Public Attitudes toward Pharmacogenomic Testing and Establishing a Statewide Pharmacogenomics Database in the State of Minnesota
Abstract
:1. Introduction
2. Materials and Methods
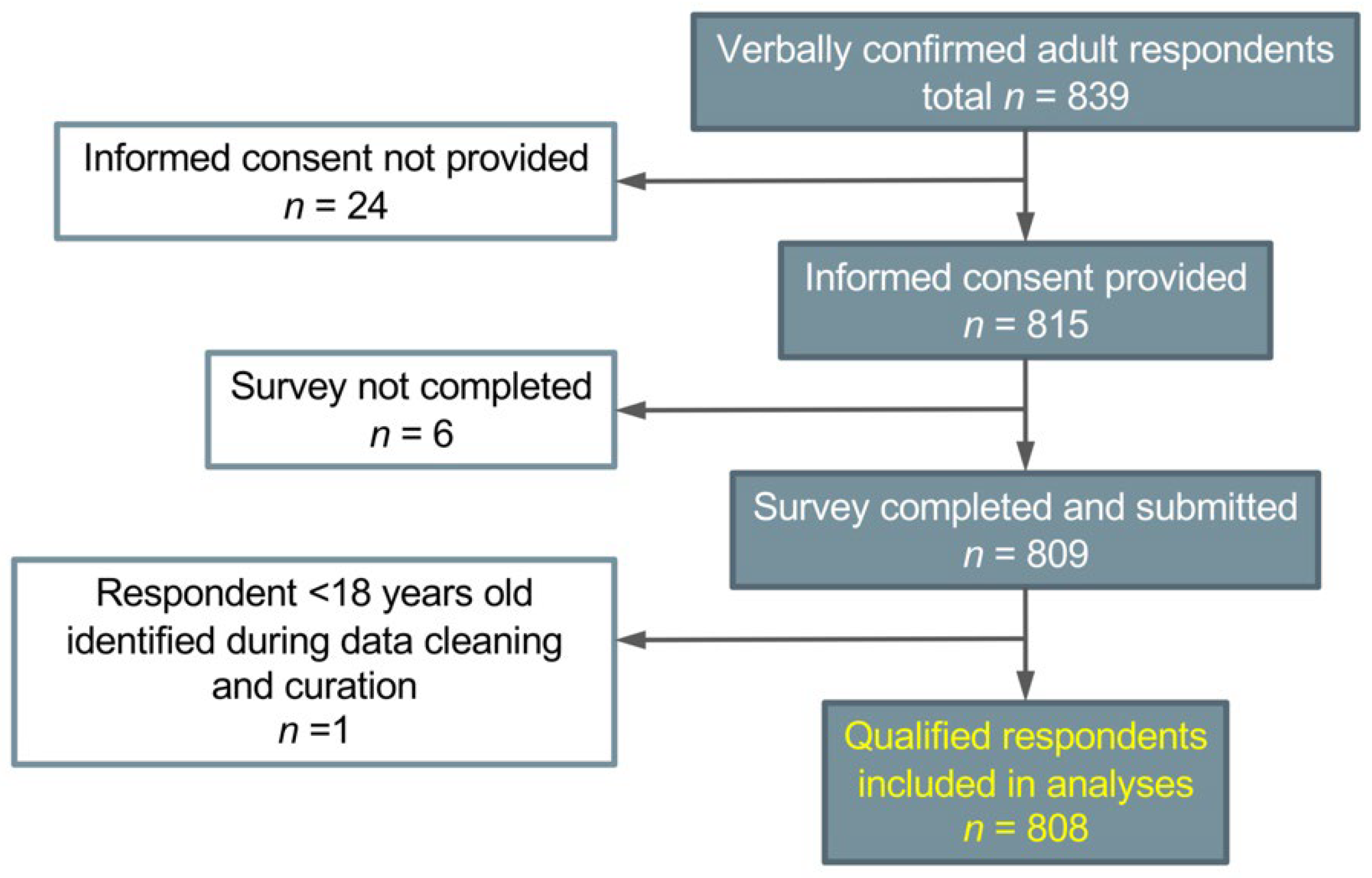
2.1. Eligibility and Recruitment
2.2. Survey Design and Measures
2.3. Data Analysis
3. Results
3.1. Participant Characteristics
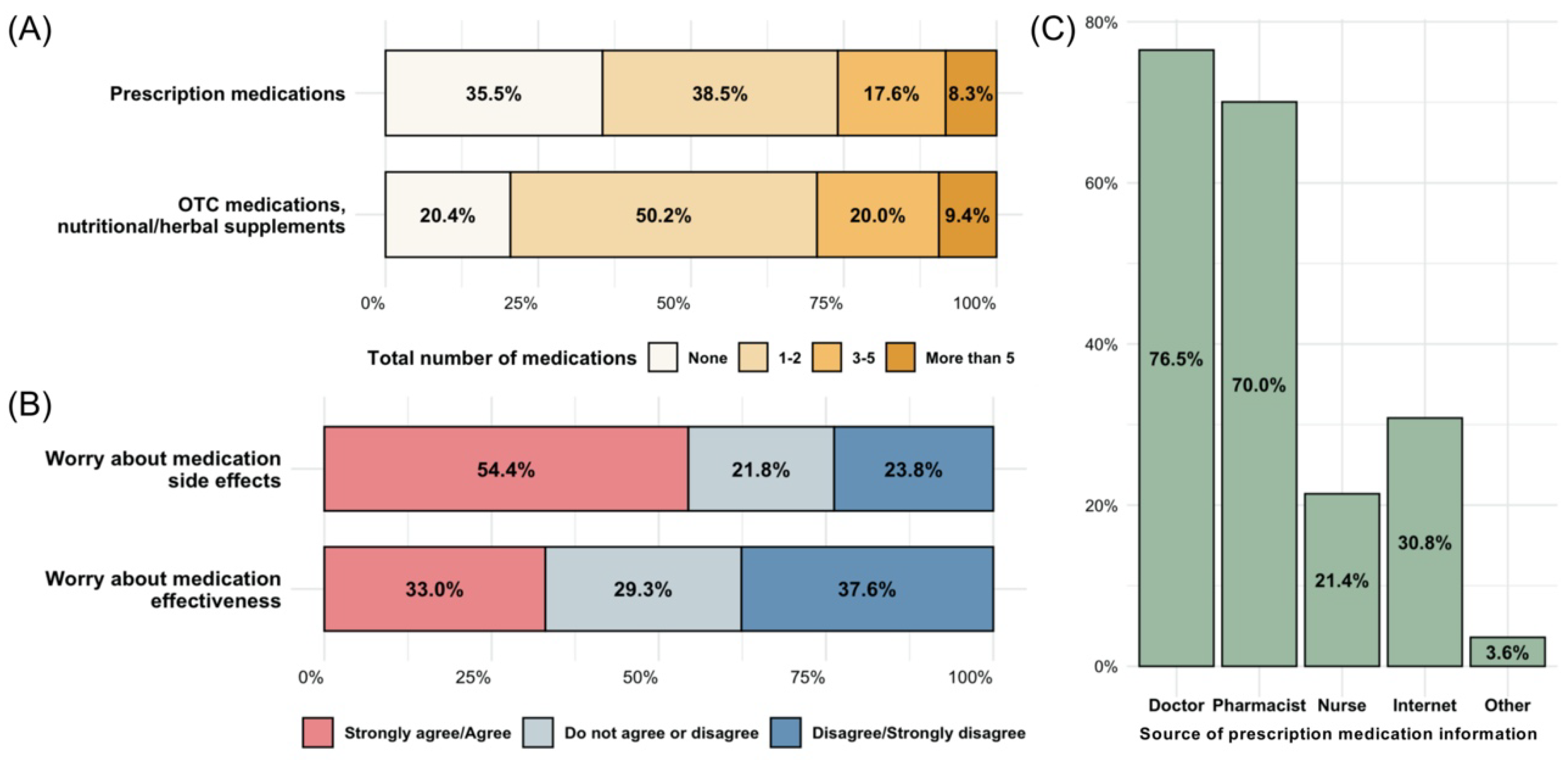
3.2. Participant Medication Experience
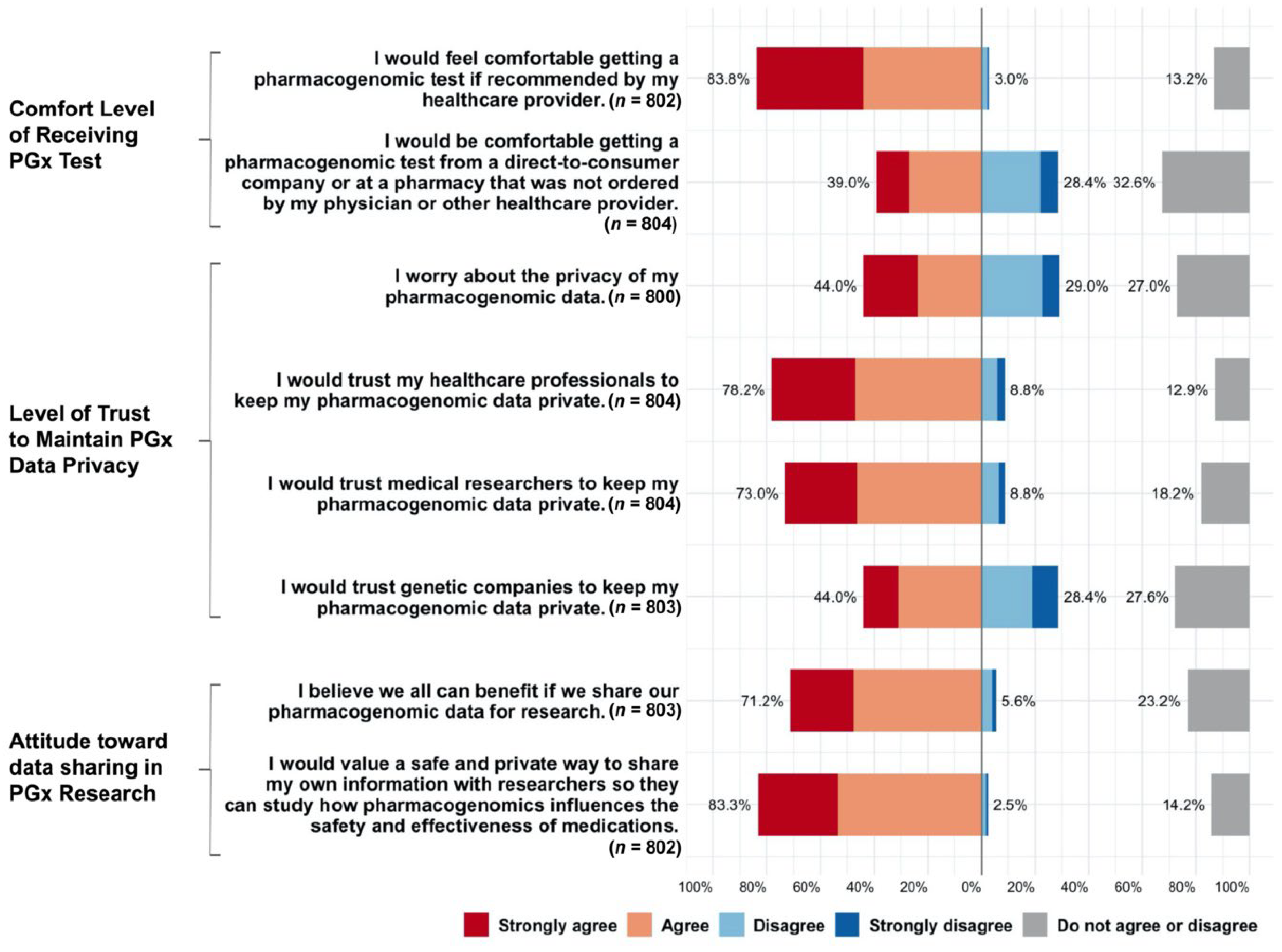
3.3. Acceptability and Perception of PGx Testing, Data Privacy, and Research
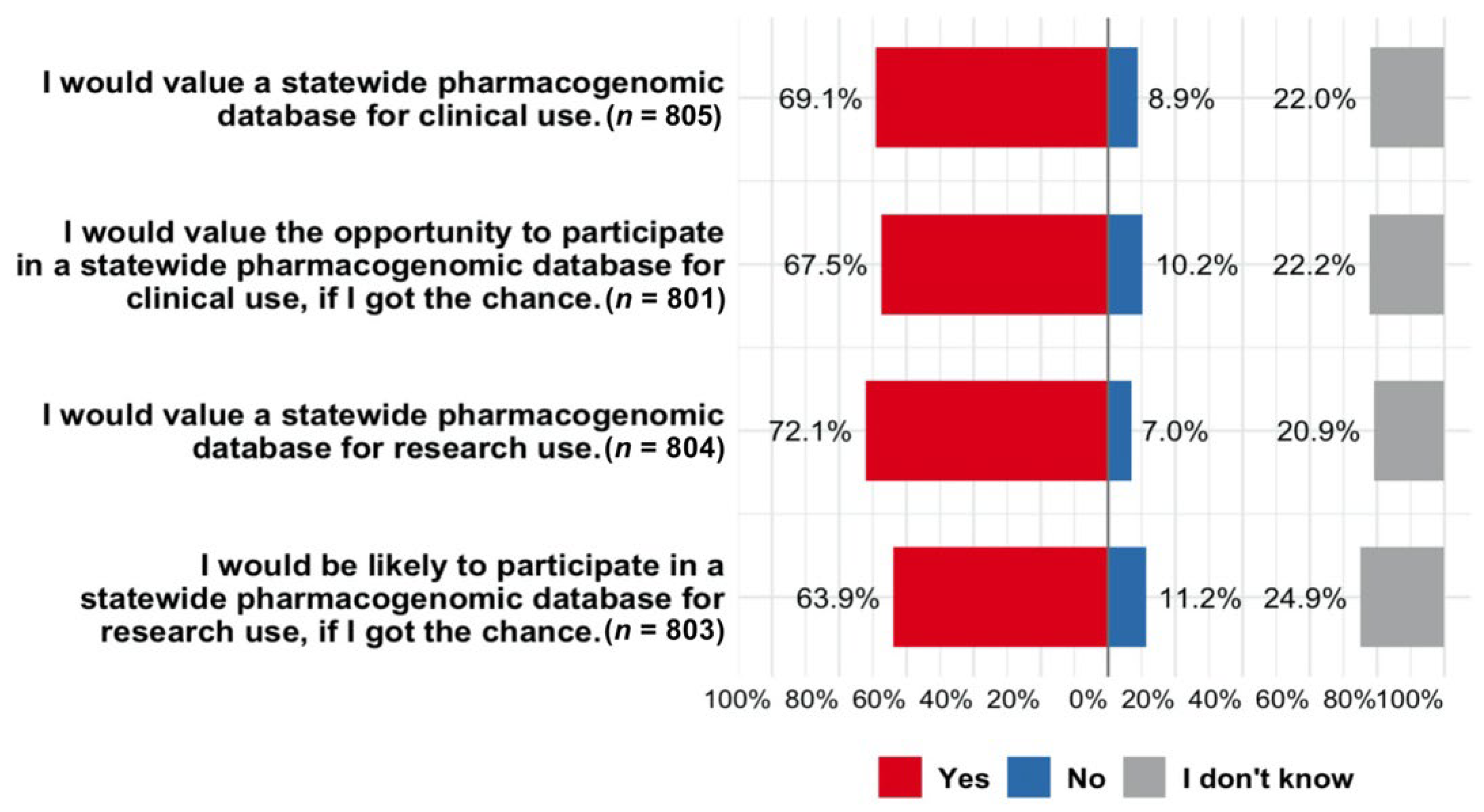
3.4. Attitudes toward Developing a Statewide PGx Database
4. Discussion
4.1. Acceptability of Receiving PGx Testing for Clinical Use
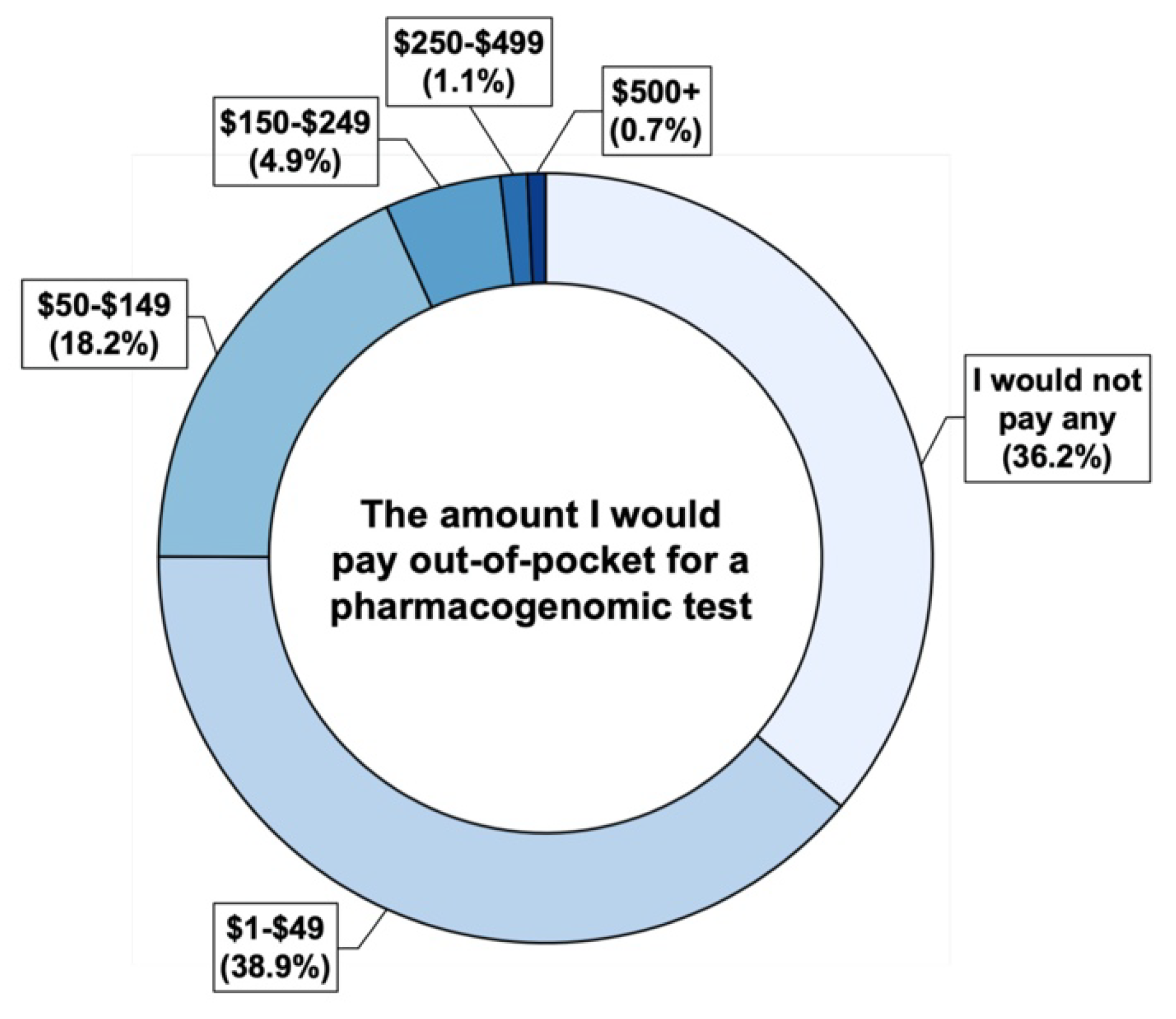
4.2. Financial Perspectives on PGx Testing
4.3. Attitudes toward PGx Data Sharing for Research
4.4. Community Support for Establishing a Statewide PGx Database
