Effectiveness of Mindfulness and Positive Strengthening mHealth Interventions for the Promotion of Subjective Emotional Wellbeing and Management of Self-Efficacy for Chronic Cardiac Diseases
Abstract
:1. Introduction
1.1. Subjective Emotional Wellbeing and Management Self-Efficacy for Cardiovascular Health
1.2. Mindfulness Interventions
1.3. Positive Strengthening Interventions
1.4. Objectives and Hypotheses
2. Materials and Methods
2.1. Participants and Procedure
2.2. Interventions
2.2.1. In-Person Intervention
2.2.2. mHealth Intervention
- Mindfulness mHealth intervention. The mindfulness intervention consisted of formal and informal mindfulness-based exercises (e.g., breathing awareness, body scan, exploration through the senses). We adapted a mindfulness-based stress reduction program [47] for instruction by messaging with the specific objective of full attention every day (see Supplemental File S1). The practice based on breathing awareness was carried out for 10 min. The goal was to develop awareness in cardiac patients through this approach based on contact with the present moment and full attention.
- Positive strengthening mHealth intervention. The program of activities was adapted by our research team from Seligman’s approach [7] (see Supplemental File S1). For this intervention, activities were proposed for a positive assessment of the main vital areas of the participant’s life (personal, family, social, and daily life). The objective of the program was to reinforce the importance of focusing on the good things that happen to us each day, combined with gratitude exercises and assessment of personal achievements from the perspective of positive psychology.
2.2.3. TAU Group
2.3. Measures
2.3.1. Positive and Negative Affect
2.3.2. Cardiovascular Management Self-Efficacy Scale (CMSES)
2.3.3. Self-Efficacy for Managing Chronic Disease Scale (SEMCD)
2.3.4. Anxiety and Depression
2.3.5. Engagement and Satisfaction
2.4. Data Analyses
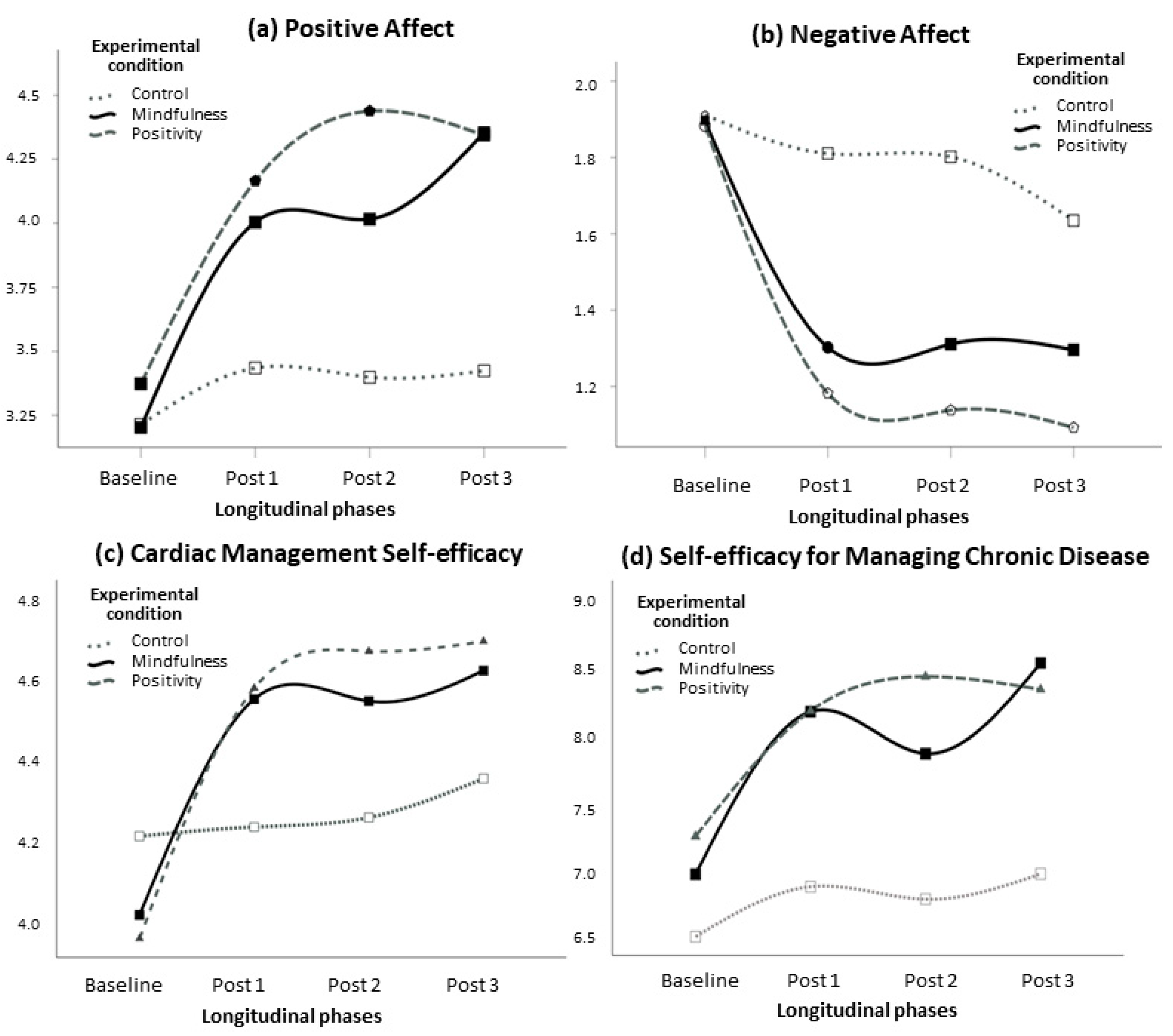
3. Results
4. Discussion
4.1. Strengths
4.2. Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). The 10 Leading Causes of Death in the World. Resource Document. World Health Organization. 2016. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 6 June 2022).
- Gupta, R.; Wood, D.A. Primary prevention of ischaemic heart disease: Populations, individuals, and health professionals. Lancet 2019, 394, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.; Herzog, A.A.; Silverman, D.I. Hearts and Minds: Stress, Anxiety, and Depression: Unsung Risk Factors for Car-diovascular Disease. Cardiol. Rev. 2019, 27, 202–207. [Google Scholar] [CrossRef]
- Pressman, S.D.; Cohen, S. Does positive affect influence health? Psychol. Bull. 2005, 131, 925–971. [Google Scholar] [CrossRef]
- Pressman, S.D.; Jenkins, B.N.; Moskowitz, J.T. Positive Affect and Health: What Do We Know and Where Next Should We Go? Annu. Rev. Psychol. 2019, 70, 627–650. [Google Scholar] [CrossRef]
