Effectiveness of Platelet-Rich Plasma Therapy in Androgenic Alopecia—A Meta-Analysis
Abstract
:1. Introduction
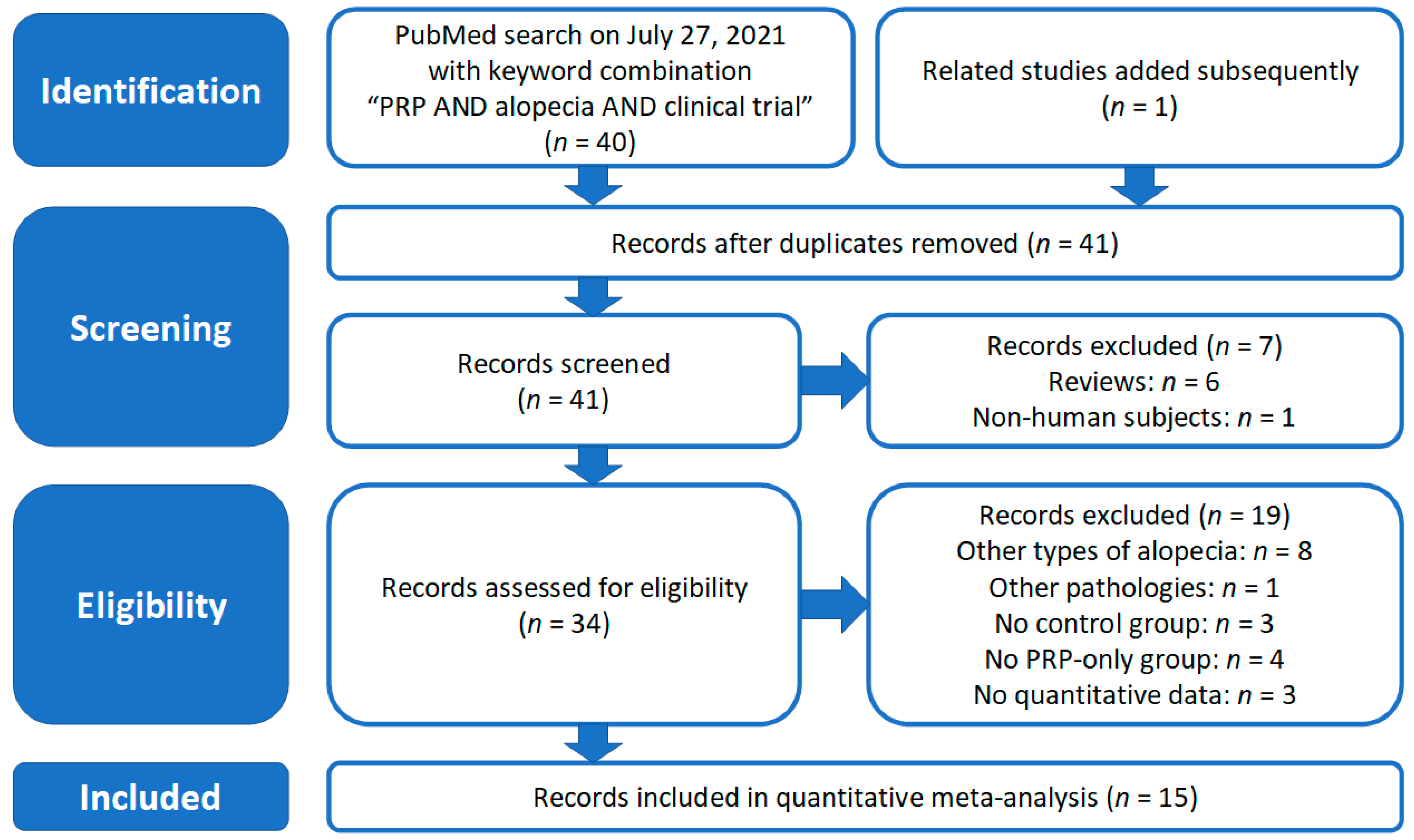
2. Materials and Methods
2.1. Literature Search and Selection of Studies
2.2. Data Analysis
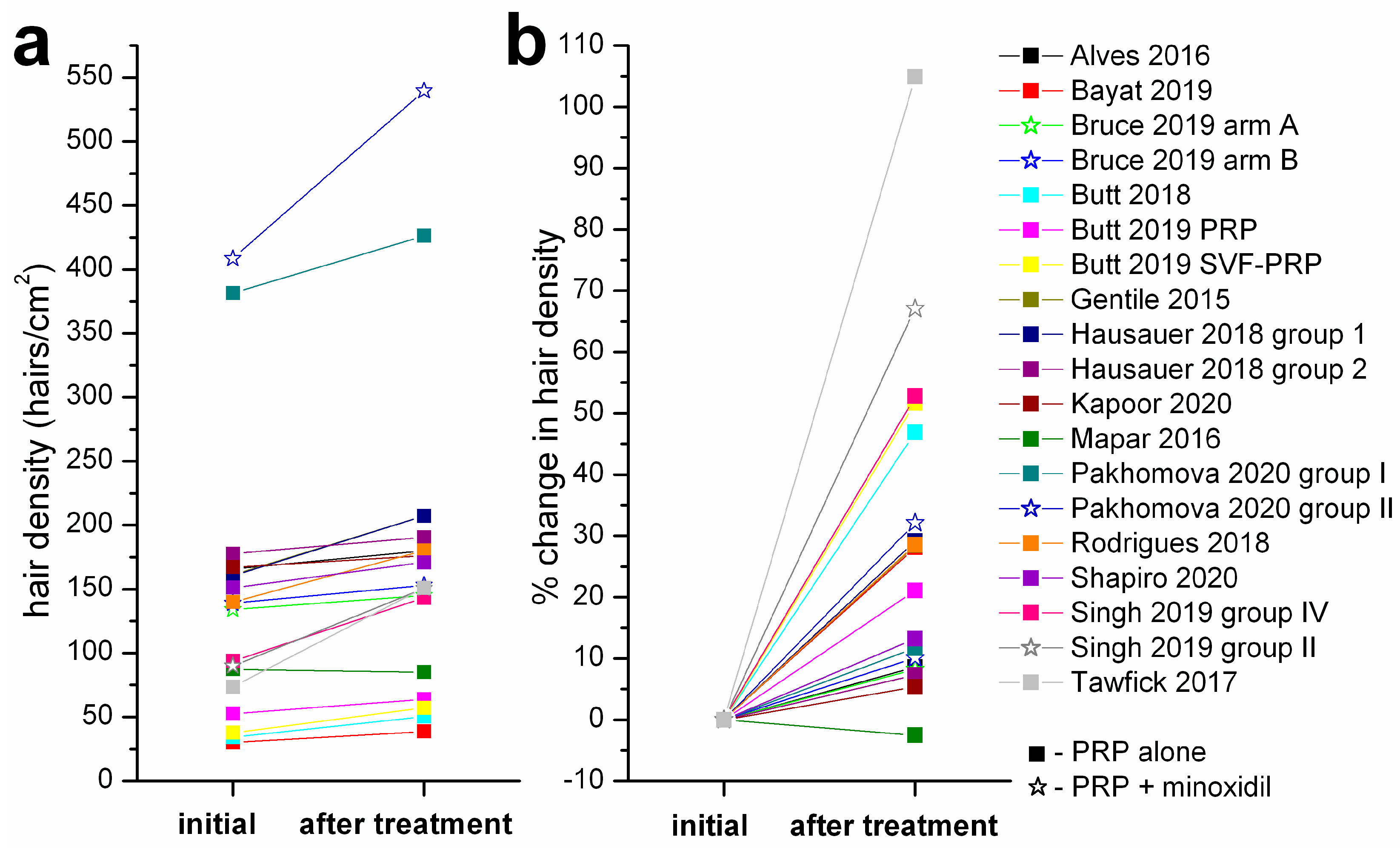
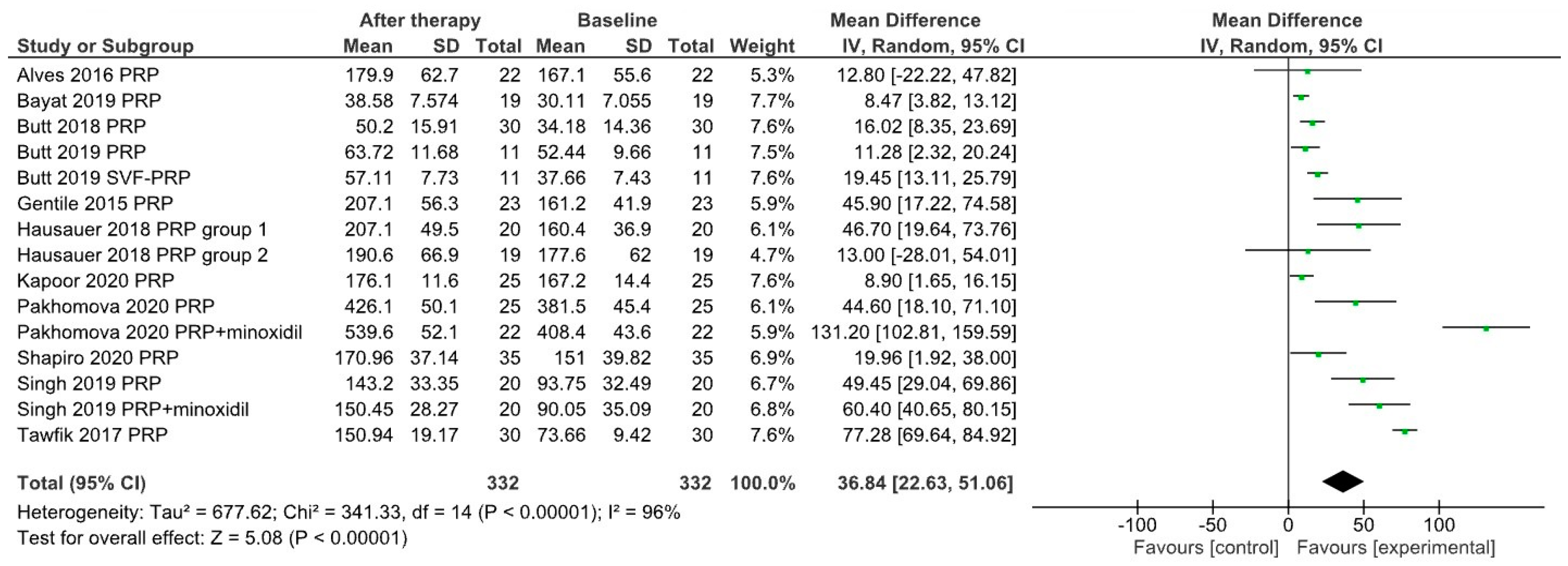
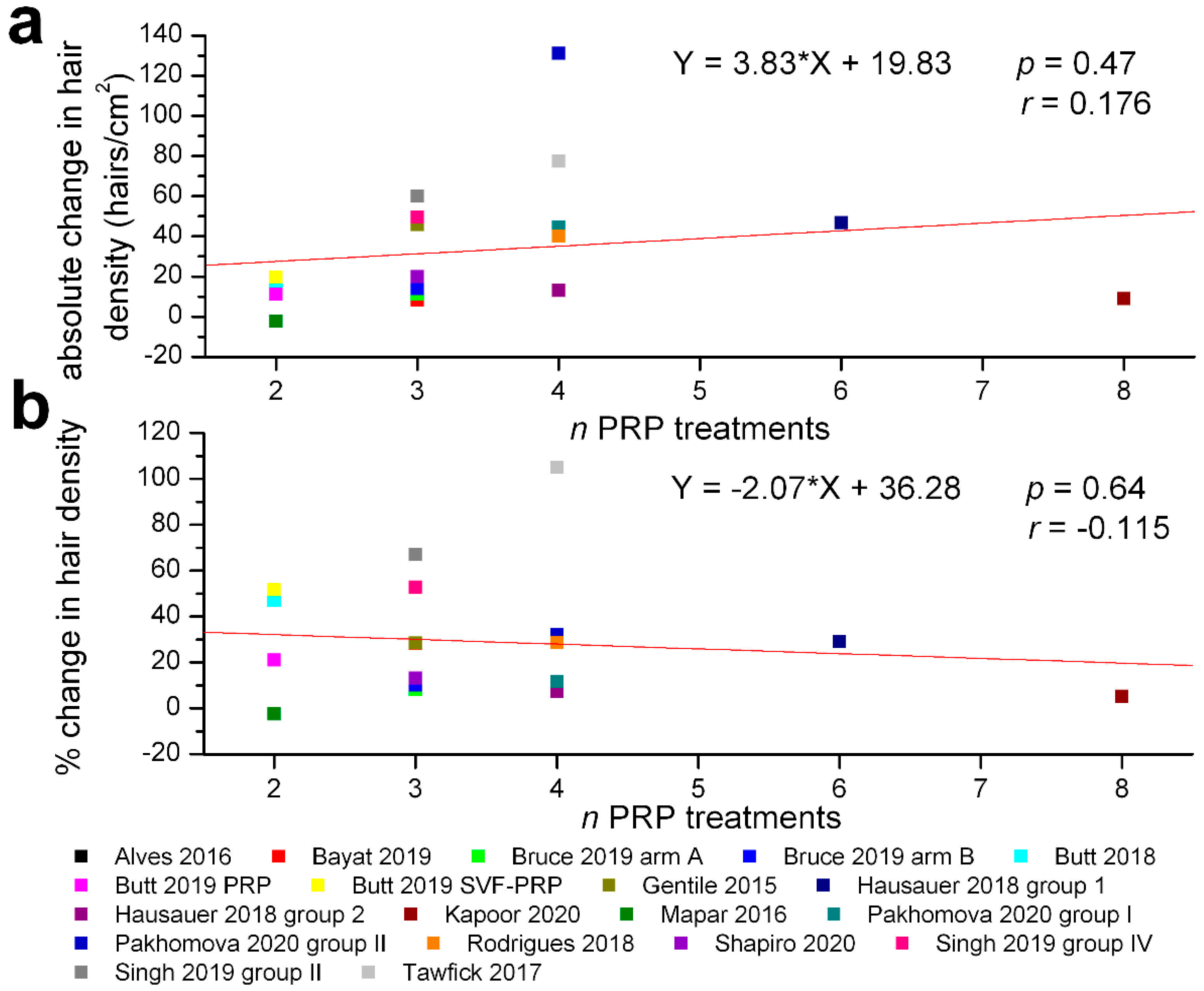
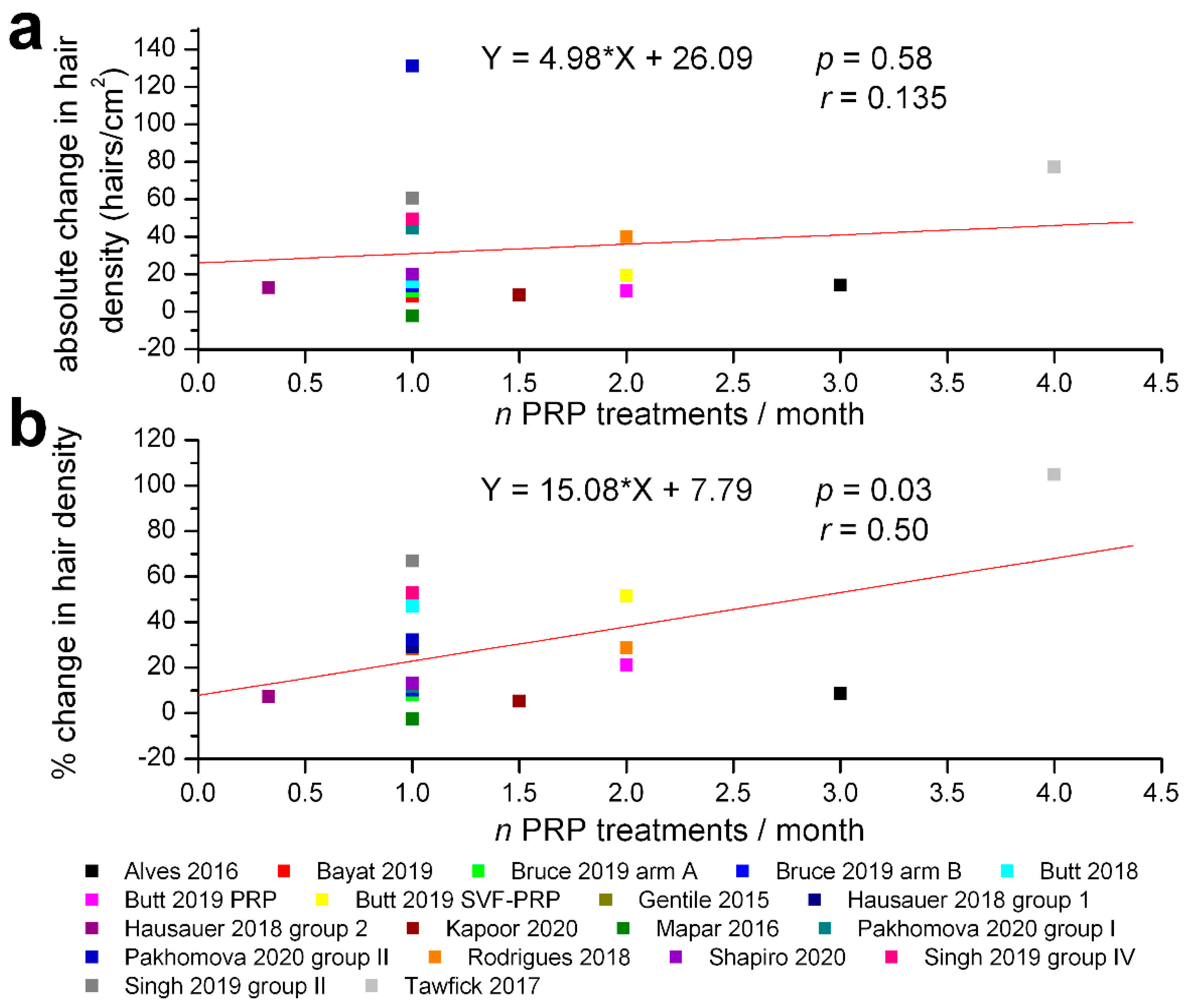
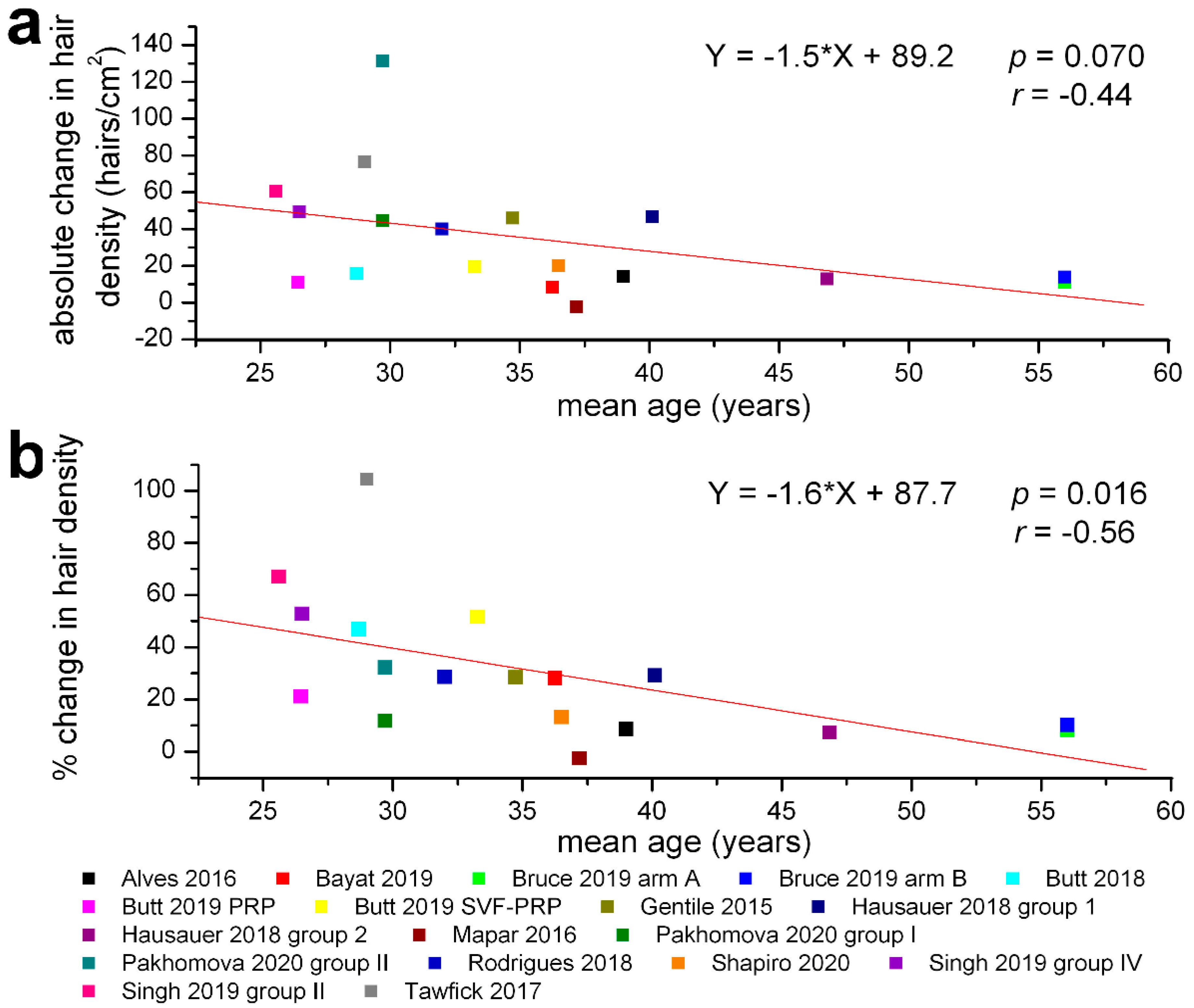
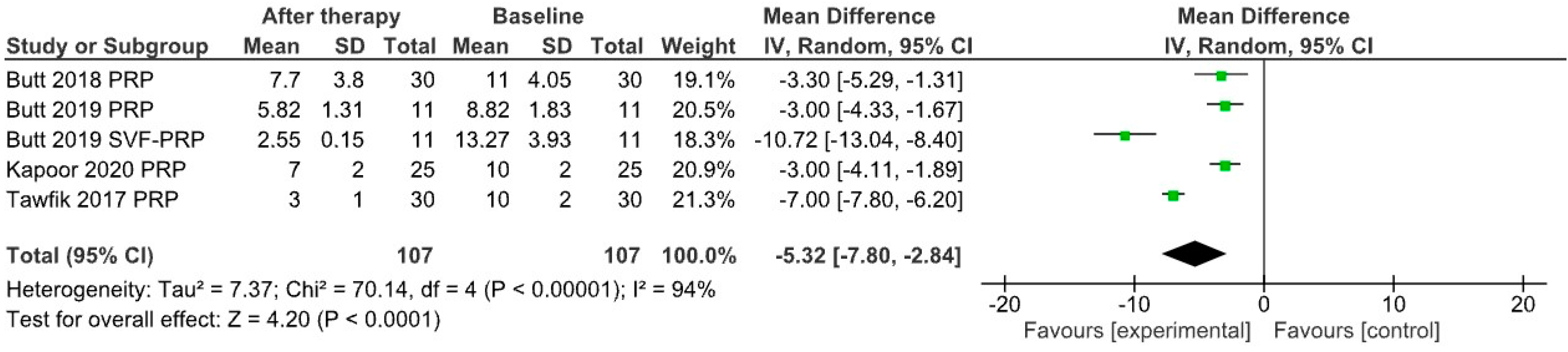
3. Results
| Study | Rando- Mized | Double- Blind | Groups | Mean Age &/Age Interval | Sex | mL PRP/ Treatment | Change in Hair Density (Hairs/cm2) Initial–Final |
|---|---|---|---|---|---|---|---|
| Alves & Grimalt 2016 [26] | Y | Y | n = 22, PRP/placebo on half-scalp 3 PRP treatments (1/month) | 39 (21–62) | M/F | 3 | 165.7–179.9 |
| Bayat et al. 2019 [33] | N | N | n = 19, 3 PRP treatments (0, 4, 8 weeks) | 36.26 (28–40) | M | 5 | 30.11–38.58 |
| Bruce et al. 2020 [34] | Y | N | arm A–PRP then minoxidil (n = 19) 3 PRP treatments over 4 weeks 8 weeks wash-out then minoxidil 12 weeks | 56 (20–79) | F | 5 | 134–145 |
| arm B–minoxidil then PRP (n = 18) minoxidil 12 weeks, 8 weeks wash-out then 3 PRP treatments over 4 weeks | 56 (20–79) | F | 5 | 139–153 | |||
| Butt et al. 2018 [35] | N | N | n = 30, 2 PRP treatments (1/month) | 28.7 (19–47) | M/F | 4 | 34.18–50.2 |
| Butt et al. 2019 [36] | Y | N | PRP group (n = 11) 2 PRP treatments at 4 weeks | 26.45 | M/F | 5 | 52.64–63.72 |
| stromal vascular fraction-PRP group (n = 11), same treatment timing | 33.27 | M/F | 5 | 37.66–57.11 | |||
| Gentile et al. 2015 [37] | Y | Y | n = 23, PRP/placebo on half-scalp 3 PRP treatments (1/month) | 34.74 (19–63) | M | 9 | 161.2–207.1 |
| Hausauer & Jones 2018 [38] | Y | single-blind | PRP group 1 (n = 20) 3 PRP treatments (1/month) + 1 booster treatment after 3 months | 40.1 (18–60) | M/F | 5 | 160.4–207.1 |
| PRP group 2 (n = 19) 2 PRP treatments at 3 months | 46.85 (18–60) | M/F | 5 | 177.6–190.6 | |||
| Kapoor et al. 2020 [16] | Y | single-blind | group B (PRP) (n = 25) 8 PRP treatments at 3 weeks | 25–50 | M | 1.5 | 167.2–176.1 |
| Mapar et al. 2016 [39] | Y | single-blind | n = 17, PRP/placebo on squares 2 PRP treatments (1/month) | 37.2 (25–45) | M | 1.5 | 87.29–85.06 |
