High-Frequency Transcranial Random Noise Stimulation Modulates Gamma-Band EEG Source-Based Large-Scale Functional Network Connectivity in Patients with Schizophrenia: A Randomized, Double-Blind, Sham-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. The Sample Size Calculation of the Participants
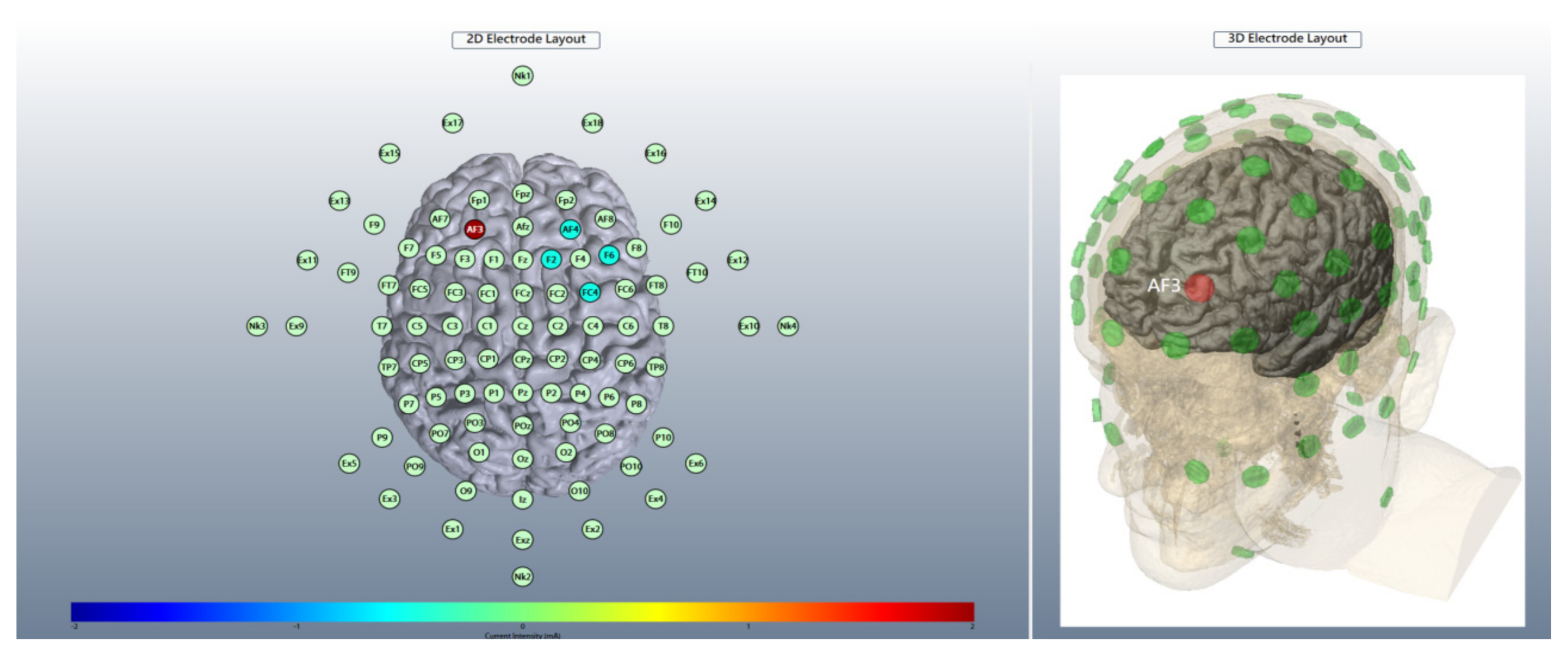
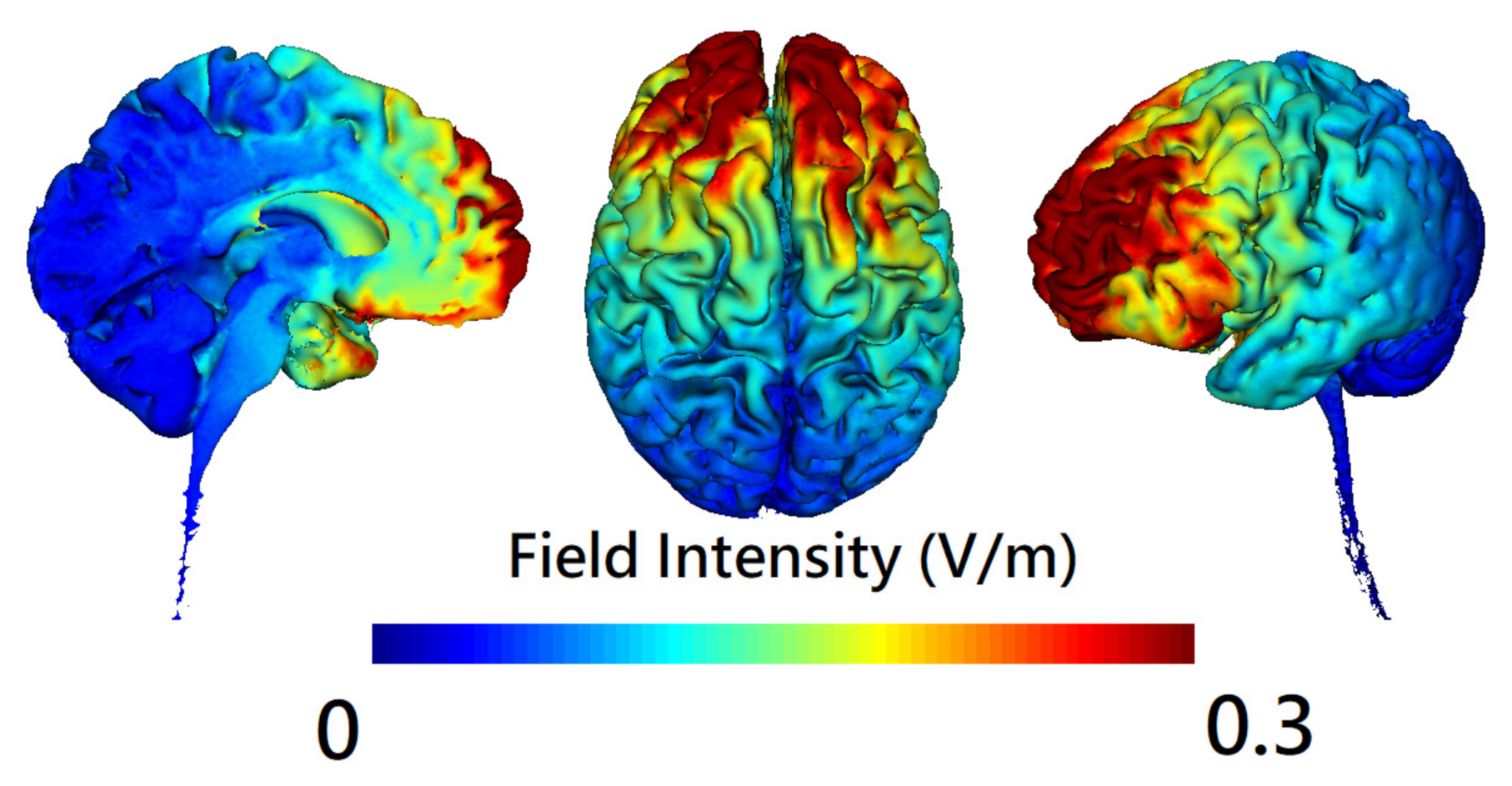
2.4. Brain Stimulation
2.5. Randomization and Blinding
2.6. EEG at Rest
2.7. Electrical Source Estimation
2.8. Source-Based Functional Connectivity
2.9. Statistical Analyses
3. Results
3.1. Effects of hf-tRNS on Scalp- and Source-Level Gamma-Band Power
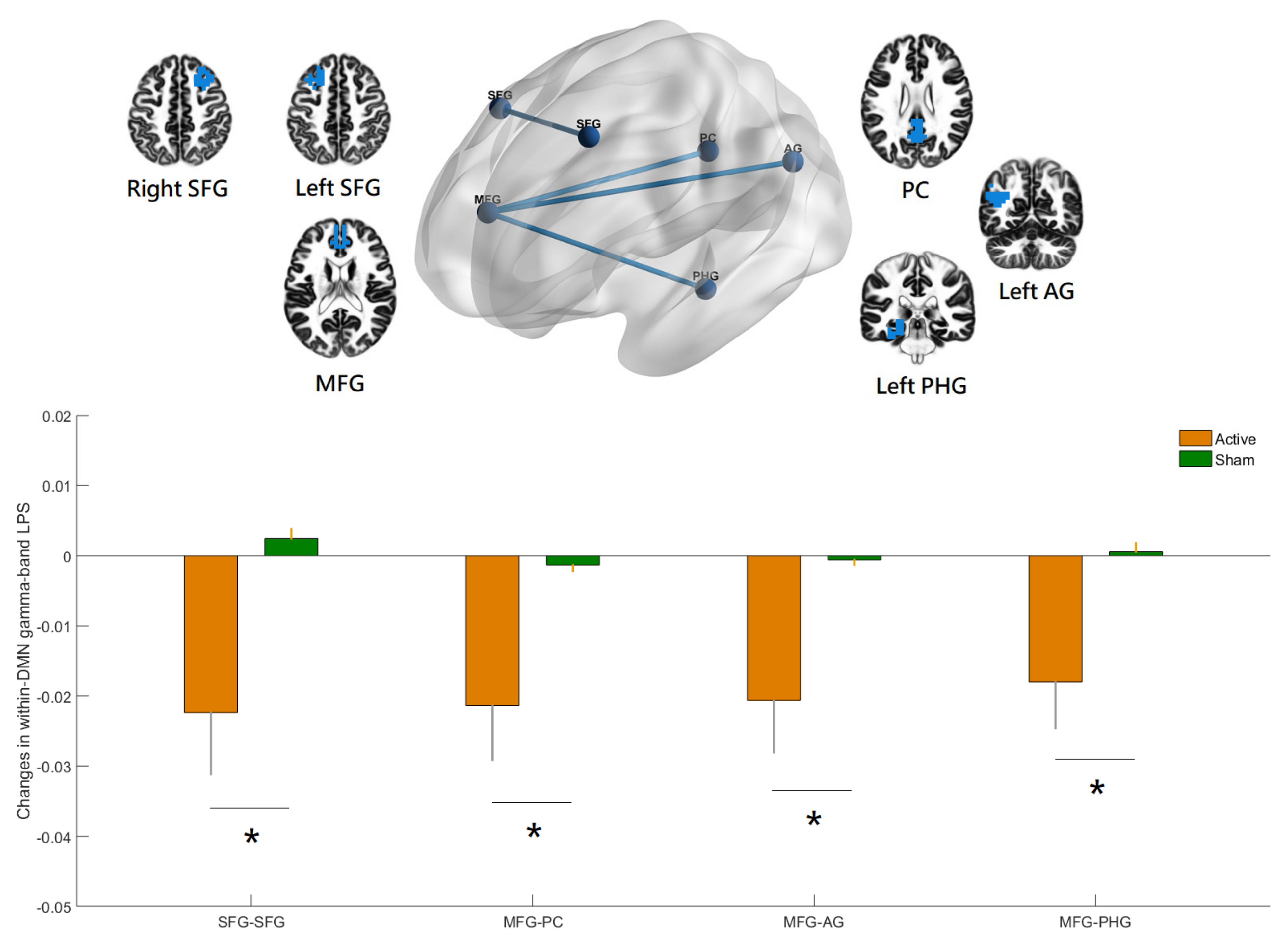
3.2. Effects of hf-tRNS on Source-Based Gamma-Band within-Network Connectivity
3.3. Effects of hf-tRNS on Source-Based Gamma-Band between-Network Connectivity
3.4. Effects of hf-tRNS on Source-Based Gamma-Band Whole-Brain Functional Connectivity
3.5. Correlation Analyses
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Papanastasiou, E.; Stahl, D.; Rocchetti, M.; Carpenter, W.; Shergill, S.; McGuire, P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr. Bull. 2015, 41, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Osoegawa, C.; Gomes, J.S.; Grigolon, R.B.; Brietzke, E.; Gadelha, A.; Lacerda, A.L.T.; Dias, A.M.; Cordeiro, Q.; Laranjeira, R.; de Jesus, D.; et al. Non-invasive brain stimulation for negative symptoms in schizophrenia: An updated systematic review and meta-analysis. Schizophr. Res. 2018, 197, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lin, Y.Y.; Tzeng, N.S.; Kao, Y.C.; Chang, H.A. Adjunct high-frequency transcranial random noise stimulation over the lateral prefrontal cortex improves negative symptoms of schizophrenia: A randomized, double-blind, sham-controlled pilot study. J. Psychiatr. Res. 2021, 132, 151–160. [Google Scholar] [CrossRef]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef] [PubMed]