- Marino, F.; Failla, C.; Carrozza, C.; Ciminata, M.; Chilà, P.; Minutoli, R.; Genovese, S.; Puglisi, A.; Arnao, A.; Tartarisco, G.; et al. Mindfulness-Based Interventions for Physical and Psychological Wellbeing in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Seligman, M.E.P.; Steen, T.A.; Park, N.; Peterson, C. Positive Psychology Progress: Empirical Validation of Interventions. Am. Psychol. 2005, 60, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Sin, N.L.; Lyubomirsky, S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: A practice-friendly meta-analysis. J. Clin. Psychol. 2009, 65, 467–487. [Google Scholar] [CrossRef] [Green Version]
- Scott-Sheldon, L.A.J.; Gathright, E.C.; Donahue, M.L.; Balletto, B.; Feulner, M.M.; DeCosta, J.; Cruess, D.G.; Wing, R.R.; Carey, M.P.; Salmoirago-Blotcher, E. Mindfulness-Based Interventions for Adults with Cardiovascular Disease: A Systematic Review and Meta-Analysis. Ann. Behav. Med. 2020, 54, 67–73. [Google Scholar] [CrossRef]
- Lee, E.K.; Yeung, N.C.; Xu, Z.; Zhang, D.; Yu, C.-P.; Wong, S.Y. Effect and Acceptability of Mindfulness-Based Stress Reduction Program on Patients with Elevated Blood Pressure or Hypertension. Hypertension 2020, 76, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Boehm, J.K.; Kubzansky, L.D. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychol. Bull. 2012, 138, 655–691. [Google Scholar] [CrossRef]
- Bandura, A.; Freeman, W.H.; Lightsey, R. Self-Efficacy: The Exercise of Control; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Banik, A.; Schwarzer, R.; Knoll, N.; Czekierda, K.; Luszczynska, A. Self-efficacy and quality of life among people with cardiovascular diseases: A meta-analysis. Rehabil. Psychol. 2018, 63, 295–312. [Google Scholar] [CrossRef]
- Castillo-Mayén, R.; Cano-Espejo, C.; Luque, B.; Cuadrado, E.; Gutiérrez-Domingo, T.; Arenas, A.; Rubio, S.; Delgado-Lista, J.; Pérez-Martínez, P.; Tabernero, C. Influence of Self-Efficacy and Motivation to Follow a Healthy Diet on Life Satisfaction of Patients with Cardiovascular Disease: A Longitudinal Study. Nutrients 2020, 12, 1903. [Google Scholar] [CrossRef]
- Akinosun, A.S.; Polson, R.; Skeete, Y.D.; De Kock, J.H.; Carragher, L.; Leslie, S.; Grindle, M.; Gorely, T. Digital Technology Interventions for Risk Factor Modification in Patients with Cardiovascular Disease: Systematic Review and Meta-analysis. JMIR mHealth uHealth 2021, 9, e21061. [Google Scholar] [CrossRef]
- Ramachandran, H.J.; Jiang, Y.; Tam, W.W.S.; Yeo, T.J.; Wang, W. Effectiveness of home-based cardiac telerehabilitation as an alternative to Phase 2 cardiac rehabilitation of coronary heart disease: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 29, 1017–1043. [Google Scholar] [CrossRef]
- Gómez-Gómez, I.; Bellón, J.; Resurrección, D.M.; Cuijpers, P.; Moreno-Peral, P.; Rigabert, A.; Maderuelo-Fernández, J.; Motrico, E. Effectiveness of universal multiple-risk lifestyle interventions in reducing depressive symptoms: Systematic review and meta-analysis. Prev. Med. 2020, 134, 106067. [Google Scholar] [CrossRef]
- Diener, E. Subjective well-being: The science of happiness and a proposal for a national index. Am. Psychol. 2000, 55, 34–43. [Google Scholar] [CrossRef]
