumerle, L.; Pirazzini, C.; Bacalini, M.G.; Maturo, M.G.; Azevedo, T.; Spasov, S.; Gómez-Garre, P.; et al. Early downregulation of hsa-miR-144-3p in serum from drug-naïve Parkinson’s disease patients. Sci. Rep. 2022, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Xiao, Y.; Huang, S.; Cen, L.; Chen, X.; Zhang, L.; Luo, Q.; Li, S.; Yang, X.; Lin, X.; et al. MicroRNA expressing profiles in A53T mutant alpha-synuclein transgenic mice and Parkinsonian. Oncotarget 2017, 8, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, Y.; Qian, J.; Wang, C. Aberrant expression of microRNA-132-3p and microRNA-146a-5p in Parkinson’s disease patients. Open Life Sci. 2020, 15, 647–653. [Google Scholar] [CrossRef]
- Nonaka, W.; Takata, T.; Iwama, H.; Komatsubara, S.; Kobara, H.; Kamada, M.; Deguchi, K.; Touge, T.; Miyamoto, O.; Nakamura, T.; et al. A cerebrospinal fluid microRNA analysis: Progressive supranuclear palsy. Mol. Med. Rep. 2022, 25, 88. [Google Scholar] [CrossRef]
- Sheinerman, K.S.; Toledo, J.; Tsivinsky, V.G.; Irwin, D.; Grossman, M.; Weintraub, D.; Hurtig, H.I.; Chen-Plotkin, A.; Wolk, D.A.; McCluskey, L.F.; et al. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimer’s Res. Ther. 2017, 9, 89. [Google Scholar] [CrossRef]
- Ding, H.; Huang, Z.; Chen, M.; Wang, C.; Chen, X.; Chen, J.; Zhang, J. Identification of a panel of five serum miRNAs as a biomarker for Parkinson’s disease. Park. Relat. Disord. 2016, 22, 68–73. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Wang, C.; Xu, F.; Wang, M.; Liu, Y. Serum miR-221 serves as a biomarker for Parkinson’s disease. Cell Biochem. Funct. 2016, 34, 511–515. [Google Scholar] [CrossRef]
- Ghit, A.; El Deeb, H. Cytokines, miRNAs, and Antioxidants as Combined Non-invasive Biomarkers for Parkinson’s Disease. J. Mol. Neurosci. 2022, 72, 1133–1140. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, R.; Hu, B.-L.; Lu, P.; Zhou, L.-L.; He, Z.-Y.; Wu, H.-M.; Zhu, J.-H. Reduced Circulating Levels of miR-433 and miR-133b Are Potential Biomarkers for Parkinson’s Disease. Front. Cell. Neurosci. 2017, 11, 170. [Google Scholar] [CrossRef] [Green Version]
- Manna, I.; Quattrone, A.; De Benedittis, S.; Vescio, B.; Iaccino, E.; Quattrone, A. Exosomal miRNA as peripheral biomarkers in Parkinson’s disease and progressive supranuclear palsy: A pilot study. Park. Relat. Disord. 2021, 93, 77–84. [Google Scholar] [CrossRef]
- He, S.; Huang, L.; Shao, C.; Nie, T.; Xia, L.; Cui, B.; Lu, F.; Zhu, L.; Chen, B.; Yang, Q. Several miRNAs derived from serum extracellular vesicles are potential biomarkers for early diagnosis and progression of Parkinson’s disease. Transl. Neurodegener. 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Soreq, L.; Salomonis, N.; Bronstein, M.; Greenberg, D.S.; Israel, Z.; Bergman, H.; Soreq, H. Small RNA sequencing-microarray analyses in Parkinson leukocytes reveal deep brain stimulation-induced splicing changes that classify brain region transcriptomes. Front. Mol. Neurosci. 2013, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, F.C.; Iop, R.D.R.; Vietta, G.G.; Kair, D.A.; Filho, P.G.; De Alvarenga, J.G.S.; Da Silva, R. microRNAs involved in Parkinson’s disease: A systematic review. Mol. Med. Rep. 2016, 14, 4015–4022. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C. miR-124 and Parkinson’s disease: A biomarker with therapeutic potential. Pharmacol. Res. 2019, 150, 104515. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Pan, X.; Zhang, J.; Ma, A.; Yang, S.; Ma, J.; Xie, A. Plasma levels of miR-137 and miR-124 are associated with Parkinson’s disease but not with Parkinson’s disease with depression. Neurol. Sci. 2017, 38, 761–767. [Google Scholar] [CrossRef]
- Ravanidis, S.; Bougea, A.; Papagiannakis, N.; Koros, C.; Simitsi, A.M.; Pachi, I.; Breza, M.; Stefanis, L.; Doxakis, E. Validation of differentially expressed brain-enriched microRNAs in the plasma of PD patients. Ann. Clin. Transl. Neurol. 2020, 7, 1594–1607. [Google Scholar] [CrossRef]
- Xie, S.; Niu, W.; Xu, F.; Wang, Y.; Hu, S.; Niu, C. Differential expression and significance of miRNAs in plasma extracellular vesicles of patients with Parkinson’s disease. Int. J. Neurosci. 2020, 1–16. [Google Scholar] [CrossRef]
- Marques, T.M.; Kuiperij, B.; Bruinsma, I.B.; Van Rumund, A.; Aerts, M.B.; Esselink, R.A.J.; Bloem, B.R.; Verbeek, M.M. MicroRNAs in Cerebrospinal Fluid as Potential Biomarkers for Parkinson’s Disease and Multiple System Atrophy. Mol. Neurobiol. 2017, 54, 7736–7745. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, S.R.; Dionísio, P.; Guedes, L.C.; Gonçalves, N.; Coelho, M.; Rosa, M.M.; Amaral, J.D.; Ferreira, J.J.; Rodrigues, C.M.P. Circulating Inflammatory miRNAs Associated with Parkinson’s Disease Pathophysiology. Biomolecules 2020, 10, 945. [Google Scholar] [CrossRef]
- Zhao, N.; Jin, L.; Fei, G.; Zheng, Z.; Zhong, C. Serum microRNA-133b is associated with low ceruloplasmin levels in Parkinson’s disease. Park. Relat. Disord. 2014, 20, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Liu, H.; Zhang, L.; Lv, W.; Hu, X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starhof, C.; Hejl, A.-M.; Heegaard, N.H.; Carlsen, A.L.; Burton, M.; Lilje, B.; Winge, K. The biomarker potential of cell-free microRNA from cerebrospinal fluid in Parkinsonian Syndromes. Mov. Disord. 2019, 34, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Chai, S.; Xiong, T.; Wei, J.; Mao, W.; Zhu, Y.; Li, X.; Wei, W.; Dai, X.; Yang, B.; et al. Aberrant Expression of Circulating MicroRNA Leads to the Dysregulation of Alpha-Synuclein and Other Pathogenic Genes in Parkinson’s Disease. Front. Cell Dev. Biol. 2021, 9, 695007. [Google Scholar] [CrossRef] [PubMed]
