Endometrial SUSD2+ Mesenchymal Stem/Stromal Cells in Tissue Engineering: Advances in Novel Cellular Constructs for Pelvic Organ Prolapse
Abstract
1. Introduction
2. Pelvic Organ Prolapse
2.1. Aetiology
2.2. Anatomy, Pathophysiology, and Biomechanics
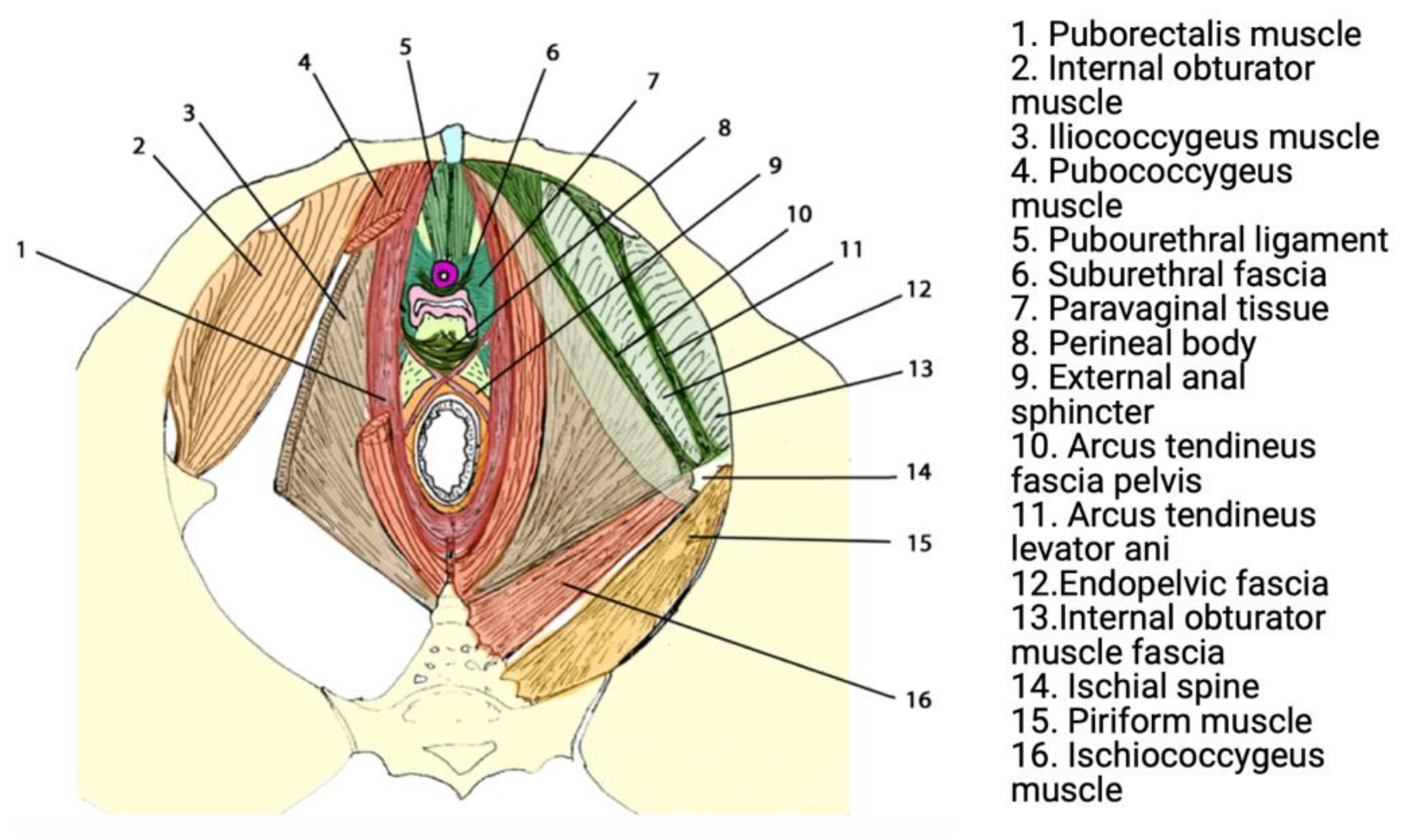
2.3. Treatment of POP
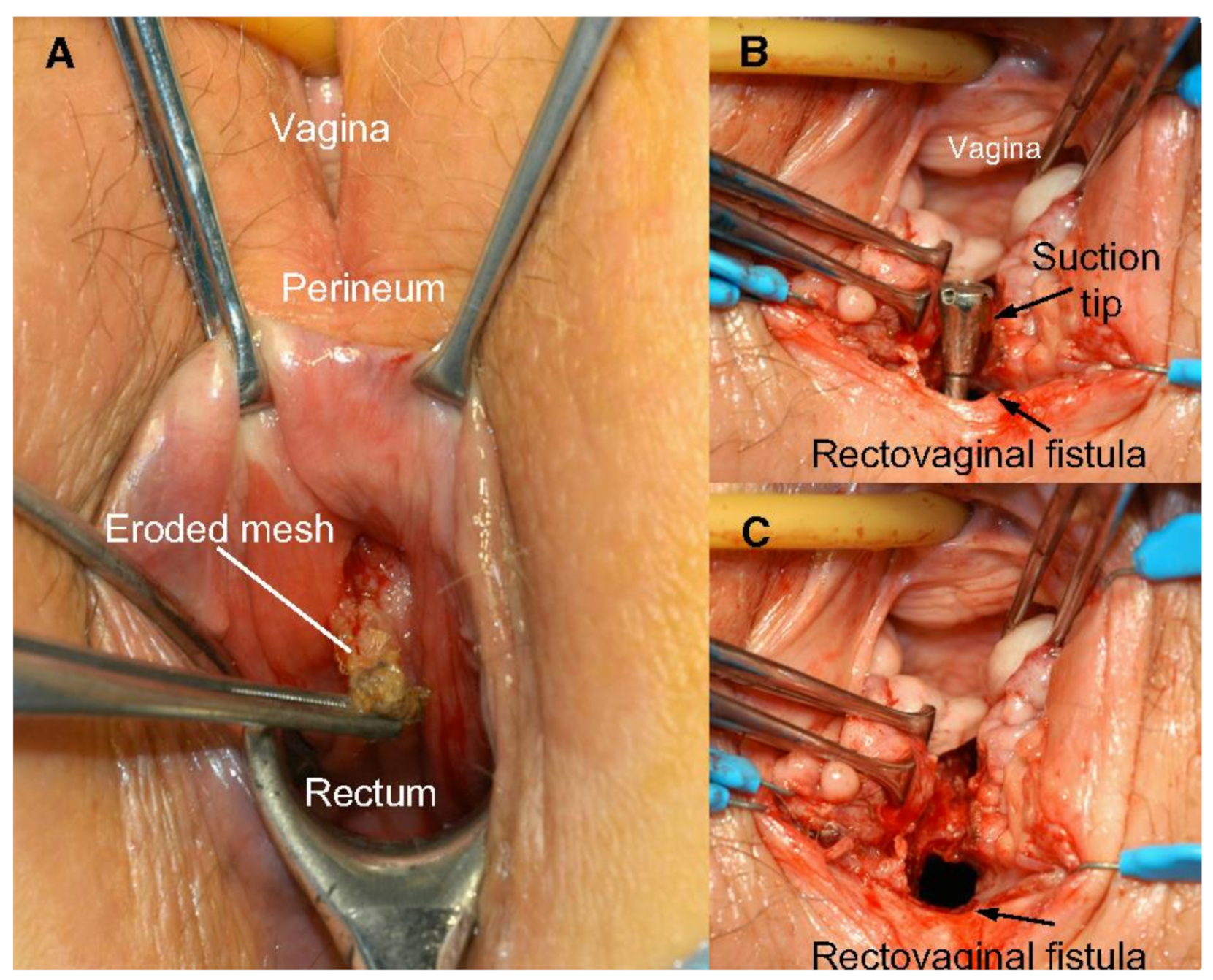
2.4. Clinical Adversities
3. Mesenchymal Stem/Progenitor Cells