- Diener, E.; Pressman, S.D.; Hunter, J.; Delgadillo-Chase, D. If, Why, and When Subjective Well-Being Influences Health, and Future Needed Research. Appl. Psychol. Heal. Well-Being 2017, 9, 133–167. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, R.; Bassett, S.M.; Boughton, S.W.; Schuette, S.A.; Shiu, E.W.; Moskowitz, J.T. Psychological Well-Being and Physical Health: Associations, Mechanisms, and Future Directions. Emot. Rev. 2018, 10, 18–29. [Google Scholar] [CrossRef]
- Kroemeke, A. Changes in well-being after myocardial infarction: Does coping matter? Qual. Life Res. 2016, 25, 2593–2601. [Google Scholar] [CrossRef] [Green Version]
- Ritter, P.L.; Lorig, K. The English and Spanish Self-Efficacy to Manage Chronic Disease Scale measures were validated using multiple studies. J. Clin. Epidemiol. 2014, 67, 1265–1273. [Google Scholar] [CrossRef]
- Steca, P.; Greco, A.; Cappelletti, E.; D’Addario, M.; Monzani, D.; Pancani, L.; Ferrari, G.; Politi, A.; Gestra, R.; Malfatto, G.; et al. Cardiovascular Management Self-efficacy: Psychometric Properties of a New Scale and Its Usefulness in a Rehabilitation Context. Ann. Behav. Med. 2015, 49, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Marogna, C.; Russo, S.E.; Caccamo, F.; Pinton, A.; Sava, V.; Carlon, R. The perception of the illness and the self-efficacy in the management of emotions in cardiac patients. Res. Psychother. Psychopathol. Process Outcome 2018, 21, 310. [Google Scholar] [CrossRef]
- Miller, G. The Smartphone Psychology Manifesto. Perspect. Psychol. Sci. 2012, 7, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Burke, L.E.; Ma, J.; Azar, K.M.; Bennett, G.G.; Peterson, E.D.; Zheng, Y.; Riley, W.J.; Stephens, J.; Shah, S.H.; Suffoletto, B.; et al. Current Science on Consumer Use of Mobile Health for Cardiovascular Disease Prevention. Circulation 2015, 132, 1157–1213. [Google Scholar] [CrossRef]
- Legler, S.; Celano, C.M.; Beale, E.E.; Hoeppner, B.B.; Huffman, J.C. Use of text messages to increase positive affect and promote physical activity in patients with heart disease. Curr. Psychol. 2018, 39, 648–655. [Google Scholar] [CrossRef]
- Tang, Y.-Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, P.; Sun, J.; Sun, Y.; Shao, D.; Cao, D.; Cao, F. Prenatal stress self-help mindfulness intervention via social media: A randomized controlled trial. J. Mental Health 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Centeno, R.P.R.; Fernandez, K.T.G. Effect of Mindfulness on Empathy and Self-Compassion: An Adapted MBCT Program on Filipino College Students. Behav. Sci. 2020, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Fissler, M.; Winnebeck, E.; Schroeter, T.; Gummersbach, M.; Huntenburg, J.M.; Gaertner, M.; Barnhofer, T. An Investigation of the Effects of Brief Mindfulness Training on Self-Reported Interoceptive Awareness, the Ability to Decenter, and Their Role in the Reduction of Depressive Symptoms. Mindfulness 2016, 7, 1170–1181. [Google Scholar] [CrossRef]