- Vallelunga, A.; Ragusa, M.; Di Mauro, S.; Iannitti, T.; Pilleri, M.; Biundo, R.; Weis, L.; Di Pietro, C.S.; De Iuliis, A.; Nicoletti, A.; et al. Identification of circulating microRNAs for the differential diagnosis of Parkinson’s disease and Multiple System Atrophy. Front. Cell. Neurosci. 2014, 8, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alieva, A.K.; Filatova, E.V.; Karabanov, A.V.; Illarioshkin, S.N.; Limborska, S.A.; Shadrina, M.I.; Slominsky, P.A. miRNA expression is highly sensitive to a drug therapy in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, T.; Li, S.; Wei, M.; Qi, H.; Shen, B.; Chang, R.C.-C.; Le, W.; Piao, F. Altered Expression Levels of MicroRNA-132 and Nurr1 in Peripheral Blood of Parkinson’s Disease: Potential Disease Biomarkers. ACS Chem. Neurosci. 2019, 10, 2243–2249. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Gao, L.; Wu, Z.; Wang, L.; Fan, L. Let-7b-5p promotes cell apoptosis in Parkinson’s disease by targeting HMGA2. Mol. Med. Rep. 2021, 24, 820. [Google Scholar] [CrossRef]
- Villar-Menéndez, I.; Porta, S.; Buira, S.P.; Pereira-Veiga, T.; Díaz-Sánchez, S.; Albasanz, J.L.; Ferrer, I.; Martín, M.; Barrachina, M. Increased striatal adenosine A2A receptor levels is an early event in Parkinson’s disease-related pathology and it is potentially regulated by miR-34b. Neurobiol. Dis. 2014, 69, 206–214. [Google Scholar] [CrossRef]
- Miñones-Moyano, E.; Porta, S.; Escaramís, G.; Rabionet, R.; Iraola-Guzmán, S.; Kagerbauer, B.; Espinosa-Parrilla, Y.; Ferrer, I.; Estivill, X.; Martí, E. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum. Mol. Genet. 2011, 20, 3067–3078. [Google Scholar] [CrossRef]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Mouradian, M.M.; Junn, E. Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett. 2014, 589, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.; Zhu, Z.; Wu, J.; Zhang, Y.; Zhang, H.; Sun, X.; Qian, C.; Wang, B.; Xie, L.; Zhang, S.; et al. MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J. 2019, 33, 8648–8665. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, Y.; Zhu, Z.; Mo, L.; Lin, C.; Wang, Q.; Wang, H.; Gong, X.; He, X.; Lu, G.; et al. MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of Parkinson’s Disease by Targeting to Bim. Brain Pathol. 2016, 26, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-D.; Tong, L.; Xu, N.; Ye, Y.; Nie, P.-Y.; Wang, Z.-Y.; Ji, L.-L. miR-124 regulates cerebromicrovascular function in APP/PS1 transgenic mice via C1ql3. Brain Res. Bull. 2019, 153, 214–222. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, B.; Tai, L.; Liu, H.; Shi, F.; Liu, N. The Neuroprotective Role of MiR-124-3p in a 6-Hydroxydopamine-Induced Cell Model of Parkinson’s Disease via the Regulation of ANAX5. J. Cell. Biochem. 2018, 119, 269–277. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Zhai, H. MicroRNA-124 Enhances Dopamine Receptor Expression and Neuronal Proliferation in Mouse Models of Parkinson’s Disease via the Hedgehog Signaling Pathway by Targeting EDN2. Neuroimmunomodulation 2019, 26, 174–187. [Google Scholar] [CrossRef]
- Yao, L.; Ye, Y.; Mao, H.; Lu, F.; He, X.; Lu, G.; Zhang, S. MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson’s disease. J. Neuroinflamm. 2018, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Geng, L.; Liu, W.; Chen, Y. miR-124-3p attenuates MPP+-induced neuronal injury by targeting STAT3 in SH-SY5Y cells. Exp. Biol. Med. 2017, 242, 1757–1764. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Fan, K.; Zhao, L.-J.; Wei, J.-M.; Gao, J.-X.; Li, Z.-F. Long non-coding RNA nuclear enriched abundant transcript 1 (NEAT1) sponges microRNA-124-3p to up-regulate phosphodiesterase 4B (PDE4B) to accelerate the progression of Parkinson’s disease. Bioengineered 2021, 12, 708–719. [Google Scholar] [CrossRef]
- Xie, S.-P.; Zhou, F.; Li, J.; Duan, S.-J. NEAT1 regulates MPP+-induced neuronal injury by targeting miR-124 in neuroblastoma cells. Neurosci. Lett. 2019, 708, 134340. [Google Scholar] [CrossRef]
- Gong, X.; Wang, H.; Ye, Y.; Shu, Y.; Deng, Y.; He, X.; Lu, G.; Zhang, S. miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson’s disease. Am. J. Transl. Res. 2016, 8, 2127–2137. [Google Scholar] [PubMed]
- Lu, Y.; Gong, Z.; Jin, X.; Zhao, P.; Zhang, Y.; Wang, Z. LncRNA MALAT1 targeting miR-124-3p regulates DAPK1 expression contributes to cell apoptosis in Parkinson’s Disease. J. Cell. Biochem. 2020, 121, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, N.; Beiping, H.; Dheen, S.T.; Tay, S. Downregulation of miR-124 in MPTP-treated mouse model of Parkinson’s disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/cdk5 pathway proteins. Neuroscience 2014, 272, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Han, X.; Jia, Y.; Zhang, B. Inhibition of long non-coding RNA HOXA11-AS against neuroinflammation in Parkinson’s disease model via targeting miR-124-3p mediated FSTL1/NF-κB axis. Aging 2021, 13, 11455–11469. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Zhao, B.; Jia, C.; Lv, Y.; Liao, J.; Li, K. Long non-coding RNA NEAT1 mediates MPTP/MPP+-induced apoptosis via regulating the miR-124/KLF4 axis in Parkinson’s disease. Open Life Sci. 2020, 15, 665–676. [Google Scholar] [CrossRef]
