Metastatic Pattern of Truncal and Extremity Leiomyosarcoma: Retrospective Analysis of Predictors, Outcomes, and Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Demographics, Presentation, and Treatment
2.2. Variables, Outcome Measures, Data Sources, and Bias
2.3. Statistical Analysis
3. Results
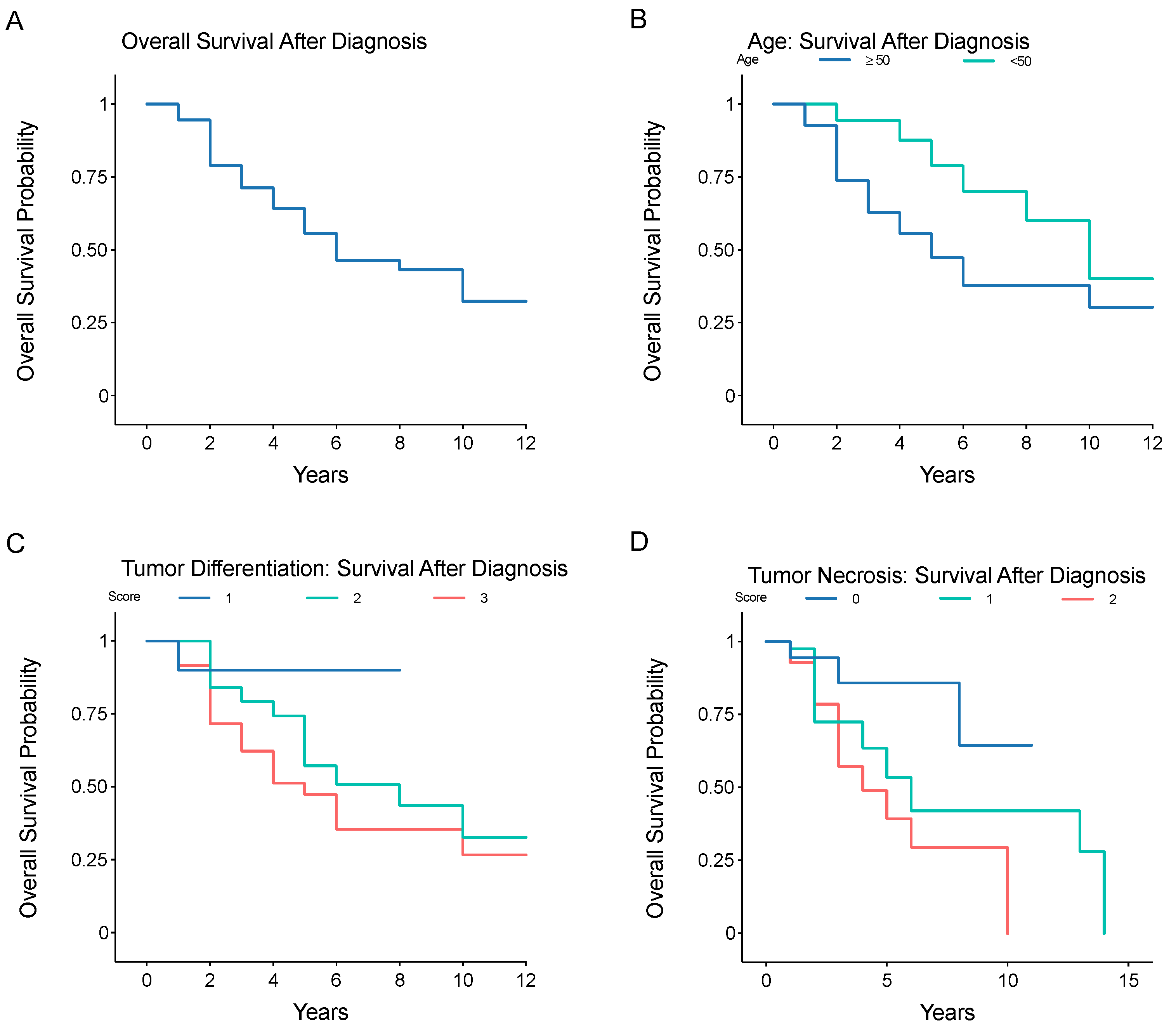
3.1. Anatomical Distribution and Frequency of Metastatic Disease in Truncal/Extremity LMS
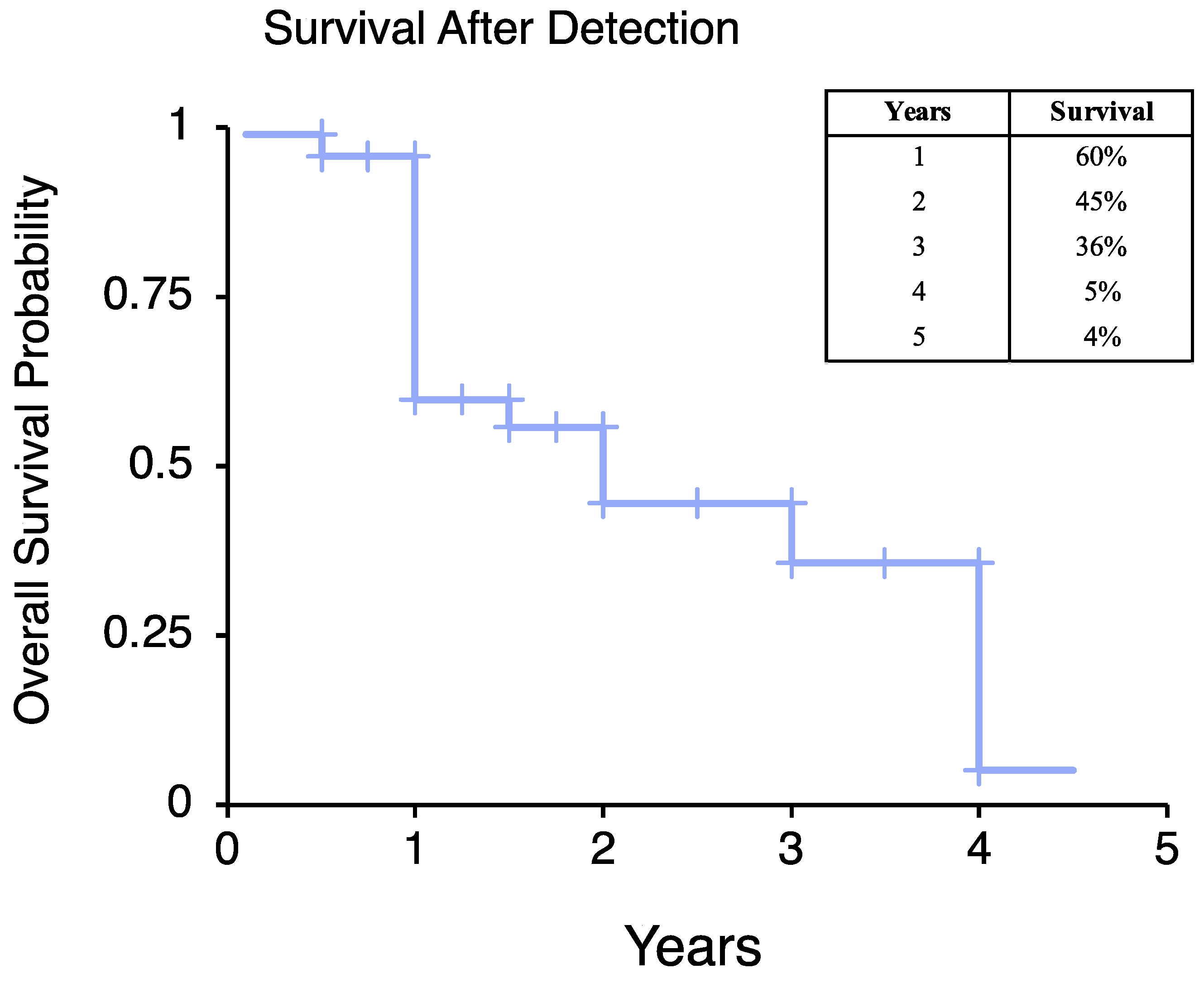
3.2. Prognostic Factors Associated with Metastatic Risk or Overall Survival after Primary Diagnosis of Truncal/Extremity LMS
3.3. Impact of Imaging Modality on Frequency of Metastatic Disease Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.; et al. SEER Cancer Statistics Review, 1975–2016; Based on November 2018 SEER Data Submission, Posted to the SEER Web Site, April 2019; National Cancer Institute: Bethesda, MD, USA, 2019. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 8 October 2021).