4.5. Study Limitations and Recommendations for Future Research
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinshilboum, R.; Wang, L. Pharmacogenomics: Bench to Bedside. Nat. Rev. Drug Discov. 2004, 3, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Evans, W.E. Pharmacogenomics in the Clinic. Nature 2015, 526, 343–350. [Google Scholar] [CrossRef]
- Roden, D.M.; McLeod, H.L.; Relling, M.V.; Williams, M.S.; Mensah, G.A.; Peterson, J.F.; Van Driest, S.L. Pharmacogenomics. Lancet 2019, 394, 521–532. [Google Scholar] [CrossRef]
- Haga, S.B.; Kantor, A. Horizon Scan of Clinical Laboratories Offering Pharmacogenetic Testing. Health Affairs 2018, 37, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Bank, P.C.D.; Caudle, K.E.; Swen, J.J.; Gammal, R.S.; Whirl-Carrillo, M.; Klein, T.E.; Relling, M.V.; Guchelaar, H.-J. Comparison of the Guidelines of the Clinical Pharmacogenetics Implementation Consortium and the Dutch Pharmacogenetics Working Group. Clin. Pharmacol. Ther. 2018, 103, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Klein, T.E.; Gammal, R.S.; Whirl-Carrillo, M.; Hoffman, J.M.; Caudle, K.E. The Clinical Pharmacogenetics Implementation Consortium: 10 Years Later. Clin. Pharmacol. Ther. 2020, 107, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Abdullah-Koolmees, H.; van Keulen, A.M.; Nijenhuis, M.; Deneer, V.H.M. Pharmacogenetics Guidelines: Overview and Comparison of the DPWG, CPIC, CPNDS and RNPGx Guidelines. Front. Pharmacol. 2021, 11, 595219. [Google Scholar] [CrossRef]
- Pratt, V.M.; Cavallari, L.H.; Del Tredici, A.L.; Gaedigk, A.; Hachad, H.; Ji, Y.; Kalman, L.V.; Ly, R.C.; Moyer, A.M.; Scott, S.A.; et al. Recommendations for Clinical CYP2D6 Genotyping Allele Selection: A Joint Consensus Recommendation of the Association for Molecular Pathology, College of American Pathologists, Dutch Pharmacogenetics Working Group of the Royal Dutch Pharmacists Association and the European Society for Pharmacogenomics and Personalized Therapy. J. Mol. Diagn. 2021, 23, 1047–1064. [Google Scholar] [CrossRef]
- Blagec, K.; Swen, J.J.; Koopmann, R.; Cheung, K.-C.; Crommentuijn-van Rhenen, M.; Holsappel, I.; Konta, L.; Ott, S.; Steinberger, D.; Xu, H.; et al. Pharmacogenomics Decision Support in the U-PGx Project: Results and Advice from Clinical Implementation across Seven European Countries. PLoS ONE 2022, 17, e0268534. [Google Scholar] [CrossRef]
- Tayeh, M.K.; Gaedigk, A.; Goetz, M.P.; Klein, T.E.; Lyon, E.; McMillin, G.A.; Rentas, S.; Shinawi, M.; Pratt, V.M.; Scott, S.A.; et al. Clinical Pharmacogenomic Testing and Reporting: A Technical Standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2022, 24, 759–768. [Google Scholar] [CrossRef]
- Clinical Pharmacogenetics Implementation Consortium. Available online: https://cpicpgx.org/ (accessed on 14 July 2022).