- Fan, J.; Wu, D.; Guo, Y.; Yang, Z. SOS1-IT1 silencing alleviates MPP+-induced neuronal cell injury through regulating the miR-124-3p/PTEN/AKT/mTOR pathway. J. Clin. Neurosci. 2022, 99, 137–146. [Google Scholar] [CrossRef]
- Han, Y.-P.; Liu, Z.-J.; Bao, H.-H.; Wang, Q.; Su, L.-L. miR-126-5p Targets SP1 to Inhibit the Progression of Parkinson’s Disease. Eur. Neurol. 2022, 85, 235–244. [Google Scholar] [CrossRef]
- Song, Z.; Xie, B. LncRNA OIP5-AS1 reduces α-synuclein aggregation and toxicity by targeting miR-126 to activate PLK2 in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 2021, 740, 135482. [Google Scholar] [CrossRef]
- Lin, Q.; Hou, S.; Dai, Y.; Jiang, N.; Lin, Y. LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson’s disease through RAB3IP. Biol. Chem. 2019, 400, 1217–1228. [Google Scholar] [CrossRef]
- Kim, W.; Noh, H.; Lee, Y.; Jeon, J.; Shanmugavadivu, A.; McPHIE, D.L.; Kim, K.-S.; Cohen, B.M.; Seo, H.; Sonntag, K.C. MiR-126 Regulates Growth Factor Activities and Vulnerability to Toxic Insult in Neurons. Mol. Neurobiol. 2016, 53, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Schulz, J.; Takousis, P.; Wohlers, I.; Itua, I.O.; Dobricic, V.; Rücker, G.; Binder, H.; Middleton, L.; Ioannidis, J.P.; Perneczky, R.; et al. Meta-analyses identify differentially expressed microRNAs in Parkinson’s disease. Ann. Neurol. 2019, 85, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Kang, Y.; Liu, S.; Wang, Y.; Wang, Y.; Wang, L. LncRNA MIAT Inhibits MPP+-Induced Neuronal Damage Through Regulating the miR-132/SIRT1 Axis in PC12 Cells. Neurochem. Res. 2021, 46, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, M.; Li, Q.; Pei, X.; Zhu, X. miR-132-5p regulates apoptosis and autophagy in MPTP model of Parkinson’s disease by targeting ULK1. Neuroreport 2020, 31, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, T.; Wang, Y.; Tang, Y.; Cui, H.; Tang, Y.; Zhang, X.; Chen, D.; Shen, N.; Le, W. miR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. J. Cell Sci. 2012, 125, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Qazi, T.J.; Lu, J.; Duru, L.; Zhao, J.; Qing, H. Upregulation of mir-132 induces dopaminergic neuronal death via activating SIRT1/P53 pathway. Neurosci. Lett. 2021, 740, 135465. [Google Scholar] [CrossRef]
- Coccia, E.; Masanas, M.; López-Soriano, J.; Segura, M.F.; Comella, J.X.; Pérez-García, M.J. FAIM Is Regulated by MiR-206, MiR-1-3p and MiR-133b. Front. Cell Dev. Biol. 2020, 8, 584606. [Google Scholar] [CrossRef]
- Niu, M.; Xu, R.; Wang, J.; Hou, B.; Xie, A. MiR-133b ameliorates axon degeneration induced by MPP+ via targeting RhoA. Neuroscience 2016, 325, 39–49. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Wang, M.-H.; Yang, H.-C.; Tian, T.; Sun, G.-F.; Ji, Y.-F.; Hu, W.-T.; Liu, X.; Wang, J.-P.; Lu, H. Dopaminergic neuron injury in Parkinson’s disease is mitigated by interfering lncRNA SNHG14 expression to regulate the miR-133b/ α-synuclein pathway. Aging 2019, 11, 9264–9279. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, J.; Lv, Y.; Song, C.; Ding, J.; Xiao, M.; Lu, M.; Hu, G. Kir6.2 Deficiency Promotes Mesencephalic Neural Precursor Cell Differentiation via Regulating miR-133b/GDNF in a Parkinson’s Disease Mouse Model. Mol. Neurobiol. 2018, 55, 8550–8562. [Google Scholar] [CrossRef]
- Dong, L.G.; Lu, F.F.; Zu, J.; Zhang, W.; Xu, C.Y.; Jin, G.L.; Yang, X.X.; Xiao, Q.H.; Cui, C.C.; Xu, R.; et al. MiR-133b inhibits MPP+-induced apoptosis in Parkinson’s disease model by inhibiting the ERK1/2 signaling pathway. Eur. Rev. Med. Pharm. Sci. 2020, 24, 11192–11198. [Google Scholar] [CrossRef]
- He, X.; Yang, L.; Huang, R.; Lin, L.; Shen, Y.; Cheng, L.; Jin, L.; Wang, S.; Zhu, R. Activation of CB2R with AM1241 ameliorates neurodegeneration via the Xist/miR-133b-3p/Pitx3 axis. J. Cell. Physiol. 2020, 235, 6032–6042. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, J.; Ji, C.; Wang, A.L. MiR-144-3p and Its Target Gene β-Amyloid Precursor Protein Regulate 1-Methyl-4-Phenyl-1,2-3,6-Tetrahydropyridine-Induced Mitochondrial Dysfunction. Mol. Cells 2016, 39, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Zhang, D.; Guo, J.; Chen, Z.; Chen, Y.; Zhang, J. Long non-coding RNA NORAD functions as a microRNA-204-5p sponge to repress the progression of Parkinson’s disease in vitro by increasing the solute carrier family 5 member 3 expression. IUBMB Life 2020, 72, 2045–2055. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Yeh, T.-H.; Chen, R.-S.; Chen, H.-C.; Huang, Y.-Z.; Weng, Y.-H.; Cheng, Y.-C.; Liu, Y.-C.; Cheng, A.-J.; Lu, Y.-C.; et al. Upregulated Expression of MicroRNA-204-5p Leads to the Death of Dopaminergic Cells by Targeting DYRK1A-Mediated Apoptotic Signaling Cascade. Front. Cell. Neurosci. 2019, 13, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Pan, X.; Wang, X.; Cao, Y.; Chen, P.; Du, C.; Huang, D. Rab6c is a new target of miR-218 that can promote the progression of bladder cancer. Mol. Med. Rep. 2021, 24, 792. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, H.; Yin, H.; Geng, S.; Liu, Y.; Liu, C.; Zhao, J.; Liu, Y.; Wang, X.; Wang, Y. Up-regulated microRNA-218-5p ameliorates the damage of dopaminergic neurons in rats with Parkinson’s disease via suppression of LASP1. Brain Res. Bull. 2021, 166, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Di Rita, A.; Maiorino, T.; Bruqi, K.; Volpicelli, F.; Bellenchi, G.C.; Strappazzon, F. miR-218 Inhibits Mitochondrial Clearance by Targeting PRKN E3 Ubiquitin Ligase. Int. J. Mol. Sci. 2020, 21, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, Y.; Zhang, H.; Yu, H.; Li, Y.; Liu, X.; Li, M. Long non-coding RNA myocardial infarction-associated transcript promotes 1-Methyl-4-phenylpyridinium ion-induced neuronal inflammation and oxidative stress in Parkinson’s disease through regulating microRNA-221-3p/ transforming growth factor /nuclear factor E2-related factor 2 axis. Bioengineered 2021, 13, 930–940. [Google Scholar] [CrossRef]