- O’Sullivan, P.J.; Harris, A.C.; Munk, P.L. Radiological imaging features of non-uterine leiomyosarcoma. Br. J. Radiol. 2008, 81, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Sultan, I.; Huang, T.T.; Rodriguez-Galindo, C.; Shehadeh, A.; Meazza, C.; Ness, K.K.; Casanova, M.; Spunt, S.L. Soft tissue sarcoma across the age spectrum: A population-based study from the Surveillance Epidemiology and End Results database. Pediatr. Blood Cancer 2011, 57, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Tirumani, S.H.; Deaver, P.; Shinagare, A.B.; Tirumani, H.; Hornick, J.L.; George, S.; Ramaiya, N.H. Metastatic pattern of uterine leiomyosarcoma: Retrospective analysis of the predictors and outcome in 113 patients. J. Gynecol. Oncol. 2014, 25, 306–312. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Serrano, C.; Hensley, M.L.; Ray-Coquard, I. Soft Tissue and Uterine Leiomyosarcoma. J. Clin. Oncol. 2018, 36, 144–150. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Landa, J.; Kuk, D.; Sanchez, A.; Lala, B.; Schmidt, N.; Okoli, C.; Chi, P.; Dickson, M.A.; Gounder, M.M.; et al. Overall Survival and Response to Systemic Therapy in Metastatic Extrauterine Leiomyosarcoma. Sarcoma 2016, 2016, 3547497. [Google Scholar] [CrossRef] [PubMed]
- Lamm, W.; Natter, C.; Schur, S.; Kostler, W.J.; Reinthaller, A.; Krainer, M.; Grimm, C.; Horvath, R.; Amann, G.; Funovics, P.; et al. Distinctive outcome in patients with non-uterine and uterine leiomyosarcoma. BMC Cancer 2014, 14, 981. [Google Scholar] [CrossRef] [PubMed]
- Kamat, N.V.; Million, L.; Yao, D.H.; Donaldson, S.S.; Mohler, D.G.; van de Rijn, M.; Avedian, R.S.; Kapp, D.S.; Ganjoo, K.N. The Outcome of Patients With Localized Undifferentiated Pleomorphic Sarcoma of the Lower Extremity Treated at Stanford University. Am. J. Clin. Oncol. 2019, 42, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Steffner, R.J.; Jang, E.S. Staging of Bone and Soft-tissue Sarcomas. J. Am. Acad. Orthop. Surg. 2018, 26, e269–e278. [Google Scholar] [CrossRef] [PubMed]
- Gladdy, R.A.; Qin, L.X.; Moraco, N.; Agaram, N.P.; Brennan, M.F.; Singer, S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann. Surg. Oncol. 2013, 20, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.M.; Ethun, C.G.; Zaidi, M.Y.; Tran, T.B.; Poultsides, G.A.; Grignol, V.P.; Howard, J.H.; Bedi, M.; Gamblin, T.C.; Tseng, J.; et al. A closer look at the natural history and recurrence patterns of high-grade truncal/extremity leiomyosarcomas: A multi-institutional analysis from the US Sarcoma Collaborative. Surg. Oncol. 2020, 34, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Nascimento, A.G. Grading in soft tissue tumors: Principles and problems. Skeletal Radiol. 2001, 30, 543–559. [Google Scholar] [PubMed]