- FDA Table of Pharmacogenetic Associations. Available online: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations (accessed on 14 July 2022).
- Bielinski, S.J.; Olson, J.E.; Pathak, J.; Weinshilboum, R.M.; Wang, L.; Lyke, K.J.; Ryu, E.; Targonski, P.V.; Van Norstrand, M.D.; Hathcock, M.A.; et al. Preemptive Genotyping for Personalized Medicine: Design of the Right Drug, Right Dose, Right Time-Using Genomic Data to Individualize Treatment Protocol. Mayo Clin. Proc. 2014, 89, 25–33. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Haidar, C.E.; Wilkinson, M.R.; Crews, K.R.; Baker, D.K.; Kornegay, N.M.; Yang, W.; Pui, C.-H.; Reiss, U.M.; Gaur, A.H.; et al. PG4KDS: A Model for the Clinical Implementation of Pre-Emptive Pharmacogenetics. Am. J. Med. Genet. C Semin Med. Genet. 2014, 166, 45–55. [Google Scholar] [CrossRef]
- Dunnenberger, H.M.; Crews, K.R.; Hoffman, J.M.; Caudle, K.E.; Broeckel, U.; Howard, S.C.; Hunkler, R.J.; Klein, T.E.; Evans, W.E.; Relling, M.V. Preemptive Clinical Pharmacogenetics Implementation: Current Programs in Five US Medical Centers. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 89–106. [Google Scholar] [CrossRef]
- Luczak, T.; Brown, S.J.; Armbruster, D.; Hundertmark, M.; Brown, J.; Stenehjem, D. Strategies and Settings of Clinical Pharmacogenetic Implementation: A Scoping Review of Pharmacogenetics Programs. Pharmacogenomics 2021, 22, 345–364. [Google Scholar] [CrossRef]
- Rollinson, V.; Turner, R.; Pirmohamed, M. Pharmacogenomics for Primary Care: An Overview. Genes 2020, 11, 1337. [Google Scholar] [CrossRef]
- Bielinski, S.J.; Sauver, J.L.S.; Olson, J.E.; Wieland, M.L.; Vitek, C.R.; Bell, E.J.; McGree, M.E.; Jacobson, D.J.; McCormick, J.B.; Takahashi, P.Y.; et al. Are Patients Willing to Incur out of Pocket Costs for Pharmacogenomic Testing? Pharm. J. 2017, 17, 1–3. [Google Scholar] [CrossRef]
- Klein, M.E.; Parvez, M.M.; Shin, J.-G. Clinical Implementation of Pharmacogenomics for Personalized Precision Medicine: Barriers and Solutions. J. Pharm. Sci. 2017, 106, 2368–2379. [Google Scholar] [CrossRef]
- Rigter, T.; Jansen, M.E.; de Groot, J.M.; Janssen, S.W.J.; Rodenburg, W.; Cornel, M.C. Implementation of Pharmacogenetics in Primary Care: A Multi-Stakeholder Perspective. Front. Genet. 2020, 11, 10. [Google Scholar] [CrossRef]
- Bishop, J.R.; Huang, R.S.; Brown, J.T.; Mroz, P.; Johnson, S.G.; Allen, J.D.; Bielinski, S.J.; England, J.; Farley, J.F.; Gregornik, D.; et al. Pharmacogenomics Education, Research and Clinical Implementation in the State of Minnesota. Pharmacogenomics 2021, 22, 681–691. [Google Scholar] [CrossRef]
- MPMC-II (2019-2021) | Minnesota Precision Medicine Collaborative. Available online: http://mpmc.umn.edu/about/mpmc-ii-2019-2021 (accessed on 14 July 2022).
- Mroz, P.; Michel, S.; Allen, J.D.; Meyer, T.; McGonagle, E.J.; Carpentier, R.; Vecchia, A.; Schlichte, A.; Bishop, J.R.; Dunnenberger, H.M.; et al. Development and Implementation of In-House Pharmacogenomic Testing Program at a Major Academic Health System. Front. Genet. 2021, 12, 712602. [Google Scholar] [CrossRef]
- PGx ECHO. Available online: https://www.pharmacy.umn.edu/degrees-and-programs/continuing-pharmacy-education/pgx-echo (accessed on 14 July 2022).
- PGx Clinical Workforce Training Program. Available online: https://www.pharmacy.umn.edu/degrees-and-programs/continuing-pharmacy-education/pgx-clinical-training (accessed on 9 August 2022).
- Minnesota State Fair Attendance. Available online: https://www.mnstatefair.org/about-the-fair/attendance/ (accessed on 6 June 2022).
- Driven to Discover Research Facility at the Minnesota State Fair Annual Report. Available online: http://d2d.umn.edu/wp-content/uploads/2022/04/2021-Annual-Report.pdf (accessed on 14 July 2022).
- Allen, J.D.; Zhang, L.; Johnson, A.N.K.; Jacobson, P.A.; McCarty, C.A.; Pittenger, A.L.; Bishop, J.R. Development and Validation of the Minnesota Assessment of Pharmacogenomic Literacy (MAPL). J. Pers. Med. 2022, 12, 1398. [Google Scholar] [CrossRef]
- USDA ERS—Rural-Urban Commuting Area Codes. Available online: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx (accessed on 7 June 2022).