| Pakhomova & Smirnova 2020 [40] | Y | single-blind | group I (PRP) (n = 25) 4 PRP treatments (1/month) | 29.7 (18–53) | M | 4 | 381.5–426.1 |
| group II (PRP + minoxidil) (n = 22) 4 PRP treatments (1/month) | M | 4 | 408.4–539.6 | ||||
| Puig et al. 2016 [41] | Y | Y | PRP group (n = 15) 1 treatment | >18 | F | 10 | no signifcant differences |
| placebo group (n = 11) 1 treatment | |||||||
| Rodrigues et al. 2020 [42] | Y | Y | PRP group (n = 15) 4 treatments at 15 days intervals | 32 (18–50) | M | 2 | 140–180 |
| placebo group (n = 11) 4 treatments at 15 days intervals | |||||||
| Shapiro et al. 2020 [43] | Y | Y | n = 35, PRP/placebo on squares 3 PRP treatments (1/month) | 36.5 (18–58) | M/F | 5 | 151–170.96 |
| Singh et al. 2020 [44] | Y | Y | PRP group (n = 20) 3 treatments (1/month) | 26.5 | M | 3–3.5 | 93.73–143.2 |
| PRP+minoxidil group (n = 20) 3 treatments (1/month) | 25.6 | M | 3–3.5 | 90.05–150.45 | |||
| Tawfick & Osman 2017 [45] | Y | Y | n = 30, PRP/placebo on areas 4 PRP treatments (1/week) | 29.3 (20–45) | F | 0.9 | 73.66–150.94 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGA | androgenic alopecia |
| CASP7 | caspase 7 |
| CD | cluster of differentiation |
| CTGF | connective tissue growth factor |
| EGF | epidermal growth factor |
| ERK | extracellular signal-regulated kinases |
| FDA | United States Food and Drug Administration |
| FGF | fibroblast growth factor |
| KGF | keratinocyte growth factor |
| PDGF | platelet-derived growth factor |
| PPP | platelet-poor plasma |
| PRF | platelet-rich fibrin |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRP | platelet-rich plasma |
| ROS | reactive oxygen species |
| TGF | transforming growth factor |
| TNF-α | tumor necrosis factor α |
| VEGF | vascular endothelial growth factor |
References
- Sadick, N.S.; Callender, V.D.; Kircik, L.H.; Kogan, S. New Insight into the Pathophysiology of Hair Loss Trigger a Paradigm Shift in the Treatment Approach. J. Drugs Dermatol. 2017, 16, s135–s140. [Google Scholar]