- Inukai, Y.; Saito, K.; Sasaki, R.; Tsuiki, S.; Miyaguchi, S.; Kojima, S.; Masaki, M.; Otsuru, N.; Onishi, H. Comparison of Three Non-Invasive Transcranial Electrical Stimulation Methods for Increasing Cortical Excitability. Front. Hum. Neurosci. 2016, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Ghin, F.; O’Hare, L.; Pavan, A. Electrophysiological aftereffects of high-frequency transcranial random noise stimulation (hf-tRNS): An EEG investigation. Exp. Brain Res. 2021, 239, 2399–2418. [Google Scholar] [CrossRef]
- Snowball, A.; Tachtsidis, I.; Popescu, T.; Thompson, J.; Delazer, M.; Zamarian, L.; Zhu, T.; Cohen Kadosh, R. Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Curr. Biol. 2013, 23, 987–992. [Google Scholar] [CrossRef]
- Xi, Y.B.; Guo, F.; Liu, W.M.; Fu, Y.F.; Li, J.M.; Wang, H.N.; Chen, F.L.; Cui, L.B.; Zhu, Y.Q.; Li, C.; et al. Triple network hypothesis-related disrupted connections in schizophrenia: A spectral dynamic causal modeling analysis with functional magnetic resonance imaging. Schizophr. Res. 2021, 233, 89–96. [Google Scholar] [CrossRef]
- Koban, L.; Gianaros, P.J.; Kober, H.; Wager, T.D. The self in context: Brain systems linking mental and physical health. Nat. Rev. Neurosci. 2021, 22, 309–322. [Google Scholar] [CrossRef]
- Hua, J.P.Y.; Karcher, N.R.; Merrill, A.M.; O’Brien, K.J.; Straub, K.T.; Trull, T.J.; Kerns, J.G. Psychosis risk is associated with decreased resting-state functional connectivity between the striatum and the default mode network. Cogn. Affect. Behav. Neurosci. 2019, 19, 998–1011. [Google Scholar] [CrossRef]
- Di Plinio, S.; Perrucci, M.G.; Aleman, A.; Ebisch, S.J.H. I am Me: Brain systems integrate and segregate to establish a multidimensional sense of self. Neuroimage 2020, 205, 116284. [Google Scholar] [CrossRef]
- Fan, Y.S.; Li, H.; Guo, J.; Pang, Y.; Li, L.; Hu, M.; Li, M.; Wang, C.; Sheng, W.; Liu, H.; et al. Tracking Positive and Negative Symptom Improvement in First-Episode Schizophrenia Treated with Risperidone Using Individual-Level Functional Connectivity. Brain Connect. 2022, 12, 454–464. [Google Scholar] [CrossRef]