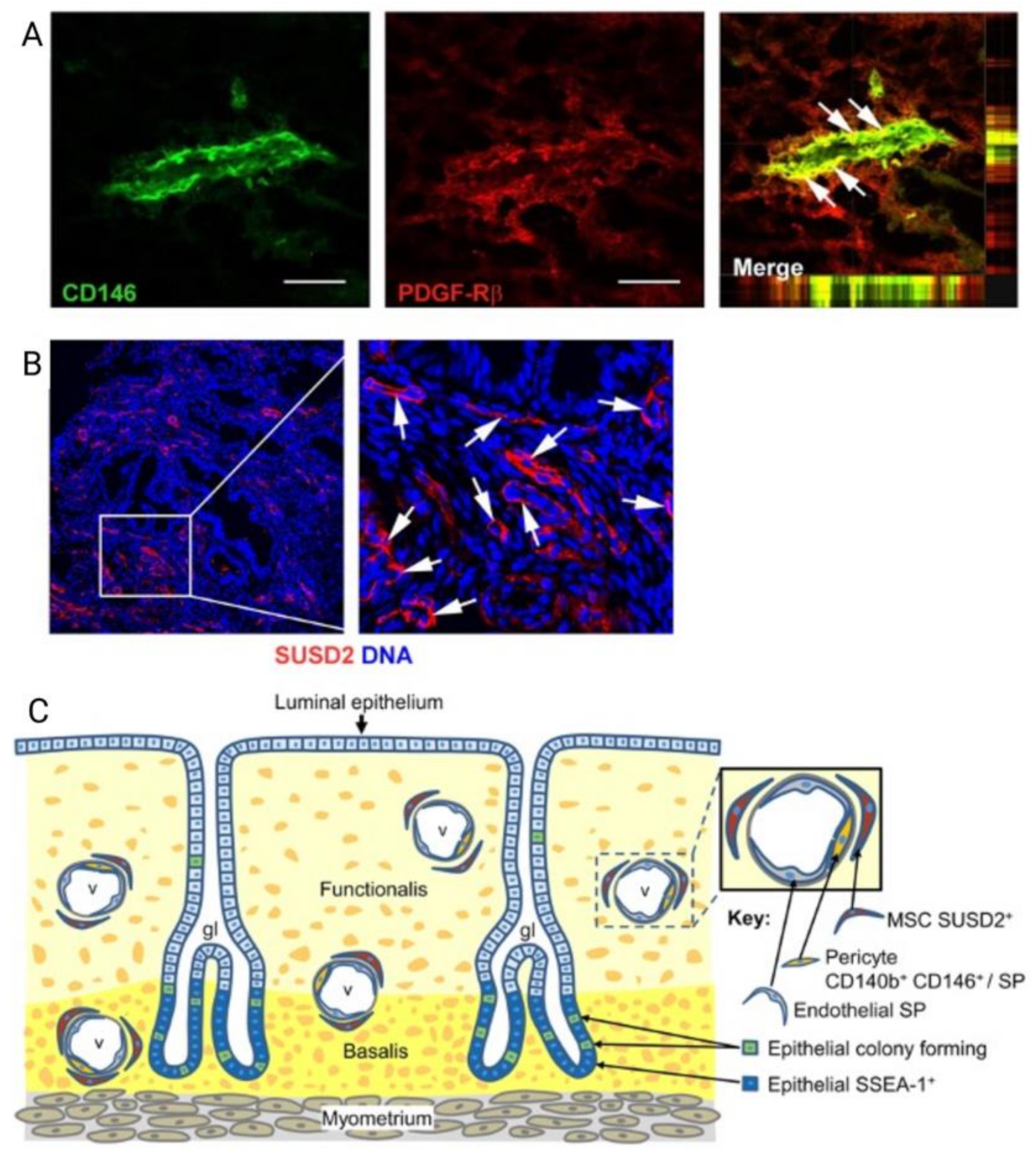
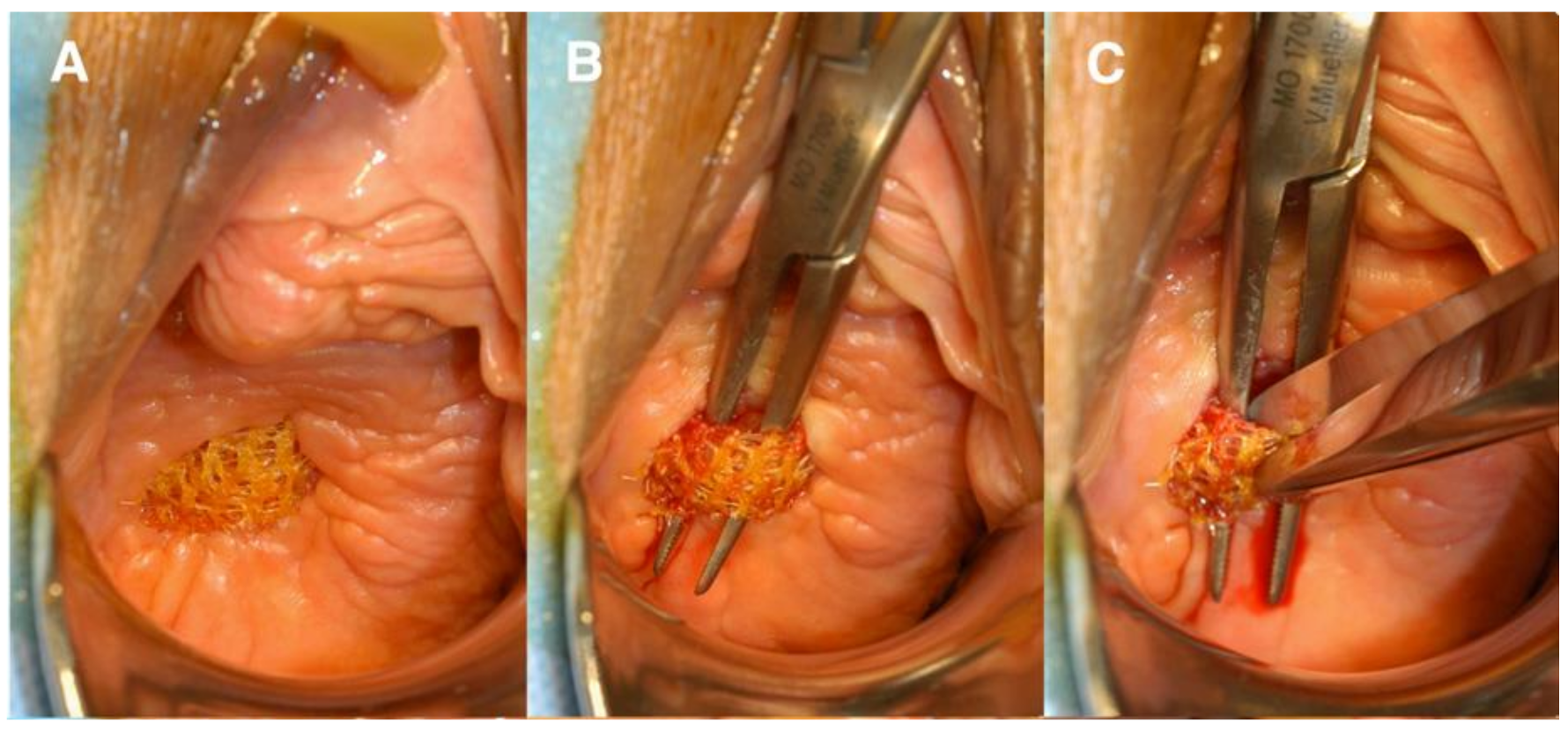
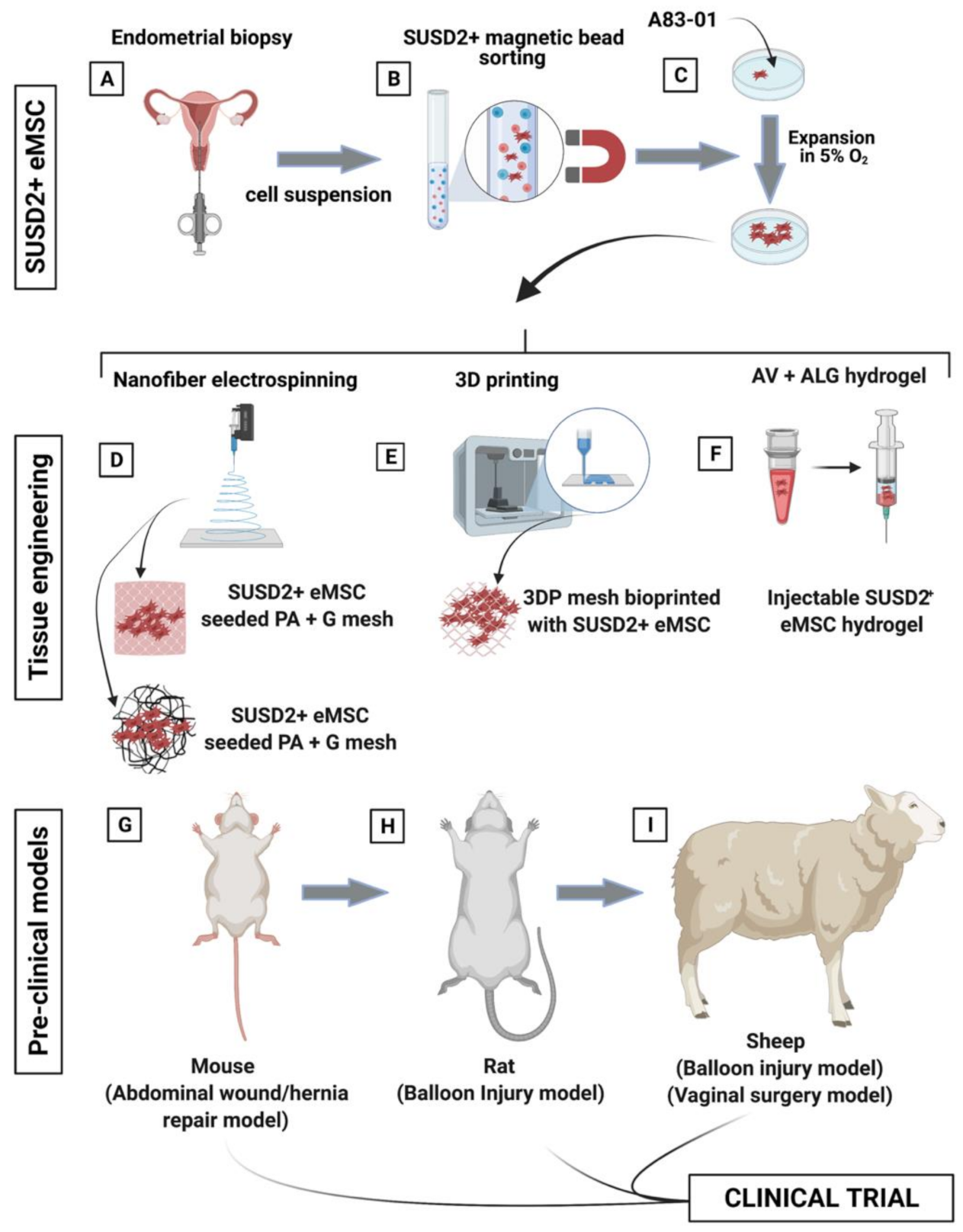
4. Engineering Novel Meshes with eMSC
4.1. eMSC Non-Degradable Tissue Engineered Mesh
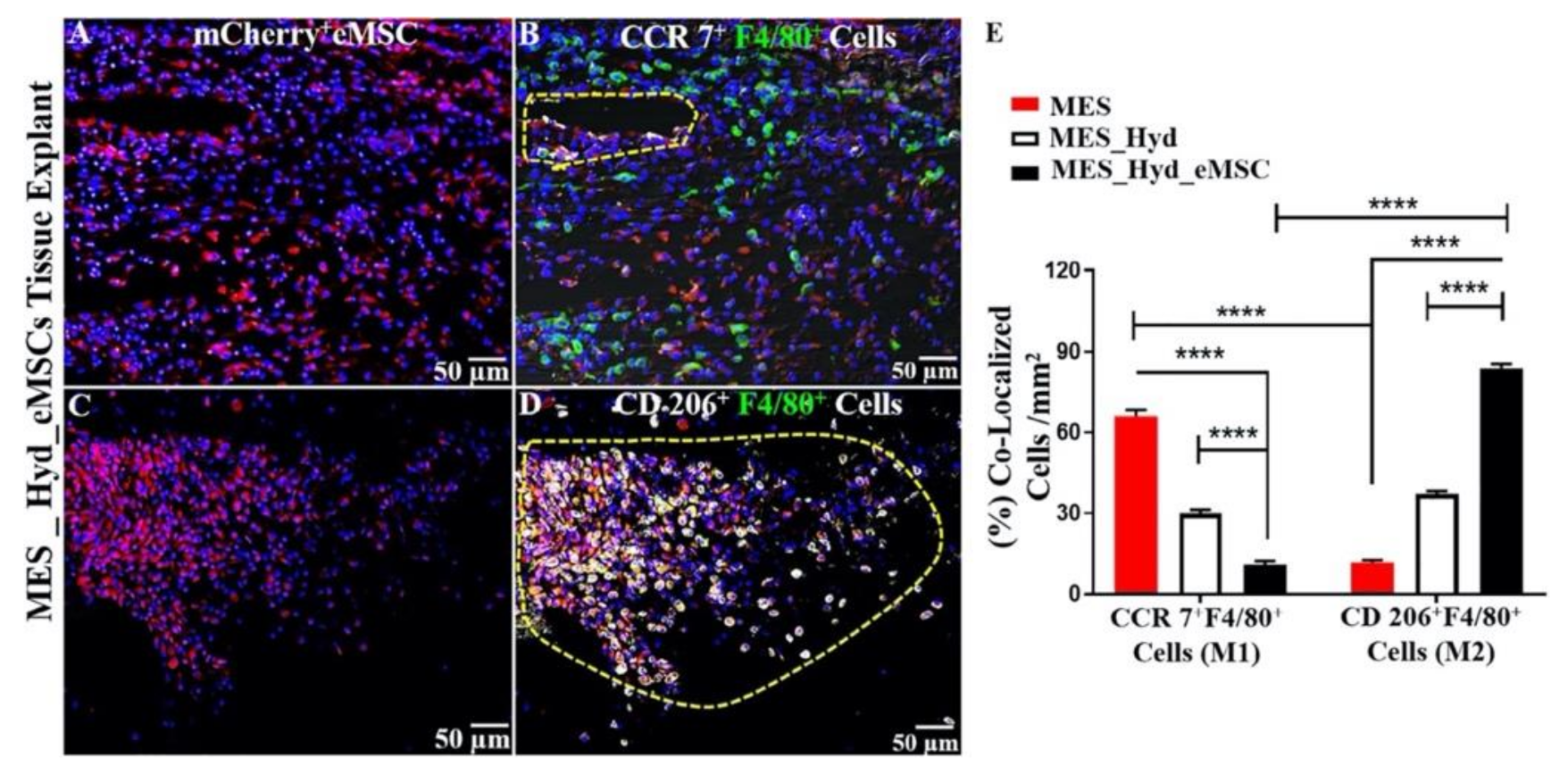
4.2. eMSC Degradable Tissue Engineered Mesh
4.3. Large Pre-Clinical Animal Models of POP
5. Potential Clinical Applications of eMSC
6. Limitations
