Sex-Specific Differences in Pre-Stroke Characteristics Reveal Vulnerability of Elderly Women
Abstract
:1. Introduction
2. Materials and Methods
3. Results
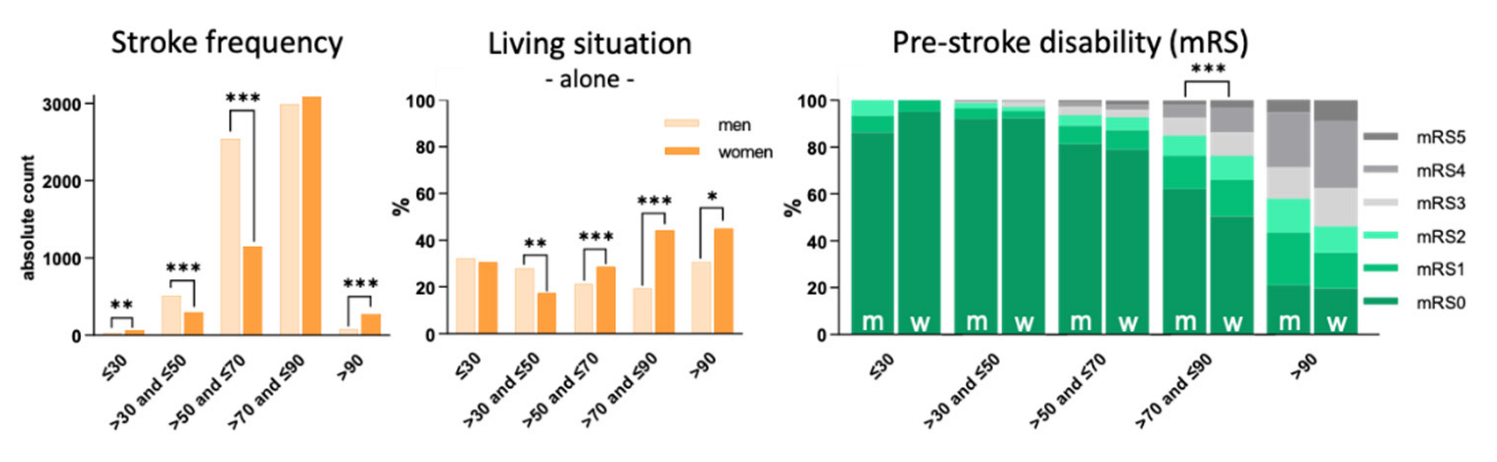
3.1. Living Situation
3.2. Pre-Stroke Disability
3.3. Risk Factors
3.4. Cerebral and Cardiac Vascular Premorbidity
3.5. Stroke Etiology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajsic, S.; Gothe, H.; Borba, H.H.; Sroczynski, G.; Vujicic, J.; Toell, T.; Siebert, U. Economic burden of stroke: A systematic review on post-stroke care. Eur. J. Health Econ. 2019, 20, 107–134. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.S. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar]
- United Nations Development Programme: Ageing, Older Persons and the 2030 Agenda for Sustainable Development. Available online: https://www.un.org/development/Desa/ageing/wpcontent/uploads/sites/24/2017/07/UNDP_AARP_HelpAge_International_AgeingOlderpersons-and-2030-Agenda-2.pdf (accessed on 22 December 2021).
- European Commission: Eurostat. Available online: https://ec.europa.eu/eurostat/documents/10186/10994376/DE-EN.pdf (accessed on 22 December 2021).