- Krukow, P.; Jonak, K.; Grochowski, C.; Plechawska-Wojcik, M.; Karakula-Juchnowicz, H. Resting-state hyperconnectivity within the default mode network impedes the ability to initiate cognitive performance in first-episode schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109959. [Google Scholar] [CrossRef]
- Choi, K.M.; Kim, J.Y.; Kim, Y.W.; Han, J.W.; Im, C.H.; Lee, S.H. Comparative analysis of default mode networks in major psychiatric disorders using resting-state EEG. Sci. Rep. 2021, 11, 22007. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100–113. [Google Scholar] [CrossRef]
- Baradits, M.; Kakuszi, B.; Balint, S.; Fullajtar, M.; Mod, L.; Bitter, I.; Czobor, P. Alterations in resting-state gamma activity in patients with schizophrenia: A high-density EEG study. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 429–437. [Google Scholar] [CrossRef]
- Andreou, C.; Nolte, G.; Leicht, G.; Polomac, N.; Hanganu-Opatz, I.L.; Lambert, M.; Engel, A.K.; Mulert, C. Increased Resting-State Gamma-Band Connectivity in First-Episode Schizophrenia. Schizophr. Bull. 2015, 41, 930–939. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Daverio, A.; Ferrentino, F.; Santarnecchi, E.; Ciabattini, F.; Monaco, L.; Lisi, G.; Barone, Y.; Di Lorenzo, C.; Niolu, C.; et al. Altered resting-state EEG source functional connectivity in schizophrenia: The effect of illness duration. Front. Hum. Neurosci. 2015, 9, 234. [Google Scholar] [CrossRef]
- Millan, M.J.; Fone, K.; Steckler, T.; Horan, W.P. Negative symptoms of schizophrenia: Clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol. 2014, 24, 645–692. [Google Scholar] [CrossRef]
- Curic, S.; Andreou, C.; Nolte, G.; Steinmann, S.; Thiebes, S.; Polomac, N.; Haaf, M.; Rauh, J.; Leicht, G.; Mulert, C. Ketamine Alters Functional Gamma and Theta Resting-State Connectivity in Healthy Humans: Implications for Schizophrenia Treatment Targeting the Glutamate System. Front. Psychiatry 2021, 12, 671007. [Google Scholar] [CrossRef]