- Alsubaie, M.; Dickens, C.; Dunn, B.D.; Gibson, A.; Ukoumunne, O.C.; Evans, A.; Vicary, R.; Gandhi, M.; Kuyken, W. Feasibility and Acceptability of Mindfulness-based Cognitive Therapy Compared with Mindfulness-based Stress Reduction and Treatment as Usual in People with Depression and Cardiovascular Disorders: A Three-Arm Randomised Controlled Trial. Mindfulness 2020, 11, 30–50. [Google Scholar] [CrossRef]
- Kraft, S.; Wolf, M.; Klein, T.; Becker, T.; Bauer, S.; Puschner, B.; Birney, A.; Kannisto, K. Text Message Feedback to Support Mindfulness Practice in People with Depressive Symptoms: A Pilot Randomized Controlled Trial. JMIR mHealth uHealth 2017, 5, e59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckenberg, R.A.; Hale, M.W.; Kent, S.; Wright, B.J. An online mindfulness-based program is effective in improving affect, over-commitment, optimism and mucosal immunity. Physiol. Behav. 2018, 199, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.C.; Millstein, R.A.; Mastromauro, C.A.; Moore, S.V.; Celano, C.M.; Bedoya, C.A.; Suarez, L.; Boehm, J.K.; Januzzi, J.L. A Positive Psychology Intervention for Patients with an Acute Coronary Syndrome: Treatment Development and Proof-of-Concept Trial. J. Happiness Stud. 2015, 17, 1985–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjuán, P.; Montalbetti, T.; Pérez-García, A.M.; Bermúdez, J.; Arranz, H.; Castro, A. A Randomised Trial of a Positive Intervention to Promote Well-Being in Cardiac Patients. Appl. Psychol. Health Well-Being 2016, 8, 64–84. [Google Scholar] [CrossRef]
- Nikrahan, G.R.; Laferton, J.A.; Asgari, K.; Kalantari, M.; Abedi, M.R.; Etesampour, A.; Rezaei, A.; Suarez, L.; Huffman, J.C. Effects of Positive Psychology Interventions on Risk Biomarkers in Coronary Patients: A Randomized, Wait-List Controlled Pilot Trial. J. Psychosom. Res. 2016, 57, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Rash, J.A.; Matsuba, M.K.; Prkachin, K.M. Gratitude and Well-Being: Who Benefits the Most from a Gratitude Intervention? Appl. Psychol. Health Well-Being 2011, 3, 350–369. [Google Scholar] [CrossRef]
- Henning, M.; Fox, G.R. Kaplan, J. Damasio, H. Damasio, A. A potential role for mu-opioids in mediating the positive effects of gratitude. Front. Psychol. 2017, 8, 868. [Google Scholar] [CrossRef] [Green Version]
- Moieni, M.; Irwin, M.R.; Haltom, K.E.B.; Jevtic, I.; Meyer, M.L.; Breen, E.C.; Cole, S.W.; Eisenberger, N.I. Exploring the role of gratitude and support-giving on inflammatory outcomes. Emotion 2019, 19, 939–949. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using SPSS: And Sex and Drugs and Rock ‘n’ roll, 5th ed.; Sage: London, UK, 2017. [Google Scholar]
- Kotrlik, J.W.; Williams, H.A. The incorporation of effect size in information technology, learning, and performance research. Inf. Technol. Learn. Perform. J. 2003, 21, 1–7. [Google Scholar]
- Bartlett, J. Introduction to Sample Size Calculation Using G* Power. 2019. Available online: https://files.osf.io/v1/resources/pcfvj/providers/osfstorage/5dcea84d5b97bd000e57aba0?action=download&version=1&direct (accessed on 21 October 2022).