- Lang, H.; Nussbaum, K.T.; Kaudel, P.; Fruhauf, N.; Flemming, P.; Raab, R. Hepatic metastases from leiomyosarcoma: A single-center experience with 34 liver resections during a 15-year period. Ann. Surg. 2000, 231, 500–505. [Google Scholar] [CrossRef]
- King, D.M.; Hackbarth, D.A.; Kilian, C.M.; Carrera, G.F. Soft-tissue sarcoma metastases identified on abdomen and pelvis CT imaging. Clin. Orthop. Relat. Res. 2009, 467, 2838–2844. [Google Scholar] [CrossRef][Green Version]
- Thompson, M.J.; Ross, J.; Domson, G.; Foster, W. Screening and surveillance CT abdomen/pelvis for metastases in patients with soft-tissue sarcoma of the extremity. Bone Jt. Res. 2015, 4, 45–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rivera, L.; Gandikota, N.; Love, C.; Yang, J.; Libes, R.; Rosen, G.; Aziz, M.; Dokken, R.; Abdel-Dayem, H. Role of F-18-FDG PET/CT in follow-up of patients with treated leiomyosarcoma. J. Nucl. Med. 2010, 51, 513. [Google Scholar]
- Park, J.Y.; Lee, J.W.; Lee, H.J.; Lee, J.J.; Moon, S.H.; Kang, S.Y.; Cheon, G.J.; Chung, H.H. Prognostic significance of preoperative (1)(8)F-FDG PET/CT in uterine leiomyosarcoma. J. Gynecol. Oncol. 2017, 28, e28. [Google Scholar] [CrossRef]
- Macpherson, R.E.; Pratap, S.; Tyrrell, H.; Khonsari, M.; Wilson, S.; Gibbons, M.; Whitwell, D.; Giele, H.; Critchley, P.; Cogswell, L.; et al. Retrospective audit of 957 consecutive (18)F-FDG PET-CT scans compared to CT and MRI in 493 patients with different histological subtypes of bone and soft tissue sarcoma. Clin. Sarcoma Res. 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Punt, S.E.; Eary, J.F.; O’Sullivan, J.; Conrad, E.U. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: Imaging characteristics. Nucl. Med. Commun. 2009, 30, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Barrette, B.A.; Baumann, K.; Gaffney, D.; Hamilton, A.L.; Kim, J.W.; Maenpaa, J.U.; Pautier, P.; Siddiqui, N.A.; Westermann, A.M.; et al. Gynecologic Cancer InterGroup (GCIG) consensus review: Uterine and ovarian leiomyosarcomas. Int. J. Gynecol. Cancer 2014, 24 (Suppl. 3), S61–S66. [Google Scholar] [CrossRef] [PubMed]

| Variable | Factor | n, Mean | %, Range |
|---|---|---|---|
| Gender | Female | 30 | 41% |
| Male | 43 | 59% | |
| Mean Age (Years) | ≥50 years | 55 | 75% |
| <50 years | 18 | 25% | |
| Tumor Size (cm) | <5 cm | 20 | 28% |
| 5–10 cm | 29 | 74% | |
| ≥10 cm | 23 | 32% | |
| Primary Tumor Site | Extremity | 50 | 68% |
| Flank/Pelvis | 13 | 18% | |
| Chest Wall/Spine | 10 | 14% | |
| Tumor Differentiation Score | 1 | 10 | 14% |
| 2 | 26 | 36% | |
| 3 | 36 | 50% | |