- Marder, S.R.; Davis, J.M.; Chouinard, G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. J. Clin. Psychiatry 1997, 58, 538–546. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsu, S.H.; Pion-Tonachini, L.; Jung, T.P. Evaluation of Artifact Subspace Reconstruction for Automatic Artifact Components Removal in Multi-Channel EEG Recordings. IEEE Trans. Biomed. Eng. 2020, 67, 1114–1121. [Google Scholar] [CrossRef]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. Neuroimage 2019, 198, 181–197. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- Olbrich, S.; Mulert, C.; Karch, S.; Trenner, M.; Leicht, G.; Pogarell, O.; Hegerl, U. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage 2009, 45, 319–332. [Google Scholar] [CrossRef]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zollei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Murillo, M.A.; Trevino, M.; Manjarrez, E. Random noise stimulation in the treatment of patients with neurological disorders. Neural Regen. Res. 2022, 17, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.S.; Ahmed, F.; Al-Fahad, R.; Moinuddin, K.A.; Yeasin, M.; Alain, C.; Bidelman, G.M. Decoding Hearing-Related Changes in Older Adults’ Spatiotemporal Neural Processing of Speech Using Machine Learning. Front. Neurosci. 2020, 14, 748. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.O., Jr.; Gonsalvez, I.; Lee, I.; Ongur, D.; Seidman, L.J.; Schmahmann, J.D.; Eack, S.M.; Keshavan, M.S.; Pascual-Leone, A.; Halko, M.A. Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. Am. J. Psychiatry 2019, 176, 512–520. [Google Scholar] [CrossRef]
- Whitton, A.E.; Deccy, S.; Ironside, M.L.; Kumar, P.; Beltzer, M.; Pizzagalli, D.A. Electroencephalography Source Functional Connectivity Reveals Abnormal High-Frequency Communication Among Large-Scale Functional Networks in Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 50–58. [Google Scholar] [CrossRef]