- Dahl, S.; Hjalmarsson, C.; Andersson, B. Sex differences in risk factors, treatment, and prognosis in acute stroke. Womens Health 2020, 16, 1745506520952039. [Google Scholar] [CrossRef]
- Medlin, F.; Amiguet, M.; Eskandari, A.; Michel, P. Sex differences in acute ischaemic stroke patients: Clinical presentation, causes and outcomes. Eur. J. Neurol. 2020, 27, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Poorthuis, M.H.; Algra, A.M.; Algra, A.; Kappelle, L.J.; Klijn, C.J. Female- and male-specific risk factors for stroke: A systematic review and meta-analysis. JAMA Neurol. 2017, 74, 75–81. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H.; Rao-Melacini, P.; Zhang, X.; Pais, P.; Agapay, S.; et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (interstroke): A case-control study. Lancet 2016, 388, 761–775. [Google Scholar] [CrossRef]
- Howard, V.J.; Madsen, T.E.; Kleindorfer, D.O.; Judd, S.E.; Rhodes, J.D.; Soliman, E.Z.; Kissela, B.M.; Safford, M.M.; Moy, C.S.; McClure, L.A.; et al. Sex and race differences in the association of incident ischemic stroke with risk factors. JAMA Neurol. 2019, 76, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Bushnell, C.; Howard, V.J.; Lisabeth, L.; Caso, V.; Gall, S.; Kleindorfer, D.; Chaturvedi, S.; Madsen, T.E.; Demel, S.L.; Lee, S.J.; et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. 2018, 17, 641–650. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Hennerici, M.G. New approach to stroke subtyping: The a-s-c-o (phenotypic) classification of stroke. Cerebrovasc. Dis. 2009, 27, 502–508. [Google Scholar] [CrossRef]
- Vyas, M.V.; Silver, F.L.; Austin, P.C.; Yu, A.Y.X.; Pequeno, P.; Fang, J.; Laupacis, A.; Kapral, M.K. Stroke incidence by sex across the lifespan. Stroke 2021, 52, 447–451. [Google Scholar] [CrossRef]
- Marzona, I.; Proietti, M.; Farcomeni, A.; Romiti, G.F.; Romanazzi, I.; Raparelli, V.; Basili, S.; Lip, G.Y.H.; Nobili, A.; Roncaglioni, M.C. Sex differences in stroke and major adverse clinical events in patients with atrial fibrillation: A systematic review and meta-analysis of 993,600 patients. Int. J. Cardiol. 2018, 269, 182–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrenfeldt, L.J.; Moller, S.; Thinggaard, M.; Christensen, K.; Lindahl-Jacobsen, R. Sex differences in comorbidity and frailty in europe. Int. J. Public Health 2019, 64, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M.; Kim, J.K.; Sole-Auro, A. Gender differences in health: Results from share, elsa and hrs. Eur. J. Public Health 2011, 21, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leigh-Hunt, N.; Bagguley, D.; Bash, K.; Turner, V.; Turnbull, S.; Valtorta, N.; Caan, W. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health 2017, 152, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Carney, M.T.; Fujiwara, J.; Emmert, B.E., Jr.; Liberman, T.A.; Paris, B. Elder orphans hiding in plain sight: A growing vulnerable population. Curr. Gerontol. Geriatr. Res. 2016, 2016, 4723250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jonsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, C.; Schmidt, H.L.; Kranaster, L.; Alonso, A. Impact of psychiatric comorbidity on the severity, short-term functional outcome, and psychiatric complications after acute stroke. Neuropsychiatr. Dis. Treat 2019, 15, 1823–1831. [Google Scholar] [CrossRef]
- Taylor-Rowan, M.; Momoh, O.; Ayerbe, L.; Evans, J.J.; Stott, D.J.; Quinn, T.J. Prevalence of pre-stroke depression and its association with post-stroke depression: A systematic review and meta-analysis. Psychol. Med. 2019, 49, 685–696. [Google Scholar] [CrossRef]
- Rutledge, T.; Linke, S.E.; Olson, M.B.; Francis, J.; Johnson, B.D.; Bittner, V.; York, K.; McClure, C.; Kelsey, S.F.; Reis, S.E.; et al. Social networks and incident stroke among women with suspected myocardial ischemia. Psychosom. Med. 2008, 70, 282–287. [Google Scholar] [CrossRef]