| Mitotic Index | 1 | 26 | 36% |
| 2 | 29 | 40% | |
| 3 | 17 | 24% | |
| Tumor Necrosis Score | 0 | 18 | 25% |
| 1 | 40 | 56% | |
| 2 | 14 | 19% | |
| Total Score | 2 | 5 | 7% |
| 3 | 2 | 3% | |
| 4 | 18 | 25% | |
| 5 | 6 | 8% | |
| 6 | 32 | 44% | |
| 7 | 9 | 13% | |
| 8 | 0 | 0% | |
| Histological Grade | I | 7 | 10% |
| II | 24 | 34% | |
| III | 39 | 56% | |
| Pathologist-Reported Margins | Positive | 21 | 32% |
| Negative | 45 | 68% | |
| Presentation Status | Primary Disease | 56 | 77% |
| Metastatic Disease | 17 | 23% | |
| Treatment | Neoadjuvant Therapy | 14 | 19% |
| Surgical Resection | 67 | 92% | |
| Adjuvant Chemotherapy | 25 | 34% | |
| Adjuvant Radiotherapy | 28 | 38% | |
| Adjuvant Chemotherapy + Radiotherapy | 15 | 21% | |
| Development of Metastatic Disease by Histological Grade | I | 1 | 14% |
| II | 17 | 71% | |
| III | 32 | 82% | |
| Time to Metastatic Disease from Diagnosis (Years) | Any Location | 50 | 69% |
| Lung | 42 | 84% | |
| Abdomen/Thorax/Visceral Organ | 26 | 52% | |
| Bone | 17 | 34% | |
| Skin/Soft Tissue | 14 | 28% | |
| Lymph Node | 3 | 6% | |
| Brain | 2 | 4% | |
| Vessel | 1 | 2% | |
| Yes | 24 | 36% | |
| No | 42 | 64% | |
| Mean Survival After Primary Diagnosis, All Grades (Years) | 4.7 | 1–14 |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variable | Level | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Any Metastasis | Age ≥ 50 years | 3.74 (1.32–12.36) | <0.001 * | 2.54 (1.18–8.98) | 0.001 * |
| Positive Margins | 1.12 (0.42–2.29) | 0.892 | 1.02 (0.52–1.49) | 0.921 | |
| Tumor Differentiation | |||||
| 2 | 4.01 (0.71–22.61) | 0.116 | 3.23 (0.82–12.36) | 0.223 | |
| 3 | 14.02 (2.54–79.65) | 0.003 * | 12.09 (2.29–67.42) | 0.002 * | |
| Mitotic Index | |||||
| 2 | 1.39 (0.47–4.15) | 0.552 | 1.17 (0.78–2.51) | 0.673 | |
| 3 | 0.83 (0.24–2.82) | 0.759 | 0.92 (0.22–2.17) | 0.889 | |
| Tumor Necrosis | |||||
| 1 | 6.85 (1.98–23.76) | 0.002 * | 4.12 (1.42–13.34) | 0.032 * | |
| 2 | 4.68 (1.04–21.04) | 0.044 * | 3.65 (0.80–16.51) | 0.026 * | |
| Tumor Size | |||||
| 5–10 cm | 1.02 (0.94–1.17) | 0.515 | 1.01 (0.96–1.12) | 0.551 | |
| ≥10 cm | 1.73 (0.83–2.09) | 0.221 | 1.34 (0.81–1.78) | 0.319 | |
| Lung Metastasis | Tumor Differentiation | ||||
| 2 | 8.32 (1.87–187.36) | 0.026 * | 5.22 (1.65–89.32) | 0.039 * | |
| 3 | 14.65 (1.29–237.42) | 0.003 * | 11.76 (1.04–143.29) | 0.004 * | |
| Variable | Level | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|
| Age | ≥50 years | 4.76 (0.06–0.75) | 0.017 * |
| Primary Site | Extremity | 0.59 (0.29–1.19) | 0.140 |
| Flank/Pelvis | 1.37 (0.65–2.90) | 0.412 | |
| Chest Wall/Spine | 1.73 (0.78–3.82) | 0.174 | |