- Gupta, A.K.; Mays, R.R.; Dotzert, M.S.; Versteeg, S.G.; Shear, N.H.; Piguet, V. Efficacy of non-surgical treatments for androgenetic alopecia: A systematic review and network meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2112–2125. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Romeo, M.; Lankinen, P. Platelet-rich plasma for androgenetic alopecia: Does it work? Evidence from meta analysis. J. Cosmet. Dermatol. 2017, 16, 374–381. [Google Scholar] [CrossRef]
- Gupta, A.K.; Carviel, J.L. Meta-analysis of efficacy of platelet-rich plasma therapy for androgenetic alopecia. J. Dermatolog. Treat. 2017, 28, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar]
- Georgescu, S.R.; Tampa, M.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour Microenvironment in Skin Carcinogenesis. Adv. Exp. Med. Biol. 2020, 1226, 123–142. [Google Scholar] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Spychalowicz, A.; Wilk, G.; Śliwa, T.; Ludew, D.; Guzik, T.J. Novel therapeutic approaches in limiting oxidative stress and inflammation. Curr. Pharm. Biotechnol. 2012, 13, 2456–2466. [Google Scholar] [CrossRef]
- Acharya, P.; Mathur, M.C. Oxidative stress in alopecia areata: A systematic review and meta-analysis. Int. J. Dermatol. 2020, 59, 434–440. [Google Scholar] [CrossRef]
- Dizen-Namdar, N.; Emel Kocak, F.; Kidir, M.; Sarici, G.; Tak, H.; Altuntas, I. Evaluation of Serum Paraoxonase, Arylesterase, Prolidase Activities and Oxidative Stress in Patients with Alopecia Areata. Skin Pharmacol. Physiol. 2019, 32, 59–64. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Ene, C.D.; Tampa, M.; Matei, C.; Benea, V.; Nicolae, I. Oxidative stress-related markers and alopecia areata through latex turbidimetric immunoassay method. Mater. Plast. 2016, 53, 522–526. [Google Scholar]
- Mustafa, A.I.; Khashaba, R.A.; Fawzy, E.; Baghdady, S.M.A.; Rezk, S.M. Cross talk between oxidative stress and inflammation in alopecia areata. J. Cosmet. Dermatol. 2021, 20, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Cwynar, A.; Olszewska-Słonina, D.M.; Czajkowski, R. The impact of oxidative stress in androgenic alopecia in women. Postepy Dermatol. Alergol. 2020, 37, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Kaya Erdogan, H.; Bulur, I.; Kocaturk, E.; Yildiz, B.; Saracoglu, Z.N.; Alatas, O. The role of oxidative stress in early-onset androgenetic alopecia. J. Cosmet. Dermatol. 