- Des Jarlais, D.C.; Lyles, C.; Crepaz, N.; the TREND Group. Improving the Reporting Quality of Nonrandomized Evaluations of Behavioral and Public Health Interventions: The TREND Statement. Am. J. Public Health 2004, 94, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Trials 2010, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabat-Zinn, J. Full Catastrophe Living; Delacorte: New York, NY, USA, 1990. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Castillo-Mayén, R.; Luque, B.; Rubio, S.J.; Cuadrado, E.; Gutiérrez-Domingo, T.; Arenas, A.; Delgado-Lista, J.; Pérez-Martínez, P.; Tabernero, C. Positive psychological profiles based on perceived health clustering in patients with cardiovascular disease: A longitudinal study. BMJ Open 2021, 11, e050818. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Mayén, R.; Luque, B.; Gutiérrez-Domingo, T.; Cuadrado, E.; Arenas, A.; Rubio, S.; Quintana-Navarro, G.M.; Delgado-Lista, J.; Tabernero, C. Emotion regulation in patients with cardiovascular disease: Development and validation of the stress and anxiety regulation strategies scale (STARTS). Anxiety Stress Coping 2020, 34, 349–364. [Google Scholar] [CrossRef]
- Selya, A.S.; Rose, J.S.; Dierker, L.C.; Hedeker, D.; Mermelstein, R.J. A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Front. Psychol. 2012, 3, 111. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, F.A.; Lu, L.M.; Sollers, J.J.; Krivoschekov, S.G.; Booth, R.J.; Consedine, N.S. Why It Pays to be Mindful: Trait Mindfulness Predicts Physiological Recovery from Emotional Stress and Greater Differentiation among Negative Emotions. Mindfulness 2015, 6, 175–185. [Google Scholar] [CrossRef]
- De Vibe, M.; Solhaug, I.; Rosenvinge, J.H.; Tyssen, R.; Hanley, A.; Garland, E. Six-year positive effects of a mindfulness-based intervention on mindfulness, coping and well-being in medical and psychology students; Results from a randomized controlled trial. PLoS ONE 2018, 13, e0196053. [Google Scholar] [CrossRef] [Green Version]
- Jacquet-Smailovic, M.; Brennsthul, M.-J.; Denis, I.; Kirche, A.; Tarquinio, C.; Tarquinio, C. Relationship between Post-traumatic Stress Disorder and subsequent myocardial infarction: A systematic review and meta-analysis. J. Affect. Disord. 2022, 297, 525–535. [Google Scholar] [CrossRef]
- Schotanus-Dijkstra, M.; Pieterse, M.E.; Drossaert, C.H.C.; Walburg, J.A.; Bohlmeijer, E.T. Possible mechanisms in a multicomponent email guided positive psychology intervention to improve mental well-being, anxiety and depression: A multiple mediation model. J. Posit. Psychol. 2019, 14, 141–155. [Google Scholar] [CrossRef]
- Tomlinson, M.; Rotheram-Borus, M.J.; Swartz, L.; Tsai, A.C. Scaling Up mHealth: Where Is the Evidence? PLOS Med. 2013, 10, e1001382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, J.K.; Chen, Y.; Qureshi, F.; Soo, J.; Umukoro, P.; Hernandez, R.; Lloyd-Jones, D.; Kubzansky, L.D. Positive emotions and favorable cardiovascular health: A 20-year longitudinal study. Prev. Med. 2020, 136, 106103. [Google Scholar] [CrossRef]