- Chen, B. Abnormal regions in functional connectivity of chronic schizophrenia. Int. J. Neurosci. 2018, 128, 1150–1156. [Google Scholar] [CrossRef]
- Lerman-Sinkoff, D.B.; Barch, D.M. Network community structure alterations in adult schizophrenia: Identification and localization of alterations. Neuroimage Clin. 2016, 10, 96–106. [Google Scholar] [CrossRef]
- Schlegelmilch, F.; Markert, S.; Berkes, S.; Schellhorn, K. Online ocular artifact removal for dc-EEG-signals: Estimation of dc-level. Biomed. Tech. 2004, 2, 340–341. [Google Scholar]

| hf-tRNS (N = 17) | Sham (N = 18) | |
|---|---|---|
| Gender (f/m) | 6/11 | 8/10 |
| Handedness (r/l) | 15/2 | 16/2 |
| Age, years old | 44.06 ± 12.50 | 43.17 ± 11.63 |
| Years of education, years | 13.53 ± 2.32 | 12.44 ± 3.52 |
| Years since diagnosis, years | 18.82 ± 9.73 | 19.11 ± 13.35 |
| Chlorpromazine equivalent dose, mg/day | 581.70 ± 310.59 | 626.10 ± 298.82 |
| PANSS total score | 69.00 ± 9.64 | 71.39 ± 9.06 |
| PANSS Factor Score for Negative Symptoms (FSNS) | 21.29 ± 3.00 | 21.94 ± 4.40 |
| PANSS Factor Score for Positive Symptoms | 11.71 ± 3.46 | 12.61 ± 3.74 |
| PANSS Factor Score for Excitement | 5.59 ± 2.48 | 5.28 ± 1.81 |
| PANSS Factor Score for Disorganization | 11.88 ± 1.27 | 12.11 ± 2.14 |
| PANSS Factor Score for Emotional distress | 5.82 ± 2.46 | 6.39 ± 1.50 |
| Network | Anatomic Structure | Side | XYZ (MNI) | ||
|---|---|---|---|---|---|
| DMN | Superior frontal gyrus | L | −27 | 23 | 48 |
| Superior frontal gyrus | R | 27 | 23 | 48 | |
| Angular gyrus | L | −41 | −60 | 29 | |
| Angular gyrus | R | 41 | −60 | 29 | |
| Middle temporal gyrus | L | −64 | −20 | −9 | |
| Middle temporal gyrus | R | 64 | −20 | −9 | |
| Medial frontal gyrus | Mid | 0 | 49 | 18 | |
| Parahippocampal gyrus | L | −25 | −32 | −18 | |
| Parahippocampal gyrus | R | 25 | −32 | −18 | |
| Posterior cingulate | Mid | 0 | −52 | 26 | |
| FPN | Frontal pole | L | −40 | 50 | 7 |
| Frontal pole | R | 40 | 50 | 7 | |
| Supramarginal gyrus | L | −43 | −50 | 46 | |