2017, 16, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Jadkauskaite, L.; Coulombe, P.A.; Schäfer, M.; Dinkova-Kostova, A.T.; Paus, R.; Haslam, I.S. Oxidative stress management in the hair follicle: Could targeting NRF2 counter age-related hair disorders and beyond? Bioessays 2017, 39, 1700029. [Google Scholar] [CrossRef]
- Kapoor, R.; Shome, D.; Vadera, S.; Ram, M.S. QR 678 & QR678 Neo Vs PRP-A randomised, comparative, prospective study. J. Cosmet. Dermatol. 2020, 19, 2877–2885. [Google Scholar] [PubMed]
- Gentile, P.; Garcovich, S. Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil®, Finasteride®, and Adult Stem Cell-Based Therapy. Int. J. Mol. Sci. 2020, 21, 2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Versteeg, S.G.; Rapaport, J.; Hausauer, A.K.; Shear, N.H.; Piguet, V. The Efficacy of Platelet-Rich Plasma in the Field of Hair Restoration and Facial Aesthetics-A Systematic Review and Meta-analysis. J. Cutan. Med. Surg. 2019, 23, 185–203. [Google Scholar] [CrossRef]
- Leo, M.S.; Kumar, A.S.; Kirit, R.; Konathan, R.; Sivamani, R.K. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J. Cosmet. Dermatol. 2015, 14, 315–323. [Google Scholar] [CrossRef]
- Bucur, M.; Constantin, C.; Neagu, M.; Zurac, S.; Dinca, O.; Vladan, C.; Cioplea, M.; Popp, C.; Nichita, L.; Ionescu, E. Alveolar blood clots and platelet-rich fibrin induce in vitro fibroblast proliferation and migration. Exp. Ther. Med. 2019, 17, 982–989. [Google Scholar] [CrossRef]
- Elghblawi, E. Platelet-rich plasma, the ultimate secret for youthful skin elixir and hair growth triggering. J. Cosmet. Dermatol. 2017, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Park, G.; Zhou, B.; Luo, D. Applications and efficacy of platelet-rich plasma in dermatology: A clinical review. J. Cosmet. Dermatol. 2018, 17, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, L.; Barone, S.; Bennardo, F.; Giudice, A. Management of Facial Pyoderma Gangrenosum Using Platelet-Rich Fibrin: A Technical Report. J. Oral Maxillofac. Surg. 2018, 76, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Maluf, G.; Caldas, R.J.; Silva Santos, P.S. Use of Leukocyte- and Platelet-Rich Fibrin in the Treatment of Medication-Related Osteonecrosis of the Jaws. J. Oral Maxillofac. Surg. 2018, 76, 88–96. [Google Scholar] [CrossRef]