- DuBois, C.; Lopez, O.V.; Beale, E.E.; Healy, B.C.; Boehm, J.K.; Huffman, J.C. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: A systematic review. Int. J. Cardiol. 2015, 195, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Vickers, A.J. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Med. Res. Methodol. 2005, 5, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.P.; Liang, W.; Guo, L.; Wienert, J.; Si, G.Y.; Lippke, S. Evaluation of a Web-Based Intervention for Multiple Health Behavior Changes in Patients with Coronary Heart Disease in Home-Based Rehabilitation: Pilot Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e12052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensorio, M.S.; Cebolla-Martí, A.; Rodilla, E.; Palomar, G.; Lisón, J.F.; Botella, C.; Fernández-Aranda, F.; Jimenez-Murcia, S.; Baños, R.M. Analysis of the efficacy of an internet-based self-administered intervention (“Living Better”) to promote healthy habits in a population with obesity and hypertension: An exploratory randomized controlled trial. Int. J. Med. Inform. 2019, 124, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, F.; Saltini, S.; Carella, E.; Carlon, R.; Marogna, C.; Sava, V. The measure of effectiveness of a short-term 2-week intensive Cardiac Rehabilitation program in decreasing levels of anxiety and depression. Monaldi Arch. Chest Dis. 2018, 88, 858. [Google Scholar] [CrossRef] [Green Version]
- Fineberg, N.A.; Baldwin, D.S.; Drummond, L.M.; Wyatt, S.; Hanson, J.; Gopi, S.; Kaur, S.; Reid, J.; Marwah, V.; Sachdev, R.A.; et al. Optimal treatment for obsessive compulsive disorder: A randomized controlled feasibility study of the clinical-effectiveness and cost-effectiveness of cognitive-behavioural therapy, selective serotonin reuptake inhibitors and their combination in the management of obsessive compulsive disorder. Int. Clin. Psychopharmacol. 2018, 33, 334–348. [Google Scholar] [CrossRef] [Green Version]
- Meyerowitz-Katz, G.; Ravi, S.; Arnolda, L.; Feng, X.; Maberly, G.; Astell-Burt, T. Rates of Attrition and Dropout in App-Based Interventions for Chronic Disease: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e20283. [Google Scholar] [CrossRef]
- Ivtzan, I.; Young, T.; Martman, J.; Jeffrey, A.; Lomas, T.; Hart, R.; Eiroa-Orosa, F.J. Integrating Mindfulness into Positive Psychology: A Randomised Controlled Trial of an Online Positive Mindfulness Program. Mindfulness 2016, 7, 1396–1407. [Google Scholar] [CrossRef]
- Diener, E.; Emmons, R.A.; Larsen, R.J.; Griffin, S. The Satisfaction with Life Scale. J. Pers. Assess. 1985, 49, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S.; Anderson, K.L. The Quality of Life Scale (QOLS): Reliability, Validity, and Utilization. Heal. Qual. Life Outcomes 2003, 1, 60. [Google Scholar] [CrossRef]