- Lang, Y.; Li, Y.; Yu, H.; Lin, L.; Chen, X.; Wang, S.; Zhang, H. HOTAIR drives autophagy in midbrain dopaminergic neurons in the substantia nigra compacta in a mouse model of Parkinson’s disease by elevating NPTX2 via miR-221-3p binding. Aging 2020, 12, 7660–7678. [Google Scholar] [CrossRef]
- Qian, C.; Ye, Y.; Mao, H.; Yao, L.; Sun, X.; Wang, B.; Zhang, H.; Xie, L.; Zhang, H.; Zhang, Y.; et al. Downregulated lncRNA-SNHG1 enhances autophagy and prevents cell death through the miR-221/222 /p27/mTOR pathway in Parkinson’s disease. Exp. Cell Res. 2019, 384, 111614. [Google Scholar] [CrossRef]
- Oh, S.E.; Park, H.-J.; He, L.; Skibiel, C.; Junn, E.; Mouradian, M.M. The Parkinson’s disease gene product DJ-1 modulates miR-221 to promote neuronal survival against oxidative stress. Redox Biol. 2018, 19, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Asci, R.; Vallefuoco, F.; Andolfo, I.; Bruno, M.; De Falco, L.; Iolascon, A. Trasferrin receptor 2 gene regulation by microRNA 221 in SH-SY5Y cells treated with MPP+ as Parkinson’s disease cellular model. Neurosci. Res. 2013, 77, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zongaro, S.; Hukema, R.; D’Antoni, S.; Davidovic, L.; Barbry, P.; Catania, M.V.; Willemsen, R.; Mari, B.; Bardoni, B. The 3’ UTR of FMR1 mRNA is a target of miR-101, miR-129-5p and miR-221: Implications for the molecular pathology of FXTAS at the synapse. Hum. Mol. Genet. 2013, 22, 1971–1982. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, C.; Tao, H.; Yao, S.; Wu, X. LINC00943 acts as miR-338-3p sponge to promote MPP+-induced SK-N-SH cell injury by directly targeting SP1 in Parkinson’s disease. Brain Res. 2022, 1782, 147814. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Hu, K.; Liu, H. Downregulation of long noncoding RNA SNHG7 protects against inflammation and apoptosis in Parkinson’s disease model by targeting the miR-425-5p/TRAF5/NF-κB axis. J. Biochem. Mol. Toxicol. 2021, 35, e22867. [Google Scholar] [CrossRef]
- Chiò, A.; Logroscino, G.; Traynor, B.; Collins, J.; Simeone, J.; Goldstein, L.; White, L. Global Epidemiology of Amyotrophic Lateral Sclerosis: A Systematic Review of the Published Literature. Neuroepidemiology 2013, 41, 118–130. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, M.K. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med. Res. Rev. 2019, 39, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, J.; Sun, Q. Aberrant Stress Granule Dynamics and Aggrephagy in ALS Pathogenesis. Cells 2021, 10, 2247. [Google Scholar] [CrossRef]
- Morgan, S.; Orrell, R.W. Pathogenesis of amyotrophic lateral sclerosis. Br. Med. Bull. 2016, 119, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and Cellular Mechanisms Affected in ALS. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef]
- Emde, A.; Eitan, C.; Liou, L.; Libby, R.T.; Rivkin, N.; Magen, I.; Reichenstein, I.; Oppenheim, H.; Eilam, R.; Silvestroni, A.; et al. Dysregulated mi RNA biogenesis downstream of cellular stress and ALS-causing mutations: A new mechanism for ALS. EMBO J. 2015, 34, 2633–2651. [Google Scholar] [CrossRef] [Green Version]
- Dardiotis, E.; Aloizou, A.-M.; Siokas, V.; Patrinos, G.P.; Deretzi, G.; Mitsias, P.; Aschner, M.; Tsatsakis, A. The Role of MicroRNAs in Patients with Amyotrophic Lateral Sclerosis. J. Mol. Neurosci. 2018, 66, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Hamzeiy, H.; Suluyayla, R.; Brinkrolf, C.; Janowski, S.J.; Hofestädt, R.; Allmer, J. Visualization and Analysis of miRNAs Implicated in Amyotrophic Lateral Sclerosis Within Gene Regulatory Pathways. Ger. Med. Data Sci. 2018, 253, 183–187. [Google Scholar] [CrossRef]
- Butti, Z.; Patten, S.A. RNA Dysregulation in Amyotrophic Lateral Sclerosis. Front. Genet. 2019, 9, 712. [Google Scholar] [CrossRef] [Green Version]
- Pansarasa, O.; Gagliardi, S.; Sproviero, D.; Cereda, C. RNA Metabolism and Therapeutics in Amyotrophic Lateral Sclerosis. In Amyotrophic Lateral Sclerosis—Recent Advances and Therapeutic Challenges; Hegde, M.L., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Rinchetti, P.; Rizzuti, M.; Faravelli, I.; Corti, S. MicroRNA Metabolism and Dysregulation in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2017, 55, 2617–2630. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.-C.; Polymenidou, M.; Cleveland, D.W. Converging Mechanisms in ALS and FTD: Disrupted RNA and Protein Homeostasis. Neuron 2013, 79, 416–438. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, K.; Mori, F.; Kakita, A.; Takahashi, H.; Utsumi, J.; Sasaki, H. Analysis of microRNA from archived formalin-fixed paraffin-embedded specimens of amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2014, 2, 173. [Google Scholar] [CrossRef] [Green Version]