| Supramarginal gyrus | R | 43 | −50 | 46 | |
| Middle temporal gyrus | L | −57 | −54 | −9 | |
| Middle temporal gyrus | R | 57 | −54 | −9 | |
| Paracingulate gyrus | Mid | 0 | 22 | 47 | |
| Cingulate gyrus | Mid | 0 | 4 | 29 | |
| Precuneus cortex | Mid | 0 | −76 | 45 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, T.-C.; Huang, C.C.-Y.; Chung, Y.-A.; Im, J.J.; Lin, Y.-Y.; Ma, C.-C.; Tzeng, N.-S.; Chang, H.-A. High-Frequency Transcranial Random Noise Stimulation Modulates Gamma-Band EEG Source-Based Large-Scale Functional Network Connectivity in Patients with Schizophrenia: A Randomized, Double-Blind, Sham-Controlled Clinical Trial. J. Pers. Med. 2022, 12, 1617. https://doi.org/10.3390/jpm12101617
Yeh T-C, Huang CC-Y, Chung Y-A, Im JJ, Lin Y-Y, Ma C-C, Tzeng N-S, Chang H-A. High-Frequency Transcranial Random Noise Stimulation Modulates Gamma-Band EEG Source-Based Large-Scale Functional Network Connectivity in Patients with Schizophrenia: A Randomized, Double-Blind, Sham-Controlled Clinical Trial. Journal of Personalized Medicine. 2022; 12(10):1617. https://doi.org/10.3390/jpm12101617
Chicago/Turabian StyleYeh, Ta-Chuan, Cathy Chia-Yu Huang, Yong-An Chung, Jooyeon Jamie Im, Yen-Yue Lin, Chin-Chao Ma, Nian-Sheng Tzeng, and Hsin-An Chang. 2022. "High-Frequency Transcranial Random Noise Stimulation Modulates Gamma-Band EEG Source-Based Large-Scale Functional Network Connectivity in Patients with Schizophrenia: A Randomized, Double-Blind, Sham-Controlled Clinical Trial" Journal of Personalized Medicine 12, no. 10: 1617. https://doi.org/10.3390/jpm12101617
APA StyleYeh, T.-C., Huang, C. C.-Y., Chung, Y.-A., Im, J. J., Lin, Y.-Y., Ma, C.-C., Tzeng, N.-S., & Chang, H.-A. (2022). High-Frequency Transcranial Random Noise Stimulation Modulates Gamma-Band EEG Source-Based Large-Scale Functional Network Connectivity in Patients with Schizophrenia: A Randomized, Double-Blind, Sham-Controlled Clinical Trial. Journal of Personalized Medicine, 12(10), 1617. https://doi.org/10.3390/jpm12101617