| Total (n = 93) | Mindfulness Group (n = 32) | Positive Strengthening Group (n = 32) | TAU Group (n = 29) | Statistical Significance | |
|---|---|---|---|---|---|
| Age (M, SD) | 63.9 (10.9) | 64.8 (10.1) | 61 (10.3) | 66.2 (12.1) | (F(2,90)= 1.87, ns) |
| Gender, n (%) | (X2 = 4.59, gl 2, ns) | ||||
| Male | 76 (82%) | 28 | 28 | 20 | |
| Female | 17 (18%) | 4 | 4 | 9 | |
| Marital status n (%) | (X2 = 17, df 10, ns) | ||||
| Single | 6 (7%) | 4 | 1 | 1 | |
| Single with couple | 2 (2%) | 2 | 0 | 0 | |
| Cohabiting partner | 1 (1%) | 1 | 0 | 0 | |
| Married | 79(85%) | 24 | 31 | 24 | |
| Separated | 2 (2%) | 1 | 0 | 1 | |
| Widowed | 3 (3%) | 0 | 0 | 3 | |
| Employment status n (%) | (X2 = 5.4, gl 8, ns) | ||||
| Retired | 60 (65%) | 21 | 17 | 22 | |
| Part-time work | 1 (1%) | 0 | 1 | 0 | |
| Full-time work | 22(24%) | 7 | 10 | 5 | |
| Unemployed | 7 (7%) | 3 | 3 | 1 | |
| Home care | 3 (3%) | 1 | 1 | 1 | |
| Educational level n (%) | (X2 = 19.28, gl 8, ns) | ||||
| Elementary school | 68 (73%) | 22 | 23 | 23 | |
| Middle school | 6 (7%) | 0 | 5 | 1 | |
| High school | 11 (12%) | 6 | 3 | 2 | |
| University | 8 (9%) | 4 | 1 | 3 | |
| Economic level | (X2 = 14.1, gl 6, ns) | ||||
| <22,000 € | 78 (84%) | 25 | 26 | 27 | |
| 22,000–43,000 € | 13 (14%) | 6 | 5 | 2 | |
| >43,000 € | 2 (2%) | 1 | 1 | 0 | |
| Age onset CVD (M, SD) | 60 (11.5) | 61.1 (10.8) | 54.1 (13.24) | 63.0 (11.8) | (F(2,90) = 4.63, p < 0.01) |
| Time with CVD (M, SD) | 3.82 (6.04) | 3.81 (5.10) | 5.41 (7.77) | 2.07 (4.27) | (F(2,39) = 0.097, ns) |
| Type of CVD n (%) | |||||
| Angina pectoris | 31 (33%) | 12 | 13 | 6 | (X2= 3.1, gl 2, ns) |
| Myocardial infarction | 42 (45%) | 11 | 16 | 15 | (X2= 2.31, gl 2, ns) |
| Hearth failure | 8 (9%) | 3 | 1 | 4 | (X2= 2.24, gl 2, ns) |
| Arrhythmia | 9 (10%) | 3 | 3 | 3 | (X2= 0.02, gl 2, ns) |
| Other | 19 (20%) | 9 | 6 | 4 | (X2= 2, gl 2, ns) |
| More than one CVD n (%) | 16 (17.2%) | 6 | 7 | 3 | |
| Level of limitation of ADL n (%) | |||||
| Level 1 | 43 | 19 | 14 | 10 | (X2= 3.9, gl 2, ns) |
| Level 2 | 27 | 8 | 9 | 10 | (X2 = 0.68, gl 8, ns) |
| Level 3 | 19 | 3 | 7 | 9 | (X2 = 4.45, gl 2, ns) |
| Level 4 | 4 | 2 | 2 | 0 | (X2 = 1.89, gl 2, ns) |
| Variable | Mean Square | F | df | Error df | p | ηp2 | Power |
|---|---|---|---|---|---|---|---|
| Positive affect | |||||||
| Time | 6.22 | 8.39 | 2.85 | 250.78 | <0.001 | 0.09 | 0.99 |
| Time × anxiety | 0.38 | 0.52 | 2.85 | 250.78 | 0.661 | 0.01 | 0.15 |
| Time × depression | 1.26 | 1.70 | 2.85 | 250.78 | 0.171 | 0.02 | 0.43 |
| Time × condition | 8.56 | 5.77 | 5.70 | 250.78 | <0.001 | 0.12 | 1.00 |
| Error | 65.29 | ||||||
| Negative affect | |||||||
| Time | 4.25 | 10.83 | 2.69 | 236.48 | <0.001 | 0.11 | 1.00 |
| Time × anxiety | 5.13 | 13.08 | 2.69 | 236.48 | <0.001 | 0.13 | 1.00 |
| Time × depression | 0.41 | 1.04 | 2.69 | 236.48 | 0.370 | 0.01 | 0.27 |
| Time × condition | 4.47 | 5.69 | 5.38 | 236.48 | <0.001 | 0.12 | 1.00 |