- Soydan, S.S.; Uckan, S. Management of bisphosphonate-related osteonecrosis of the jaw with a platelet-rich fibrin membrane: Technical report. J. Oral Maxillofac. Surg. 2014, 72, 322–326. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. Randomized Placebo-Controlled, Double-Blind, Half-Head Study to Assess the Efficacy of Platelet-Rich Plasma on the Treatment of Androgenetic Alopecia. Dermatol. Surg. 2016, 42, 491–497. [Google Scholar] [CrossRef]
- Varothai, S.; Bergfeld, W.F. Androgenetic alopecia: An evidence-based treatment update. Am. J. Clin. Dermatol. 2014, 15, 217–230. [Google Scholar] [CrossRef]
- Motofei, I.G.; Rowland, D.L.; Baconi, D.L.; Tampa, M.; Sârbu, M.I.; Păunică, S.; Constantin, V.D.; Bălălău, C.; Păunică, I.; Georgescu, S.R. Androgenetic alopecia; drug safety and therapeutic strategies. Expert Opin. Drug Saf. 2018, 17, 407–412. [Google Scholar] [CrossRef]
- Talavera-Adame, D.; Newman, D.; Newman, N. Conventional and novel stem cell based therapies for androgenic alopecia. Stem Cells Cloning 2017, 10, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- The Cochrane Collaboration. Review Manager (RevMan); Vesion 5.3; The Nordic Cochrane Centre: Copenhagen, Denmark, 2014. [Google Scholar]
- Bayat, M.; Yazdanpanah, M.J.; Hamidi Alamdari, D.; Banihashemi, M.; Salehi, M. The effect of platelet-rich plasma injection in the treatment of androgenetic alopecia. J. Cosmet. Dermatol. 2019, 18, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.J.; Pincelli, T.P.; Heckman, M.G.; Desmond, C.M.; Arthurs, J.R.; Diehl, N.N.; Douglass, E.J.; Bruce, C.J.; Shapiro, S.A. A Randomized, Controlled Pilot Trial Comparing Platelet-Rich Plasma to Topical Minoxidil Foam for Treatment of Androgenic Alopecia in Women. Dermatol. Surg. 2020, 46, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Butt, G.; Hussain, I.; Ahmed, F.J.; Choudhery, M.S. Efficacy of platelet-rich plasma in androgenetic alopecia patients. J. Cosmet. Dermatol. 2018, 18, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Butt, G.; Hussain, I.; Ahmad, F.J.; Choudhery, M.S. Stromal vascular fraction-enriched platelet-rich plasma therapy reverses the effects of androgenetic alopecia. J. Cosmet. Dermatol. 2019, 19, 1078–1085. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef]