- Rizzuti, M.; Filosa, G.; Melzi, V.; Calandriello, L.; Dioni, L.; Bollati, V.; Bresolin, N.; Comi, G.P.; Barabino, S.; Nizzardo, M.; et al. MicroRNA expression analysis identifies a subset of downregulated miRNAs in ALS motor neuron progenitors. Sci. Rep. 2018, 8, 10105. [Google Scholar] [CrossRef]
- Hawley, Z.C.E.; Campos-Melo, D.; Droppelmann, C.A.; Strong, M.J. MotomiRs: miRNAs in Motor Neuron Function and Disease. Front. Mol. Neurosci. 2017, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Romero, C.; Hur, J.; Lunn, J.S.; Paez-Colasante, X.; Bender, D.E.; Yung, R.; Sakowski, S.A.; Feldman, E.L. Expression of microRNAs in human post-mortem amyotrophic lateral sclerosis spinal cords provides insight into disease mechanisms. Mol. Cell. Neurosci. 2016, 71, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, R.; Santini, L.; Colantoni, A.; Peruzzi, G.; de Turris, V.; Alfano, V.; Bozzoni, I.; Rosa, A. FUS Mutant Human Motoneurons Display Altered Transcriptome and microRNA Pathways with Implications for ALS Pathogenesis. Stem Cell Rep. 2017, 9, 1450–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Erchia, A.M.; Gallo, A.; Manzari, C.; Raho, S.; Horner, D.S.; Chiara, M.; Valletti, A.; Aiello, I.; Mastropasqua, F.; Ciaccia, L.; et al. Massive transcriptome sequencing of human spinal cord tissues provides new insights into motor neuron degeneration in ALS. Sci. Rep. 2017, 7, 10046. [Google Scholar] [CrossRef] [PubMed]
- Campos-Melo, D.; Droppelmann, C.A.; He, Z.; Volkening, K.; Strong, M.J. Altered microRNA expression profile in amyotrophic lateral sclerosis: A role in the regulation of NFL mRNA levels. Mol. Brain 2013, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Campos-Melo, D.; Hawley, Z.C.E.; Strong, M.J. Dysregulation of human NEFM and NEFH mRNA stability by ALS-linked miRNAs. Mol. Brain 2018, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Almeida, S.; Lu, Y.; Nishimura, A.L.; Peng, L.; Sun, D.; Wu, B.; Karydas, A.M.; Tartaglia, M.C.; Fong, J.C.; et al. Downregulation of MicroRNA-9 in iPSC-Derived Neurons of FTD/ALS Patients with TDP-43 Mutations. PLoS ONE 2013, 8, e76055. [Google Scholar] [CrossRef] [Green Version]
- Hawley, Z.C.; Campos-Melo, D.; Strong, M.J. MiR-105 and miR-9 regulate the mRNA stability of neuronal intermediate filaments. Implications for the pathogenesis of amyotrophic lateral sclerosis (ALS). Brain Res. 2019, 1706, 93–100. [Google Scholar] [CrossRef]
- Otaegi, G.; Pollock, A.; Hong, J.; Sun, T. MicroRNA miR-9 Modifies Motor Neuron Columns by a Tuning Regulation of FoxP1 Levels in Developing Spinal Cords. J. Neurosci. 2011, 31, 809–818. [Google Scholar] [CrossRef] [Green Version]
- Cong, C.; Liang, W.; Zhang, C.; Wang, Y.; Yang, Y.; Wang, X.; Wang, S.; Huo, D.; Wang, H.; Wang, D.; et al. PAK4 suppresses motor neuron degeneration in hSOD1 G93A-linked amyotrophic lateral sclerosis cell and rat models. Cell Prolif. 2021, 54, e13003. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Martone, J.; Lepore, E.; Casola, I.; Petrucci, A.; Inghilleri, M.; Morlando, M.; Colantoni, A.; Scicchitano, B.M.; Calvo, A.; et al. A longitudinal study defined circulating microRNAs as reliable biomarkers for disease prognosis and progression in ALS human patients. Cell Death Discov. 2021, 7, 4. [Google Scholar] [CrossRef]
- Vrabec, K.; Boštjančič, E.; Koritnik, B.; Leonardis, L.; Grošelj, L.D.; Zidar, J.; Rogelj, B.; Glavač, D.; Ravnik-Glavač, M. Differential Expression of Several miRNAs and the Host Genes AATK and DNM2 in Leukocytes of Sporadic ALS Patients. Front. Mol. Neurosci. 2018, 11, 106. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, C.; Guan, Y.; Chen, Y.; Lu, Q.; Jie, L.; Gao, H.; Du, H.; Zhang, H.; Liu, Y.; et al. Screening the expression characteristics of several miRNAs in G93A-SOD1 transgenic mouse: Altered expression of miRNA-124 is associated with astrocyte differentiation by targeting Sox2 and Sox9. J. Neurochem. 2018, 145, 51–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laneve, P.; Tollis, P.; Caffarelli, E. RNA Deregulation in Amyotrophic Lateral Sclerosis: The Noncoding Perspective. Int. J. Mol. Sci. 2021, 22, 10285. [Google Scholar] [CrossRef]
- Han, D.; Dong, X.; Zheng, D.; Nao, J. MiR-124 and the Underlying Therapeutic Promise of Neurodegenerative Disorders. Front. Pharmacol. 2020, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.R.; Vizinha, D.; Morais, H.; Colaço, A.R.; Loch-Neckel, G.; Barbosa, M.; Brites, D. Overexpression of miR-124 in Motor Neurons Plays a Key Role in ALS Pathological Processes. Int. J. Mol. Sci. 2021, 22, 6128. [Google Scholar] [CrossRef] [PubMed]
- Waller, R.; Wyles, M.; Heath, P.R.; Kazoka, M.; Wollff, H.; Shaw, P.; Kirby, J. Small RNA Sequencing of Sporadic Amyotrophic Lateral Sclerosis Cerebrospinal Fluid Reveals Differentially Expressed miRNAs Related to Neural and Glial Activity. Front. Neurosci. 2018, 11, 731. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Q.; Chen, X.; Li, C.; Cao, B.; Ou, R.; Hadano, S.; Shang, H.-F. Aberration of miRNAs Expression in Leukocytes from Sporadic Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2016, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Koval, E.D.; Shaner, C.; Zhang, P.; du Maine, X.; Fischer, K.; Tay, J.; Chau, B.N.; Wu, G.F.; Miller, T.M. Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum. Mol. Genet. 2013, 22, 4127–4135. [Google Scholar] [CrossRef] [Green Version]