| Error | 34.53 | ||||||
| CMSE | |||||||
| Time | 3.73 | 10.78 | 2.12 | 185.39 | <0.001 | 0.11 | 0.99 |
| Time × anxiety | 0.21 | 0.61 | 2.12 | 185.39 | 0.552 | 0.01 | 0.15 |
| Time × depression | 1.09 | 3.16 | 2.12 | 185.39 | 0.042 | 0.04 | 0.62 |
| Time × condition | 4.58 | 6.62 | 4.21 | 185.39 | <0.001 | 0.13 | 0.99 |
| Error | 30.46 | ||||||
| SEMCD | |||||||
| Time | 20.78 | 7.18 | 2.98 | 262.35 | <0.001 | 0.08 | 0.98 |
| Time × anxiety | 3.70 | 1.28 | 2.98 | 262.35 | 0.281 | 0.01 | 0.34 |
| Time × depression | 8.74 | 3.02 | 2.98 | 262.35 | 0.031 | 0.03 | 0.71 |
| Time × condition | 14.05 | 2.43 | 5.96 | 262.35 | 0.027 | 0.05 | 0.82 |
| Error | 254.55 |
| Mindfulness M (SD) | Positive Strengthening M (SD) | ANOVA F (1,62); p | |
|---|---|---|---|
| Post-test 1 | |||
| Engagement | 4.54 (0.66) | 4.33 (0.85) | 1.29, 0.26 |
| General satisfaction | 9.09 (0.92) | 8.73 (1.44) | 1.51, 0.22 |
| Post-test 2 | |||
| Engagement | 3.67 (1.02) | 4.03 (0.86) | 2.42, 0.13 |
| General satisfaction | 8.85 (1.44) | 8.97 (1.03) | 0.15, 0.70 |
| Post-test 3 | |||
| Engagement | 3.88 (0.98) | 3.75 (0.88) | 0.29, 0.59 |
| General satisfaction | 9.31 (1.18) | 9.00 (1.02) | 1.29, 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabernero, C.; Gutiérrez-Domingo, T.; Steca, P.; Castillo-Mayén, R.; Cuadrado, E.; Rubio, S.J.; Farhane-Medina, N.Z.; Luque, B. Effectiveness of Mindfulness and Positive Strengthening mHealth Interventions for the Promotion of Subjective Emotional Wellbeing and Management of Self-Efficacy for Chronic Cardiac Diseases. J. Pers. Med. 2022, 12, 1953. https://doi.org/10.3390/jpm12121953
Tabernero C, Gutiérrez-Domingo T, Steca P, Castillo-Mayén R, Cuadrado E, Rubio SJ, Farhane-Medina NZ, Luque B. Effectiveness of Mindfulness and Positive Strengthening mHealth Interventions for the Promotion of Subjective Emotional Wellbeing and Management of Self-Efficacy for Chronic Cardiac Diseases. Journal of Personalized Medicine. 2022; 12(12):1953. https://doi.org/10.3390/jpm12121953
Chicago/Turabian StyleTabernero, Carmen, Tamara Gutiérrez-Domingo, Patrizia Steca, Rosario Castillo-Mayén, Esther Cuadrado, Sebastián J. Rubio, Naima Z. Farhane-Medina, and Bárbara Luque. 2022. "Effectiveness of Mindfulness and Positive Strengthening mHealth Interventions for the Promotion of Subjective Emotional Wellbeing and Management of Self-Efficacy for Chronic Cardiac Diseases" Journal of Personalized Medicine 12, no. 12: 1953. https://doi.org/10.3390/jpm12121953
APA StyleTabernero, C., Gutiérrez-Domingo, T., Steca, P., Castillo-Mayén, R., Cuadrado, E., Rubio, S. J., Farhane-Medina, N. Z., & Luque, B. (2022). Effectiveness of Mindfulness and Positive Strengthening mHealth Interventions for the Promotion of Subjective Emotional Wellbeing and Management of Self-Efficacy for Chronic Cardiac Diseases. Journal of Personalized Medicine, 12(12), 1953. https://doi.org/10.3390/jpm12121953