| Tumor Margins | Positive | 1.32 (0.61–2.01) | 0.424 |
| Tumor Differentiation | 2 | 9.02 (0.99–81.58) | 0.051 |
| 3 | 15.92 (1.81–140.17) | 0.013 * | |
| Mitotic Index | 2 | 1.91 (0.65–5.60) | 0.239 |
| 3 | 0.82(0.24–2.81) | 0.748 | |
| Tumor Necrosis | 1 | 3.52 (1.05–11.76) | 0.041 * |
| 2 | 4.68 (1.04–21.04) | 0.044 * | |
| Tumor Size | 5–10 cm | 2.12 (0.66–6.78) | 0.020 |
| ≥10 cm | 1.92 (1.31–4.22) | 0.029 * | |
| Surveillance Frequency | (≤4 mo) | 2.72 (1.17–4.79) | 0.010 * |
| Variable | n | % |
|---|---|---|
| Initial Staging Studies | ||
| CT Chest/Abdomen/Pelvis | 47 | 56% |
| PET/CT | 25 | 30% |
| Nuclear Bone Scan | 7 | 8% |
| Chest X-ray | 5 | 6% |
| Radiological Surveillance Studies | ||
| CT chest/abdomen/pelvis | 343 | 52% |
| PET/CT | 166 | 25% |
| Chest X-ray | 134 | 20% |
| Nuclear Bone Scan | 22 | 3% |
| Mean Annual Surveillance Frequency (Months) | 5 | 1–13 |
| Mean Number of Lifetime Surveillance Scans | 11 | 1–51 |
| Imaging Paradigm | Imaging Modality | Frequency Used | Frequency of Tumor Detection | Chi-Square Statistic | p-Value |
|---|---|---|---|---|---|
| Initial Staging | CT CAP | 47 | 14 | 16.5 | <0.0001 * |
| PET/CT | 25 | 20 | |||
| Radiological Surveillance | CT CAP | 343 | 24 | 23.2 | <0.0001 * |
| PET/CT | 166 | 36 | |||
| Staging + Surveillance | CT CAP | 390 | 38 | 36.2 | <0.0001 * |
| PET/CT | 191 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tigchelaar, S.S.; Frey, C.; Sivaraj, D.; Segovia, N.A.; Mohler, D.G.; Steffner, R.J.; Avedian, R.S. Metastatic Pattern of Truncal and Extremity Leiomyosarcoma: Retrospective Analysis of Predictors, Outcomes, and Detection. J. Pers. Med. 2022, 12, 345. https://doi.org/10.3390/jpm12030345
Tigchelaar SS, Frey C, Sivaraj D, Segovia NA, Mohler DG, Steffner RJ, Avedian RS. Metastatic Pattern of Truncal and Extremity Leiomyosarcoma: Retrospective Analysis of Predictors, Outcomes, and Detection. Journal of Personalized Medicine. 2022; 12(3):345. https://doi.org/10.3390/jpm12030345
Chicago/Turabian StyleTigchelaar, Seth S., Christopher Frey, Dharshan Sivaraj, Nicole A. Segovia, David G. Mohler, Robert J. Steffner, and Raffi S. Avedian. 2022. "Metastatic Pattern of Truncal and Extremity Leiomyosarcoma: Retrospective Analysis of Predictors, Outcomes, and Detection" Journal of Personalized Medicine 12, no. 3: 345. https://doi.org/10.3390/jpm12030345
APA StyleTigchelaar, S. S., Frey, C., Sivaraj, D., Segovia, N. A., Mohler, D. G., Steffner, R. J., & Avedian, R. S. (2022). Metastatic Pattern of Truncal and Extremity Leiomyosarcoma: Retrospective Analysis of Predictors, Outcomes, and Detection. Journal of Personalized Medicine, 12(3), 345. https://doi.org/10.3390/jpm12030345