- Chinn, D.; McCarthy, C. All Aspects of Health Literacy Scale (AAHLS): Developing a Tool to Measure Functional, Communicative and Critical Health Literacy in Primary Healthcare Settings. Patient Educ. Couns. 2013, 90, 247–253. [Google Scholar] [CrossRef]
- Rogausch, A.; Prause, D.; Schallenberg, A.; Brockmöller, J.; Himmel, W. Patients’ and Physicians’ Perspectives on Pharmacogenetic Testing. Pharmacogenomics 2006, 7, 49–59. [Google Scholar] [CrossRef]
- Fargher, E.A.; Eddy, C.; Newman, W.; Qasim, F.; Tricker, K.; Elliott, R.A.; Payne, K. Patients’ and Healthcare Professionals’ Views on Pharmacogenetic Testing and Its Future Delivery in the NHS. Pharmacogenomics 2007, 8, 1511–1519. [Google Scholar] [CrossRef]
- Haddy, C.A.; Ward, H.M.; Angley, M.T.; McKinnon, R.A. Consumers’ Views of Pharmacogenetics—A Qualitative Study. Res. Soc. Adm. Pharm. 2010, 6, 221–231. [Google Scholar] [CrossRef]
- Lee, I.-H.; Kang, H.-Y.; Suh, H.S.; Lee, S.; Oh, E.S.; Jeong, H. Awareness and Attitude of the Public toward Personalized Medicine in Korea. PLoS ONE 2018, 13, e0192856. [Google Scholar] [CrossRef]
- Zubiaur, P.; Prósper-Cuesta, D.N.; Novalbos, J.; Mejía-Abril, G.; Navares-Gómez, M.; Villapalos-García, G.; Soria-Chacartegui, P.; Abad-Santos, F. Patients’ Perceptions of Pharmacogenetic Testing and Access to Their Results: State of the Art in Spain and Systematic Review. J. Pers. Med. 2022, 12, 270. [Google Scholar] [CrossRef]
- Haga, S.B.; O’Daniel, J.M.; Tindall, G.M.; Lipkus, I.R.; Agans, R. Survey of US Public Attitudes toward Pharmacogenetic Testing. Pharm. J. 2012, 12, 197–204. [Google Scholar] [CrossRef]
- Payne, K.; Fargher, E.A.; Roberts, S.A.; Tricker, K.; Elliott, R.A.; Ratcliffe, J.; Newman, W.G. Valuing Pharmacogenetic Testing Services: A Comparison of Patients’ and Health Care Professionals’ Preferences. Value Health 2011, 14, 121–134. [Google Scholar] [CrossRef]
- Rasmussen-Torvik, L.J.; Stallings, S.C.; Gordon, A.S.; Almoguera, B.; Basford, M.A.; Bielinski, S.J.; Brautbar, A.; Brilliant, M.H.; Carrell, D.S.; Connolly, J.J.; et al. Design and Anticipated Outcomes of the EMERGE-PGx Project: A Multicenter Pilot for Preemptive Pharmacogenomics in Electronic Health Record Systems. Clin. Pharmacol. Ther. 2014, 96, 482–489. [Google Scholar] [CrossRef]
- Weitzel, K.W.; Alexander, M.; Bernhardt, B.A.; Calman, N.; Carey, D.J.; Cavallari, L.H.; Field, J.R.; Hauser, D.; Junkins, H.A.; Levin, P.A.; et al. The IGNITE Network: A Model for Genomic Medicine Implementation and Research. BMC Med. Genomics 2016, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Jameson, A.; Fylan, B.; Bristow, G.C.; Sagoo, G.S.; Dalton, C.; Cardno, A.; Sohal, J.; McLean, S.L. What Are the Barriers and Enablers to the Implementation of Pharmacogenetic Testing in Mental Health Care Settings? Front. Genet. 2021, 12, 740216. [Google Scholar] [CrossRef] [PubMed]
- Virelli, C.R.; Mohiuddin, A.G.; Kennedy, J.L. Barriers to Clinical Adoption of Pharmacogenomic Testing in Psychiatry: A Critical Analysis. Transl. Psychiatry 2021, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Pittenger, A.L.; Bishop, J.R. A Scoping Review of Attitudes and Experiences with Pharmacogenomic Testing among Patients and the General Public: Implications for Patient Counseling. J. Pers. Med. 2022, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.L.; Hohmeier, K.C.; Smith, C.T. Pharmacogenomics Testing in a Community Pharmacy: Patient Perceptions and Willingness-to-Pay. Pharmacogenomics 2017, 18, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Howe, N.; Giles, E.; Newbury-Birch, D.; McColl, E. Systematic Review of Participants’ Attitudes towards Data Sharing: A Thematic Synthesis. J. Health Serv. Res. Policy 2018, 23, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.J.; Baker, R.; Milner, L.C.; Devaney, S.; Hudson, K.L. A Survey of U.S Adults’ Opinions about Conduct of a Nationwide Precision Medicine Initiative® Cohort Study of Genes and Environment. PLoS ONE 2016, 11, e0160461. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, S.C.; Brothers, K.B.; Mercaldo, N.D.; Clayton, E.W.; Antommaria, A.H.M.; Aufox, S.A.; Brilliant, M.H.; Campos, D.; Carrell, D.S.; Connolly, J.; et al. Public Attitudes toward Consent and Data Sharing in Biobank Research: A Large Multi-Site Experimental Survey in the US. Am. J. Hum. Genet. 2017, 100, 414–427. [Google Scholar] [CrossRef]
- Moriarty, K.; Wolf, S.M.; Veach, P.M.; LeRoy, B.; MacFarlane, I.M.; Zierhut, H.A. A Roadmap for Precision Medicine Research Recruitment: Empirical Assessment of the Public’s Willingness to Participate. Per. Med. 2020, 17, 345–359. [Google Scholar] [CrossRef]
- Bloss, C.S.; Stoler, J.; Schairer, C.E.; Rosenthal, S.B.; Cheung, C.; Rus, H.M.; Block, J.L.; Yang, J.-A.J.; Morton, D.; Bixenman, H.; et al. Characteristics of Likely Precision Medicine Initiative Participants Drawn from A Large Blood Donor Population. Health Aff. 2018, 37, 786–792. [Google Scholar] [CrossRef]
- Kaphingst, K.A.; Blanchard, M.; Milam, L.; Pokharel, M.; Elrick, A.; Goodman, M.S. Relationships Between Health Literacy and Genomics-Related Knowledge, Self-Efficacy, Perceived Importance and Communication in a Medically Underserved Population. J. Health Commun. 2016, 21, 58–68. [Google Scholar] [CrossRef]
- Veilleux, S.; Bouffard, M. Knowledge and Understanding of Pharmacogenomic Testing among Patients and Health Care Professionals: A Scoping Review. Patient Educ. Couns. 2019, 102, 2001–2009. [Google Scholar] [CrossRef]
- MN Educational Attainment ACS 2016. Available online: https://public.tableau.com/views/MNEducationalAttainmmentACS2016_0/Story1?:embed=y&:showVizHome=no&:host_url=https%3A%2F%2Fpublic.tableau.com%2F&:embed_code_version=3&:tabs=no&:toolbar=yes&:animate_transition=yes&:display_static_image=no&:display_spinner=no&:display_overlay=yes&:display_count=yes&publish=yes&:loadOrderID=0 (accessed on 18 July 2022).