- Hausauer, A.K.; Jones, D.H. Evaluating the Efficacy of Different Platelet-Rich Plasma Regimens for Management of Androgenetic Alopecia: A Single-Center, Blinded, Randomized Clinical Trial. Dermatol. Surg. 2018, 44, 1191–1200. [Google Scholar] [CrossRef]
- Mapar, M.A.; Shahriari, S.; Haghighizadeh, M.H. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: A pilot randomized controlled trial. J. Cosmet. Laser Ther. 2016, 18, 452–455. [Google Scholar] [CrossRef]
- Pakhomova, E.E.; Smirnova, I.O. Comparative Evaluation of the Clinical Efficacy of PRP-Therapy, Minoxidil, and Their Combination with Immunohistochemical Study of the Dynamics of Cell Proliferation in the Treatment of Men with Androgenetic Alopecia. Int. J. Mol. Sci. 2020, 21, 6516. [Google Scholar] [CrossRef]
- Puig, C.J.; Reese, R.; Peters, M. Double-Blind, Placebo-Controlled Pilot Study on the Use of Platelet-Rich Plasma in Women With Female Androgenetic Alopecia. Dermatol. Surg. 2016, 42, 1243–1247. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; Montalvão, S.A.L.; Cancela, R.B.B.; Silva, F.A.R.; Urban, A.; Huber, S.C.; Júnior, J.; Lana, J.; Annichinno-Bizzacchi, J.M. Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J. Am. Acad. Dermatol. 2018, 80, 694–700. [Google Scholar] [CrossRef]
- Shapiro, J.; Ho, A.; Sukhdeo, K.; Yin, L.; Lo Sicco, K. Evaluation of platelet-rich plasma as a treatment for androgenetic alopecia: A randomized controlled trial. J. Am. Acad. Dermatol. 2020, 83, 1298–1303. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, V.; Rai, T. Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: A randomized, double-blind placebo control trial. Indian J. Dermatol. Venereol. Leprol. 2020, 86, 150–157. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Osman, M.A.R. The effect of autologous activated platelet-rich plasma injection on female pattern hair loss: A randomized placebo-controlled study. J. Cosmet. Dermatol. 2017, 17, 47–53. [Google Scholar] [CrossRef]