- Raheja, R.; Regev, K.; Healy, B.C.; Mazzola, M.A.; Beynon, V.; Von Glehn, F.; Paul, A.; Diaz-Cruz, C.; Gholipour, T.; Glanz, B.I.; et al. Correlating serum micrornas and clinical parameters in amyotrophic lateral sclerosis. Muscle Nerve 2018, 58, 261–269. [Google Scholar] [CrossRef]
- Tasca, E.; Pegoraro, V.; Merico, A.; Angelini, C. Circulating microRNAs as biomarkers of muscle differentiation and atrophy in ALS. Clin. Neuropathol. 2016, 35, 22–30. [Google Scholar] [CrossRef]
- Gupta, N.; Jadhav, S.; Tan, K.-L.; Saw, G.; Mallilankaraman, K.B.; Dheen, S.T. miR-142-3p Regulates BDNF Expression in Activated Rodent Microglia Through Its Target CAMK2A. Front. Cell. Neurosci. 2020, 14, 132. [Google Scholar] [CrossRef]
- Yardeni, T.; Fine, R.; Joshi, Y.; Gradus-Pery, T.; Kozer, N.; Reichenstein, I.; Yanowski, E.; Nevo, S.; Weiss-Tishler, H.; Eisenberg-Bord, M.; et al. High content image analysis reveals function of miR-124 upstream of Vimentin in regulating motor neuron mitochondria. Sci. Rep. 2018, 8, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandolesi, G.; De Vito, F.; Musella, A.; Gentile, A.; Bullitta, S.; Fresegna, D.; Sepman, H.; Di Sanza, C.; Haji, N.; Mori, F.; et al. miR-142-3p Is a Key Regulator of IL-1β-Dependent Synaptopathy in Neuroinflammation. J. Neurosci. 2017, 37, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-M.; Wen, X.; Han, X.-R.; Wang, S.; Wang, Y.-J.; Shen, M.; Fan, S.-H.; Zhuang, J.; Zhang, Z.-F.; Shan, Q.; et al. MiR-142-3p Enhances Cell Viability and Inhibits Apoptosis by Targeting CDKN1B and TIMP3 Following Sciatic Nerve Injury. Cell. Physiol. Biochem. 2018, 46, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Paladino, S.; Conte, A.; Caggiano, R.; Pierantoni, G.M.; Faraonio, R. Nrf2 Pathway in Age-Related Neurological Disorders: Insights into MicroRNAs. Cell. Physiol. Biochem. 2018, 47, 1951–1976. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Villegas, J.; Ferraiuolo, L.; Mead, R.; Shaw, P.; Cuadrado, A.; Rojo, A. NRF2 as a therapeutic opportunity to impact in the molecular roadmap of ALS. Free Radic. Biol. Med. 2021, 173, 125–141. [Google Scholar] [CrossRef]
- Matamala, J.M.; Arias-Carrasco, R.; Sanchez, C.; Uhrig, M.; Bargsted, L.; Matus, S.; Maracaja-Coutinho, V.; Abarzua, S.; van Zundert, B.; Verdugo, R.; et al. Genome-wide circulating microRNA expression profiling reveals potential biomarkers for amyotrophic lateral sclerosis. Neurobiol. Aging 2018, 64, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Allen, S.; Goodall, E.; Kramer, S.; Ponger, L.-L.; Heath, P.R.; Milo, M.; Hollinger, H.C.; Walsh, T.; Highley, R.; et al. Gene expression signatures in motor neurone disease fibroblasts reveal dysregulation of metabolism, hypoxia-response and RNA processing functions. Neuropathol. Appl. Neurobiol. 2015, 41, 201–226. [Google Scholar] [CrossRef]
- De Luna, N.; Turon-Sans, J.; Cortes-Vicente, E.; Carrasco-Rozas, A.; Illán-Gala, I.; Dols-Icardo, O.; Clarimón, J.; Lleó, A.; Gallardo, E.; Illa, I.; et al. Downregulation of miR-335-5P in Amyotrophic Lateral Sclerosis Can Contribute to Neuronal Mitochondrial Dysfunction and Apoptosis. Sci. Rep. 2020, 10, 4308. [Google Scholar] [CrossRef]
- Fan, W.; Liang, C.; Ou, M.; Zou, T.; Sun, F.; Zhou, H.; Cui, L. MicroRNA-146a Is a Wide-Reaching Neuroinflammatory Regulator and Potential Treatment Target in Neurological Diseases. Front. Mol. Neurosci. 2020, 13, 90. [Google Scholar] [CrossRef]
- Banack, S.A.; Dunlop, R.A.; Cox, P.A. An miRNA fingerprint using neural-enriched extracellular vesicles from blood plasma: Towards a biomarker for amyotrophic lateral sclerosis/motor neuron disease. Open Biol. 2020, 10, 200116. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Guedes, J.R.; de Almeida, L.P.; de Lima, M.C.P. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 2011, 135, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Louafi, F.; Martinez-Nunez, R.T.; Sanchez-Elsner, T. MicroRNA-155 Targets SMAD2 and Modulates the Response of Macrophages to Transforming Growth Factor-β. J. Biol. Chem. 2010, 285, 41328–41336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, D.; Kim, S.-W.; McKeller, M.R.; Dahia, P.L.M.; Aguiar, R.C.T. Targeting of SMAD5 links microRNA-155 to the TGF-β pathway and lymphomagenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3111–3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paez-Colasante, X.; Figueroa-Romero, C.; Sakowski, S.A.; Goutman, S.; Feldman, E. Amyotrophic lateral sclerosis: Mechanisms and therapeutics in the epigenomic era. Nat. Rev. Neurol. 2015, 11, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Jedrychowski, M.P.; Cialic, R.; Krasemann, S.; Murugaiyan, G.; Fanek, Z.; Greco, D.J.; Wu, P.M.; Doykan, C.E.; Kiner, O.; et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann. Neurol. 2015, 77, 75–99. [Google Scholar] [CrossRef]
- Hoye, M.L.; Regan, M.R.; Jensen, L.A.; Lake, A.M.; Reddy, L.V.; Vidensky, S.; Richard, J.-P.; Maragakis, N.J.; Rothstein, J.D.; Dougherty, J.D.; et al. Motor neuron-derived microRNAs cause astrocyte dysfunction in amyotrophic lateral sclerosis. Brain 2018, 141, 2561. [Google Scholar] [CrossRef]
- Reichenstein, I.; Eitan, C.; Diaz-Garcia, S.; Haim, G.; Magen, I.; Siany, A.; Hoye, M.L.; Rivkin, N.; Olender, T.; Toth, B.; et al. Human genetics and neuropathology suggest a link between miR-218 and amyotrophic lateral sclerosis pathophysiology. Sci. Transl. Med. 2019, 11, eaav5264. [Google Scholar] [CrossRef]