- U.S. Census Bureau QuickFacts: Minnesota. Available online: https://www.census.gov/quickfacts/MN (accessed on 18 July 2022).
- Holzer, K.; Culhane-Pera, K.A.; Straka, R.J.; Wen, Y.F.; Lo, M.; Lee, K.; Xiong, T.; Peng, K.; Bishop, J.; Thyagarajan, B.; et al. Hmong Participants’ Reactions to Return of Individual and Community Pharmacogenetic Research Results: “A Positive Light for Our Community”. J. Community Genet. 2021, 12, 53–65. [Google Scholar] [CrossRef]
- Carroll, D.M.; Murphy, S.; Meier, E.; Rhodes, K.; Dorr, C.; Braaten, G.; Jacobson, P.A.; Frizzell, L.; Tyndale, R.F.; Hatsukami, D.; et al. Exploring Potential for a Personalized Medicine Approach to Smoking Cessation with an American Indian Tribe. Nicotine Tob. Res. 2022, ntac141. [Google Scholar] [CrossRef]
| n | % | |
|---|---|---|
| Gender | ||
| Men | 290 | 35.9 |
| Women | 510 | 63.2 |
| Non-binary | 3 | 0.4 |
| Unknown/Prefer not to answer | 4 | 0.5 |
| Age | ||
| 18–29 | 203 | 27.5 |
| 30–41 | 103 | 14.0 |
| 42–53 | 147 | 19.9 |
| 54–65 | 195 | 26.4 |
| 66–77 | 82 | 11.1 |
| 78+ | 8 | 1.1 |
| Race | ||
| American Indian or Alaska Native | 6 | 0.7 |
| Asian | 59 | 7.3 |
| Black or African American | 8 | 1.0 |
| Native Hawaiian or Pacific Islanders | 2 | 0.2 |
| White | 679 | 84.3 |
| Multiracial | 18 | 2.2 |
| Other | 15 | 1.9 |
| Unknown/Prefer not to answer | 18 | 2.2 |
| Hispanic, Latino, or Spanish origin | ||
| Yes | 44 | 5.5 |
| No | 746 | 92.6 |
| Unknown/Prefer not to answer | 16 | 2.0 |
| Education attainment | ||
| No college | 78 | 9.7 |
| Some college, no degree | 160 | 19.8 |
| Associate degree | 107 | 13.2 |
| Bachelor’s degree | 286 | 35.4 |
| Master’s degree | 117 | 14.5 |
| Doctoral degree | 58 | 7.2 |
| Unknown/Prefer not to answer | 2 | 0.2 |
| Geographical regions b | ||
| Metro | 713 | 90.5 |
| Non-metro | 75 | 9.5 |
| Having health insurance | ||
| No | 46 | 5.7 |
| Yes | 757 | 94.3 |
| Insurance type | ||
| Public health plan (Medicare/Medicaid) | 183 | |
| Private health plan | 132 | |
| Employer health plan | 546 | |
| Military health plan | 22 | |
| Indian Health Service health plan | 1 | |
| Having primary care provider | ||
| Yes | 672 | 83.7 |
| No | 131 | 16.3 |
| Having commercial genetic testing performed in the past | ||
| Yes | 141 | 17.5 |
| No | 663 | 82.5 |
| Support Statewide PGx Database for Clinical Use | Willingness to Participate in Statewide PGx Database for Clinical Use | Support Statewide PGx Database for Research Use | Willingness to Participate in Statewide PGx Database for Research Use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r a | p Value | n | r | p Value | n | r | p Value | n | r | p Value | n | |
| Gender | −2.36 × 10−4 | 0.995 | 804 | −2.90 × 10−4 | 0.993 | 800 | 3.85 × 10−3 | 0.913 | 803 | 0.047 | 0.180 | 802 |
| Age | 0.018 | 0.633 | 736 | −0.087 | 0.018 | 732 | −0.059 | 0.110 | 735 | −0.073 | 0.049 | 734 |
| White vs. non-White | −0.104 | 0.003 | 802 | −0.089 | 0.012 | 798 | −0.030 | 0.396 | 801 | −0.032 | 0.366 | 800 |
| Metro vs. non-metro | −0.071 | 0.047 | 785 | −0.053 | 0.136 | 782 | −0.096 | 0.007 | 784 | −0.052 | 0.144 | 783 |
| Education | 0.175 | 5.99 × 10−7 | 805 | 0.150 | 1.96 × 10−5 | 801 | 0.187 | 9.13 × 10−8 | 804 | 0.155 | 1.04 × 10−5 | 803 |
| Having health insurance | 0.148 | 2.67 × 10−5 | 800 | 0.174 | 8.35 × 10−7 | 796 | 0.147 | 3.20 × 10−5 | 799 | 0.128 | 2.78 × 10−4 | 798 |
| Having primary care provider | 0.054 | 0.127 | 800 | 0.011 | 0.753 | 796 | 0.035 | 0.327 | 799 | 0.040 | 0.255 | 798 |
| Having prior genetic testing performed | 0.090 | 0.011 | 803 | 0.103 | 3.51 × 10−3 | 799 | 0.074 | 0.036 | 802 | 0.108 | 2.14 × 10−3 | 801 |