- McCarron, M.O.; Armstrong, M.; McCarron, P. Potential for quality improvement of acute stroke management in a district general hospital. Emerg. Med. J. 2008, 25, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Lisabeth, L.D.; Brown, D.L.; Hughes, R.; Majersik, J.J.; Morgenstern, L.B. Acute stroke symptoms: Comparing women and men. Stroke 2009, 40, 2031–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski, C.; Mays-Wilson, K.; Miller, J.; Penstone, P.; Miller, D.J.; Bakoulas, K.; Mitsias, P. Safety and outcomes in stroke mimics after intravenous tissue plasminogen activator administration: A single-center experience. J. Stroke Cerebrovasc. Dis. 2015, 24, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.A.; Sorlie, P.D.; Wolz, M.; Thom, T.; Fields, L.E.; Roccella, E.J. Trends in hypertension prevalence, awareness, treatment, and control rates in united states adults between 1988-1994 and 1999–2004. Hypertension 2008, 52, 818–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.A.E.; Carcel, C.; Millett, E.R.C.; Woodward, M. Sex differences in the association between major risk factors and the risk of stroke in the UK biobank cohort study. Neurology 2020, 95, e2715–e2726. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.M.; Ellis, C. Heterogeneity among women with stroke: Health, demographic and healthcare utilization differentials. BMC Womens Health 2021, 21, 160. [Google Scholar] [CrossRef]
- Leppert, M.H.; Ho, P.M.; Burke, J.; Madsen, T.E.; Kleindorfer, D.; Sillau, S.; Daugherty, S.; Bradley, C.J.; Poisson, S.N. Young women had more strokes than young men in a large, united states claims sample. Stroke 2020, 51, 3352–3355. [Google Scholar] [CrossRef]
- Hirani, S.P.; Beynon, M.; Cartwright, M.; Rixon, L.; Doll, H.; Henderson, C.; Bardsley, M.; Steventon, A.; Knapp, M.; Rogers, A.; et al. The effect of telecare on the quality of life and psychological well-being of elderly recipients of social care over a 12-month period: The whole systems demonstrator cluster randomised trial. Age Ageing 2014, 43, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Ollevier, A.; Aguiar, G.; Palomino, M.; Simpelaere, I.S. How can technology support ageing in place in healthy older adults? A systematic review. Public Health Rev. 2020, 41, 26. [Google Scholar] [CrossRef]
- Nabavi, D.G.; Koennecke, H.C.; Ossenbrink, M.; Grau, A.; Busse, O.; Stroke Unit Kommission; Zertifizierungsausschuss der DSG; Vorstand der DSG; Zertifizierungsausschuss der DSG. Certification criteria for stroke units in germany: Update 2018. Nervenarzt 2019, 90, 335–342. [Google Scholar] [CrossRef]
- Courtin, E.; Knapp, M. Social isolation, loneliness and health in old age: A scoping review. Health Soc. Care Commun. 2017, 25, 799–812. [Google Scholar] [CrossRef]
| ≤30 | >30 and ≤50 | >50 and ≤70 | >70 and ≤90 | >90 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | p-Value [OR] | Men | Women | p-Value [OR] | Men | Women | p-Value [OR] | Men | Women | p-Value [OR] | Men | Women | p-Value [OR] | |
| Risk Factor | |||||||||||||||
| Hypertension, n (%) | 3 (10.3) | 3 (5.0) | 0.387 [0.46] | 229 (44.6) | 116 (39.3) | 0.141 [0.80] | 1945 (76.7) | 897 (78.2) | 0.303 [1.09] | 2570 (86.0) | 2741 (88.8) | 0.001 ** [1.30] | 68 (87.2) | 237 (87.8) | 0.888 [1.06] |
| Diabetes, n (%) | 0 (0.0) | 1 (1.7) | 1.000 [1.02] | 73 (14.2) | 30 (10.2) | 0.096 [0.68] | 766 (30.2) | 322 (28.1) | 0.192 [0.90] | 1021 (34.2) | 971 (31.5) | 0.026 * [0.88] | 17 (21.8) | 56 (20.7) | 0.840 [0.94] |
| Hyperlipidemia, n (%) | 4 (13.8) | 4 (6.7) | 0.430 [0.45] | 150 (29.2) | 50 (16.9) | <0.001 *** [0.49] | 931 (36.7) | 427 (37.2) | 0.757 [1.02] | 1060 (35.5) | 1004 (32.5) | 0.016 * [0.88] | 18 (23.1) | 59 (21.9) | 0.818 [0.93] |