- Thiebes, K.P.; Nam, H.; Cambronne, X.; Shen, R.; Glasgow, S.M.; Cho, H.-H.; Kwon, J.-S.; Goodman, R.H.; Lee, J.W.; Lee, S.; et al. miR-218 is essential to establish motor neuron fate as a downstream effector of Isl1–Lhx3. Nat. Commun. 2015, 6, 7718. [Google Scholar] [CrossRef] [Green Version]
- De Felice, B.; Manfellotto, F.; Fiorentino, G.; Annunziata, A.; Biffali, E.; Pannone, R.; Federico, A. Wide-Ranging Analysis of MicroRNA Profiles in Sporadic Amyotrophic Lateral Sclerosis Using Next-Generation Sequencing. Front. Genet. 2018, 9, 310. [Google Scholar] [CrossRef]
- De Felice, B.; Guida, M.; Guida, M.; Coppola, C.; De Mieri, G.; Cotrufo, R. A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene 2012, 508, 35–40. [Google Scholar] [CrossRef]
- Aschrafi, A.; Kar, A.N.; Natera-Naranjo, O.; MacGibeny, M.A.; Gioio, A.E.; Kaplan, B.B. MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encoding subunits of the oxidative phosphorylation machinery. Cell. Mol. Life Sci. 2012, 69, 4017–4027. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Annunziata, A.; Fiorentino, G.; Borra, M.; Biffali, E.; Coppola, C.; Cotrufo, R.; Brettschneider, J.; Giordana, M.L.; Dalmay, T.; et al. miR-338-3p is over-expressed in blood, CFS, serum and spinal cord from sporadic amyotrophic lateral sclerosis patients. Neurogenetics 2014, 15, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Saucier, D.; Wajnberg, G.; Roy, J.; Beauregard, A.-P.; Chacko, S.; Crapoulet, N.; Fournier, S.; Ghosh, A.; Lewis, S.; Marrero, A.; et al. Identification of a circulating miRNA signature in extracellular vesicles collected from amyotrophic lateral sclerosis patients. Brain Res. 2019, 1708, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hou, J.; Lin, L.; Wang, C.; Liu, X.; Li, D.; Ma, F.; Wang, Z.; Cao, X. Inducible microRNA-155 Feedback Promotes Type I IFN Signaling in Antiviral Innate Immunity by Targeting Suppressor of Cytokine Signaling 1. J. Immunol. 2010, 185, 6226–6233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.-F.; Thai, T.-H.; Calado, D.; Chaudhry, A.; Kubo, M.; Tanaka, K.; Loeb, G.B.; Lee, H.; Yoshimura, A.; Rajewsky, K.; et al. Foxp3-Dependent MicroRNA155 Confers Competitive Fitness to Regulatory T Cells by Targeting SOCS1 Protein. Immunity 2009, 30, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Wang, H.; Zhang, Y.; Wei, Y. Neuroprotective effects of miR-142-5p downregulation against isoflurane-induced neurological impairment. Diagn. Pathol. 2020, 15, 70. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, L.; Lu, Y.; Zhang, M.; Zhang, Z.; Wang, K.; Lv, J. Down-regulation of microRNA-142-5p attenuates oxygen-glucose deprivation and reoxygenation-induced neuron injury through up-regulating Nrf2/ARE signaling pathway. Biomed. Pharmacother. 2017, 89, 1187–1195. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Bahroudi, Z.; Abak, A.; Majidpoor, J.; Taheri, M. An update on the role of miR-124 in the pathogenesis of human disorders. Biomed. Pharmacother. 2021, 135, 111198. [Google Scholar] [CrossRef]
- Amini, J.; Bibak, B.; Afshar, A.R.; Sahebkar, A. Evaluation role of miR-124 in neurodegenerative diseases: Literature review and in silico analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Sun, K.-H.; de Pablo, Y.; Vincent, F.; Shah, K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J. Neurochem. 2008, 107, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Vosler, P.S.; Brennan, C.S.; Chen, J. Calpain-Mediated Signaling Mechanisms in Neuronal Injury and Neurodegeneration. Mol. Neurobiol. 2008, 38, 78–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Bi, G.; Han, S.; Huang, R. MicroRNAs Play a Role in Parkinson’s Disease by Regulating Microglia Function: From Pathogenetic Involvement to Therapeutic Potential. Front. Mol. Neurosci. 2022, 14, 358. [Google Scholar] [CrossRef]
- Sadr, N.K.S.; Shafiei, M.; Galehdari, H.; Khirolah, A. The Effect of Sialic Acid on the Expression of miR-218, NF-kB, MMP-9, and TIMP-1. Biochem. Genet. 2020, 58, 883–900. [Google Scholar] [CrossRef]
- Torres-Berrío, A.; Nouel, D.; Cuesta, S.; Parise, E.M.; Restrepo-Lozano, J.M.; Larochelle, P.; Nestler, E.J.; Flores, C. MiR-218: A molecular switch and potential biomarker of susceptibility to stress. Mol. Psychiatry 2020, 25, 951–964. [Google Scholar] [CrossRef]
- Nies, Y.H.; Najib, N.H.M.; Lim, W.L.; Kamaruzzaman, M.A.; Yahaya, M.F.; Teoh, S.L. MicroRNA Dysregulation in Parkinson’s Disease: A Narrative Review. Front. Neurosci. 2021, 15, 660379. [Google Scholar] [CrossRef]
- Rosenblum, L.T.; Trotti, D. EAAT2 and the Molecular Signature of Amyotrophic Lateral Sclerosis. Adv. Neurobiol. 2017, 16, 117–136. [Google Scholar] [CrossRef]
- Qian, Y.; Song, J.; Ouyang, Y.; Han, Q.; Chen, W.; Zhao, X.; Xie, Y.; Chen, Y.; Yuan, W.; Fan, C. Advances in Roles of miR-132 in the Nervous System. Front. Pharmacol. 2017, 8, 770. [Google Scholar] [CrossRef]
- Zhang, M.; Bian, Z. Alzheimer’s Disease and microRNA-132: A Widespread Pathological Factor and Potential Therapeutic Target. Front. Neurosci. 2021, 15, 617. [Google Scholar] [CrossRef]