| Total number of prescription medications | 0.039 | 0.269 | 802 | −0.009 | 0.806 | 798 | 0.013 | 0.722 | 801 | 0.022 | 0.540 | 800 |
| Outcome Variable a | Model 1 b Support a Statewide PGx Database for Clinical Use | Model 2 Would Participate in Statewide PGx Database for Clinical Use | Model 3 Support a Statewide PGx Database for Research Use | Model 4 Would Participate in Statewide PGx Database for Research Use | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | Coefficient | SE | Coefficient | SE | |
| Female (reference: Male) | −0.236 | 0.189 | −0.125 | 0.186 | −0.094 | 0.193 | 0.128 | 0.178 |
| Age (reference: 18–29 years) | ||||||||
| 30–41 | −0.190 | 0.289 | −0.685 * | 0.293 | −0.335 | 0.309 | −0.450 | 0.283 |
| 42–53 | −0.384 | 0.271 | −0.794 ** | 0.279 | −0.665 * | 0.286 | −0.571 * | 0.265 |
| 54–65 | −0.067 | 0.255 | −0.639 * | 0.260 | −0.510 | 0.264 | −0.405 | 0.246 |
| 66–77 | −0.243 | 0.333 | −1.091 ** | 0.329 | −0.522 | 0.345 | −0.888 ** | 0.314 |
| 78+ | −0.394 | 0.945 | 0.446 | 1.180 | −1.464 | 0.882 | 0.810 | 1.148 |
| Non-White (reference: White) | −0.597 * | 0.239 | −0.501 * | 0.244 | −0.179 | 0.258 | −0.030 | 0.243 |
| Non-metro (reference: Metro) | −0.488 | 0.292 | −0.088 | 0.299 | −0.458 | 0.296 | −0.154 | 0.293 |
| Education (reference: No college) | ||||||||
| Some college, no degree | 1.018 ** | 0.336 | 1.087 ** | 0.341 | 0.738 * | 0.331 | 0.915 ** | 0.339 |
| Associate degree | 1.250 ** | 0.365 | 1.045 ** | 0.365 | 0.921 * | 0.360 | 0.992 ** | 0.362 |
| Bachelor’s degree | 1.613 *** | 0.322 | 1.610 *** | 0.326 | 1.487 *** | 0.321 | 1.509 *** | 0.323 |
| Master’s degree | 1.454 *** | 0.376 | 1.433 *** | 0.375 | 1.376 *** | 0.380 | 1.151 ** | 0.367 |
| Doctoral degree | 1.935 *** | 0.479 | 1.715 *** | 0.455 | 2.136 *** | 0.527 | 2.495 *** | 0.511 |
| Having health insurance | 0.778 | 0.406 | 1.344 ** | 0.416 | 0.870 * | 0.404 | 1.019 * | 0.413 |
| Having primary care provider | 0.196 | 0.255 | −0.053 | 0.265 | 0.245 | 0.265 | 0.122 | 0.248 |
| Having commercial genetic testing performed in the past | 0.484 | 0.250 | 0.757 ** | 0.253 | 0.472 | 0.258 | 0.695 ** | 0.239 |
| Total number of prescription medications (reference: None) | ||||||||
| 1–2 | 0.338 | 0.210 | 0.183 | 0.211 | 0.247 | 0.219 | 0.283 | 0.201 |
| 3+ | 0.031 | 0.235 | −0.146 | 0.235 | −0.043 | 0.242 | 0.077 | 0.228 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jacobson, P.A.; Johnson, A.N.K.; Gregornik, D.B.; Johnson, S.G.; McCarty, C.A.; Bishop, J.R. Public Attitudes toward Pharmacogenomic Testing and Establishing a Statewide Pharmacogenomics Database in the State of Minnesota. J. Pers. Med. 2022, 12, 1615. https://doi.org/10.3390/jpm12101615
Zhang L, Jacobson PA, Johnson ANK, Gregornik DB, Johnson SG, McCarty CA, Bishop JR. Public Attitudes toward Pharmacogenomic Testing and Establishing a Statewide Pharmacogenomics Database in the State of Minnesota. Journal of Personalized Medicine. 2022; 12(10):1615. https://doi.org/10.3390/jpm12101615
Chicago/Turabian StyleZhang, Lusi, Pamala A. Jacobson, Alyssa N. K. Johnson, David B. Gregornik, Steven G. Johnson, Catherine A. McCarty, and Jeffrey R. Bishop. 2022. "Public Attitudes toward Pharmacogenomic Testing and Establishing a Statewide Pharmacogenomics Database in the State of Minnesota" Journal of Personalized Medicine 12, no. 10: 1615. https://doi.org/10.3390/jpm12101615
APA StyleZhang, L., Jacobson, P. A., Johnson, A. N. K., Gregornik, D. B., Johnson, S. G., McCarty, C. A., & Bishop, J. R. (2022). Public Attitudes toward Pharmacogenomic Testing and Establishing a Statewide Pharmacogenomics Database in the State of Minnesota. Journal of Personalized Medicine, 12(10), 1615. https://doi.org/10.3390/jpm12101615