| Smoking, n (%) | 10 (34.5) | 18 (30.0) | 0.669 [0.81] | 225 (43.9) | 119 (40.3) | 0.330 [0.86] | 790 (31.1) | 298 (26.0) | 0.001 ** [0.78] | 239 (8.0) | 131 (4.2) | <0.001 *** [0.51] | 1 (1.3) | 3 (1.1) | 1.000 [0.86] |
| Premorbidity | |||||||||||||||
| Stroke, n (%) | 1 (3.4) | 4 (6.7) | 1.000 [2.00] | 45 (8.8) | 20 (6.8) | 0.316 [0.76] | 418 (16.5) | 198 (17.3) | 0.554 [1.06] | 725 (24.3) | 658 (21.3) | 0.007 ** [0.85] | 14 (17.9) | 65 (24.1) | 0.255 [1.45] |
| Heart Attack, n (%) | 1 (3.4) | 0 (0.0) | 0.326 [0.97] | 19 (3.7) | 5 (1.7) | 0.105 [0.45] | 235 (9.3) | 48 (4.2) | <0.001 *** [0.43] | 365 (12.2) | 204 (6.6) | <0.001 *** [0.51] | 8 (10.3) | 21 (7.8) | 0.485 [0.74] |
| Atrial Fibrillation, n (%) | 0 (0.0) | 1 (1.7) | 1.000 [1.02] | 17 (3.3) | 7 (2.4) | 0.448 [0.71] | 336 (13.2) | 156 (13.6) | 0.768 [1.03] | 927 (31.0) | 1211 (39.3) | <0.001 *** [1.44] | 45 (57.7) | 149 (55.2) | 0.695 [0.90] |
| Coronary Heart Disease, n (%) | 0 (0.0) | 0 (0.0) | - | 25 (4.9) | 3 (1.0) | 0.004 ** [0.20] | 328 (12.9) | 88 (7.7) | <0.001 *** [0.56] | 636 (21.3) | 376 (12.2) | <0.001 *** [0.51] | 12 (15.4) | 33 (12.2) | 0.463 [0.77] |
| ≤30 | >30 and ≤50 | >50 and ≤70 | >70 and ≤90 | >90 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | p-Value [OR] | Men | Women | p-Value [OR] | Men | Women | p-Value [OR] | Men | Women | p-Value [OR] | Men | Women | p-Value [OR] | |
| Stroke Etiology | |||||||||||||||
| Small Vessel Disease, n (%) | 3 (10.3) | 5 (8.3) | 0.712 [0.79] | 113 (22.0) | 64 (21.7) | 0.912 [0.98] | 668 (26.3) | 283 (24.7) | 0.287 [0.92] | 566 (18.9) | 518 (16.8) | 0.029 * [0.86] | 7 (9.0) | 25 (9.3) | 0.939 [1.03] |
| Atherosclerosis, n (%) | 3 (10.3) | 8 (13.3) | 1.000 [1.33] | 99 (19.3) | 54 (18.3) | 0.729 [0.94] | 517 (20.4) | 221 (19.3) | 0.435 [0.93] | 564 (18.9) | 380 (12.3) | <0.001 *** [0.60] | 3 (3.8) | 19 (7.0) | 0.431 [1.89] |
| Cardiac Source, n (%) | 4 (13.8) | 12 (20.0) | 0.475 [1.56] | 77 (15.0) | 35 (11.9) | 0.213 [0.76] | 558 (22.0) | 245 (21.4) | 0.666 [0.96] | 1057 (35.4) | 1347 (43.7) | <0.001 *** [1.42] | 48 (61.5) | 160 (59.3) | 0.718 [0.91] |
| ESUS, n (%) | 6 (20.7) | 15 (25.0) | 0.654 [1.28] | 60 (11.7) | 36 (12.2) | 0.830 [1.05] | 280 (11.0) | 139 (12.1) | 0.338 [1.11] | 281 (9.4) | 295 (9.6) | 0.830 [1.02] | 6 (7.7) | 21 (7.8) | 0.980 [1.01] |
| Other, n (%) | 7 (24.1) | 9 (15.0) | 0.293 [0.55] | 59 (11.5) | 52 (17.6) | 0.015 * [1.65] | 140 (5.5) | 76 (6.6) | 0.185 [1.21] | 115 (3.8) | 103 (3.3) | 0.287 [0.86] | 1 (1.3) | 3 (1.1) | 1.000 [0.86] |
| Unidentified Cause, n (%) | 6 (20.7) | 11 (18.3) | 0.791 [0.86] | 105 (20.5) | 54 (18.3) | 0.457 [0.87] | 374 (14.7) | 183 (16.0) | 0.341 [1.10] | 406 (13.6) | 442 (14.3) | 0.403 [1.06] | 13 (16.7) | 42 (15.6) | 0.813 [0.92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyer, C.; Schlenker, J.; Sandikci, V.; Ebert, A.; Wittayer, M.; Platten, M.; Szabo, K. Sex-Specific Differences in Pre-Stroke Characteristics Reveal Vulnerability of Elderly Women. J. Pers. Med. 2022, 12, 344. https://doi.org/10.3390/jpm12030344
Hoyer C, Schlenker J, Sandikci V, Ebert A, Wittayer M, Platten M, Szabo K. Sex-Specific Differences in Pre-Stroke Characteristics Reveal Vulnerability of Elderly Women. Journal of Personalized Medicine. 2022; 12(3):344. https://doi.org/10.3390/jpm12030344
Chicago/Turabian StyleHoyer, Carolin, Jan Schlenker, Vesile Sandikci, Anne Ebert, Matthias Wittayer, Michael Platten, and Kristina Szabo. 2022. "Sex-Specific Differences in Pre-Stroke Characteristics Reveal Vulnerability of Elderly Women" Journal of Personalized Medicine 12, no. 3: 344. https://doi.org/10.3390/jpm12030344
APA StyleHoyer, C., Schlenker, J., Sandikci, V., Ebert, A., Wittayer, M., Platten, M., & Szabo, K. (2022). Sex-Specific Differences in Pre-Stroke Characteristics Reveal Vulnerability of Elderly Women. Journal of Personalized Medicine, 12(3), 344. https://doi.org/10.3390/jpm12030344