- Shaik, M.M.; Tamargo, I.A.; Abubakar, M.B.; Kamal, M.A.; Greig, N.H.; Gan, S.H. The Role of microRNAs in Alzheimer’s Disease and Their Therapeutic Potentials. Genes 2018, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishtiaq, M.; Campos-Melo, D.; Volkening, K.; Strong, M.J. Analysis of Novel NEFL mRNA Targeting microRNAs in Amyotrophic Lateral Sclerosis. PLoS ONE 2014, 9, e85653. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.M.; Yu, D. MicroRNAs in common diseases and potential therapeutic applications. Clin. Exp. Pharmacol. Physiol. 2010, 37, 102–107. [Google Scholar] [CrossRef]
- Hébert, S.S.; De Strooper, B. Molecular biology: miRNAs in neurodegeneration. Science 2007, 317, 1179–1180. [Google Scholar] [CrossRef] [PubMed]
- Leggio, L.; Vivarelli, S.; L’ Episcopo, F.; Tirolo, C.; Caniglia, S.; Testa, N.; Marchetti, B.; Iraci, N. microRNAs in Parkinson’s Disease: From Pathogenesis to Novel Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2017, 18, 2698. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Ramaswamy, P.; Pal, P.K.; Christopher, R. Clinical application of circulating micrornas in parkinson’s disease: The challenges and opportunities as diagnostic biomarker. Ann. Indian Acad. Neurol. 2020, 23, 84–97. [Google Scholar] [CrossRef]
- Recasens, A.; Perier, C.; Sue, C.M. Role of microRNAs in the Regulation of alpha-Synuclein Expression: A Systematic Review. Front. Mol. Neurosci. 2016, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Malacarne, C.; Galbiati, M.; Giagnorio, E.; Cavalcante, P.; Salerno, F.; Andreetta, F.; Cagnoli, C.; Taiana, M.; Nizzardo, M.; Corti, S.; et al. Dysregulation of Muscle-Specific MicroRNAs as Common Pathogenic Feature Associated with Muscle Atrophy in ALS, SMA and SBMA: Evidence from Animal Models and Human Patients. Int. J. Mol. Sci. 2021, 22, 5673. [Google Scholar] [CrossRef]
- Garza, M.T.G. MicroRNAs in Amyotrophic Lateral Sclerosis. In Update on Amyotrophic Lateral Sclerosis; Sibat, H.F., de Fatima Ibañez Valdés, L., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Daneshafrooz, N.; Joghataei, M.T.; Mehdizadeh, M.; Alavi, A.; Barati, M.; Panahi, B.; Teimourian, S.; Zamani, B. Identification of let-7f and miR-338 as plasma-based biomarkers for sporadic amyotrophic lateral sclerosis using meta-analysis and empirical validation. Sci. Rep. 2022, 12, 1372. [Google Scholar] [CrossRef]
- Di Gregorio, S.E.; Volkening, K.; Strong, M.J.; Duennwald, M.L. Inclusion Formation and Toxicity of the ALS Protein RGNEF and Its Association with the Microtubule Network. Int. J. Mol. Sci. 2020, 21, 5597. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, S.; Zheng, G.; Chen, D.; Li, P.; Yang, M.; Luo, K.; Yin, J.; Gu, Y.; Zhang, Z.; et al. FOXO3a-driven miRNA signatures suppresses VEGF-A/NRP1 signaling and breast cancer metastasis. Oncogene 2020, 40, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Pyrzewicz, W.; Want, A.; Leszek, J.; Wojda, U. Antisense oligonucleotides for Alzheimer’s disease therapy: From the mRNA to miRNA paradigm. eBioMedicine 2021, 74, 103691. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, M.; Zheng, Q.; Hao, S.; Yang, L.; Xia, Q.; Qi, C.; Ge, J. MicroRNAs in Alzheimer’s disease: Potential diagnostic markers and therapeutic targets. Biomed. Pharmacother. 2022, 148, 112681. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V.; Martinez, B. MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches. Neural Regen. Res. 2022, 17, 728. [Google Scholar] [CrossRef] [PubMed]
- Mathis, S.; Le Masson, G. RNA-Targeted Therapies and Amyotrophic Lateral Sclerosis. Biomedicines 2018, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.; Wang, J.; Zhang, X.; Geng, Y.; Hu, Z.; Rudd, J.A.; Ling, S.; Chen, W.; Han, S. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicol. Lett. 2012, 209, 94–105. [Google Scholar] [CrossRef]
- Gan, L.; Li, Z.; Lv, Q.; Huang, W. Rabies virus glycoprotein (RVG29)-linked microRNA-124-loaded polymeric nanoparticles inhibit neuroinflammation in a Parkinson’s disease model. Int. J. Pharm. 2019, 567, 118449. [Google Scholar] [CrossRef]
- Saraiva, C.; Paiva, J.M.; Santos, T.; Ferreira, L.; Bernardino, L. MicroRNA-124 loaded nanoparticles enhance brain repair in Parkinson’s disease. J. Control. Release 2016, 235, 291–305. [Google Scholar] [CrossRef]
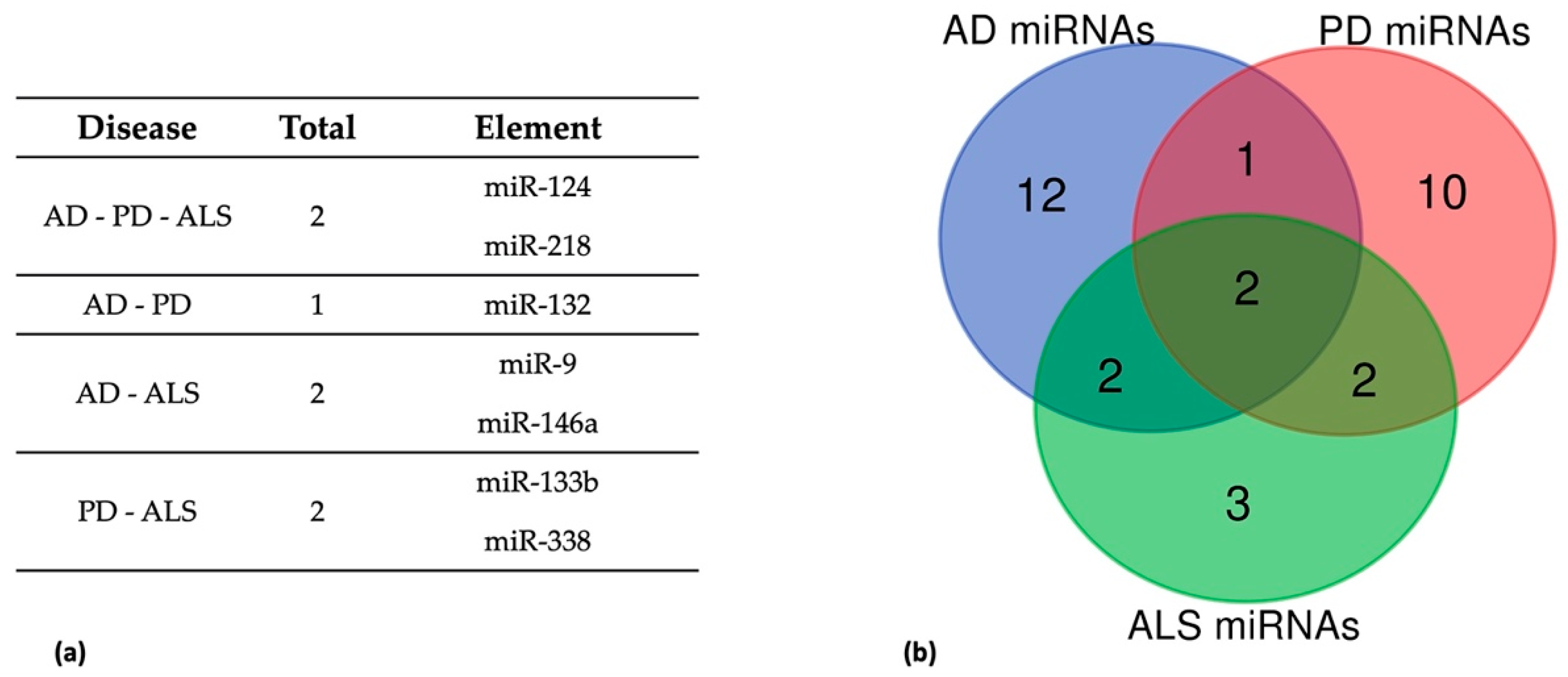
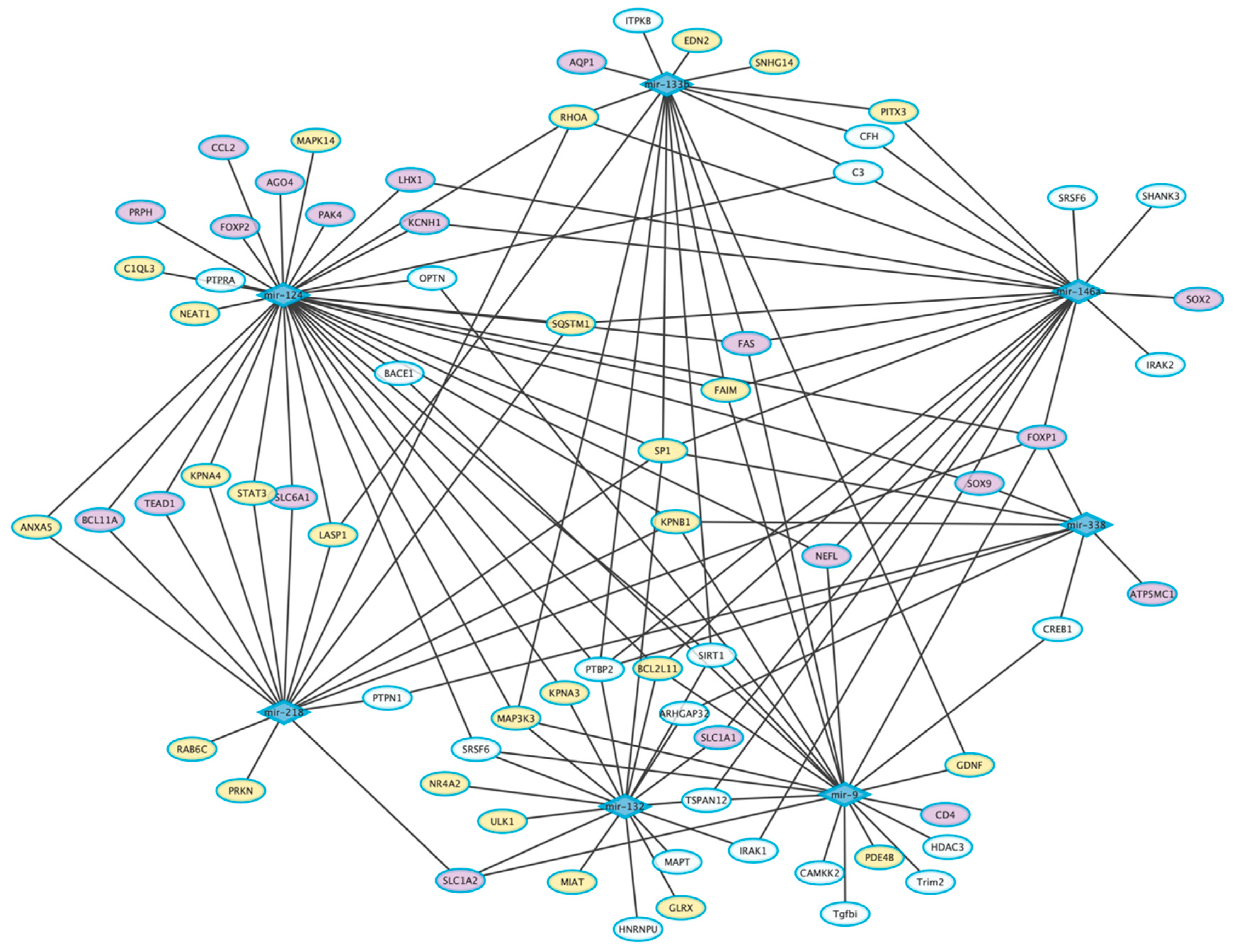
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, G.; Morello, G.; La Cognata, V.; Guarnaccia, M.; Conforti, F.L.; Cavallaro, S. Dysregulated miRNAs as Biomarkers and Therapeutical Targets in Neurodegenerative Diseases. J. Pers. Med. 2022, 12, 770. https://doi.org/10.3390/jpm12050770
Gentile G, Morello G, La Cognata V, Guarnaccia M, Conforti FL, Cavallaro S. Dysregulated miRNAs as Biomarkers and Therapeutical Targets in Neurodegenerative Diseases. Journal of Personalized Medicine. 2022; 12(5):770. https://doi.org/10.3390/jpm12050770
Chicago/Turabian StyleGentile, Giulia, Giovanna Morello, Valentina La Cognata, Maria Guarnaccia, Francesca Luisa Conforti, and Sebastiano Cavallaro. 2022. "Dysregulated miRNAs as Biomarkers and Therapeutical Targets in Neurodegenerative Diseases" Journal of Personalized Medicine 12, no. 5: 770. https://doi.org/10.3390/jpm12050770
APA StyleGentile, G., Morello, G., La Cognata, V., Guarnaccia, M., Conforti, F. L., & Cavallaro, S. (2022). Dysregulated miRNAs as Biomarkers and Therapeutical Targets in Neurodegenerative Diseases. Journal of Personalized Medicine, 12(5), 770. https://doi.org/10.3390/jpm12050770