- Motofei, I.G.; Rowland, D.L.; Georgescu, S.R.; Tampa, M.; Baconi, D.; Stefanescu, E.; Baleanu, B.C.; Balalau, C.; Constantin, V.; Paunica, S. Finasteride adverse effects in subjects with androgenic alopecia: A possible therapeutic approach according to the lateralization process of the brain. J. Dermatolog. Treat. 2016, 27, 495–497. [Google Scholar] [CrossRef]
- Motofei, I.G.; Rowland, D.L.; Georgescu, S.R.; Tampa, M.; Paunica, S.; Constantin, V.D.; Balalau, C.; Manea, M.; Baleanu, B.C.; Sinescu, I. Post-Finasteride Adverse Effects in Male Androgenic Alopecia: A Case Report of Vitiligo. Skin Pharmacol. Physiol. 2017, 30, 42–45. [Google Scholar] [CrossRef]
- Pereira, A.F.J.R.; Coelho, T.O.A. Post-finasteride syndrome. An. Bras. Dermatol. 2020, 95, 271–277. [Google Scholar] [CrossRef]
- Vasserot, A.P.; Geyfman, M.; Poloso, N.J. Androgenetic alopecia: Combing the hair follicle signaling pathways for new therapeutic targets and more effective treatment options. Expert Opin. Ther. Targets 2019, 23, 755–771. [Google Scholar] [CrossRef]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin Ther. Lett. 2019, 24, 1–6. [Google Scholar]
- Gupta, A.K.; Cole, J.; Deutsch, D.P.; Everts, P.A.; Niedbalski, R.P.; Panchaprateep, R.; Rinaldi, F.; Rose, P.T.; Sinclair, R.; Vogel, J.E.; et al. Platelet-Rich Plasma as a Treatment for Androgenetic Alopecia. Dermatol. Surg. 2019, 45, 1262–1273. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.E.; Keaney, T.C. Systematic review of platelet-rich plasma (PRP) preparation and composition for the treatment of androgenetic alopecia. J. Cosmet. Dermatol. 2018, 17, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Qi, F.; Gong, Y.; Zhang, C.; Zhao, S.; Yang, X.; He, Y. Platelet-Rich Plasma in Female Androgenic Alopecia: A Comprehensive Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 642980. [Google Scholar] [CrossRef]
- Litchman, G.; Nair, P.A.; Badri, T.; Kelly, S.E. Microneedling; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ocampo-Garza, S.S.; Fabbrocini, G.; Ocampo-Candiani, J.; Cinelli, E.; Villani, A. Micro needling: A novel therapeutic approach for androgenetic alopecia, A Review of Literature. Dermatol. Ther. 2020, 33, e14267. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Carviel, J. A Mechanistic Model of Platelet-Rich Plasma Treatment for Androgenetic Alopecia. Dermatol. Surg. 2016, 42, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Mahé, Y.F.; Michelet, J.F.; Billoni, N.; Jarrousse, F.; Buan, B.; Commo, S.; Saint-Léger, D.; Bernard, B.A. Androgenetic alopecia and microinflammation. Int. J. Dermatol. 2000, 39, 576–584. [Google Scholar] [CrossRef]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef]
- Tosti, A.; Piraccini, B.M. Finasteride and the hair cycle. J. Am. Acad. Dermatol. 2000, 42, 848–849. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Goszka, M.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryć, R.; Dołęgowska, B. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2021, 6101, 00090–00093. [Google Scholar] [CrossRef]
- Inbarajan, A.; Veeravalli, P.T.; Seenivasan, M.K.; Natarajan, S.; Sathiamurthy, A.; Ahmed, S.R.; Vaidyanathan, A.K. Platelet-Rich Plasma and Platelet-Rich Fibrin as a Regenerative Tool. J. Pharm. Bioallied Sci. 2021, 13, S1266–S1267. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgescu, S.R.; Amuzescu, A.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Constantin, C.; Tampa, M.; Neagu, M. Effectiveness of Platelet-Rich Plasma Therapy in Androgenic Alopecia—A Meta-Analysis. J. Pers. Med. 2022, 12, 342. https://doi.org/10.3390/jpm12030342
Georgescu SR, Amuzescu A, Mitran CI, Mitran MI, Matei C, Constantin C, Tampa M, Neagu M. Effectiveness of Platelet-Rich Plasma Therapy in Androgenic Alopecia—A Meta-Analysis. Journal of Personalized Medicine. 2022; 12(3):342. https://doi.org/10.3390/jpm12030342
Chicago/Turabian StyleGeorgescu, Simona Roxana, Andreea Amuzescu, Cristina Iulia Mitran, Madalina Irina Mitran, Clara Matei, Carolina Constantin, Mircea Tampa, and Monica Neagu. 2022. "Effectiveness of Platelet-Rich Plasma Therapy in Androgenic Alopecia—A Meta-Analysis" Journal of Personalized Medicine 12, no. 3: 342. https://doi.org/10.3390/jpm12030342
APA StyleGeorgescu, S. R., Amuzescu, A., Mitran, C. I., Mitran, M. I., Matei, C., Constantin, C., Tampa, M., & Neagu, M. (2022). Effectiveness of Platelet-Rich Plasma Therapy in Androgenic Alopecia—A Meta-Analysis. Journal of Personalized Medicine, 12(3), 342. https://doi.org/10.3390/jpm12030342