7. Summary and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dietz, H.P. Pelvic floor trauma in childbirth. Aust. N. Z. J. Obstet. Gynaecol. 2013, 53, 220–230. [Google Scholar] [CrossRef]
- Nygaard, I. Prevalence of Symptomatic Pelvic Floor Disorders in US Women. JAMA 2008, 300, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Zwain, O.; Aoun, J.; Eisenstein, D. Minimally invasive surgery in pelvic floor repair. Curr. Opin. Obstet. Gynecol. 2017, 29, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.; Holman, C.D.J.; Moorin, R.; Tsokos, N. Lifetime Risk of Undergoing Surgery for Pelvic Organ Prolapse. Obstet. Gynecol. 2010, 116, 1096–1100. [Google Scholar] [CrossRef]
- Wu, J.M.; Matthews, C.A.; Conover, M.; Pate, V.; Funk, M.J. Lifetime Risk of Stress Urinary Incontinence or Pelvic Organ Prolapse Surgery. Obstet. Gynecol. 2014, 123, 1201–1206. [Google Scholar] [CrossRef]
- Olsen, A.L.; Smith, V.J.; Bergstrom, J.O.; Colling, J.C.; Clark, A.L. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol. 1997, 89, 501–506. [Google Scholar] [CrossRef]
- Vinchant, M.; Bitumba, I.; Letouzey, V.; Fernandez, H.; de Tayrac, R.; Deffieux, X. Reoperation rate and outcomes following the placement of polypropylene mesh by the vaginal route for cystocele: Very long-term follow-up. Int. Urogynecol. J. 2021, 32, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.; Unger, C.A.; Evans, J.M.; Jallad, K.; Mishra, K.; Karram, M.M.; Iglesia, C.B.; Rardin, C.R.; Barber, M.D. Evaluation and management of complications from synthetic mesh after pelvic reconstructive surgery: A multicenter study. Am. J. Obstet. Gynecol. 2014, 210, 163.e1–163.e8. [Google Scholar] [CrossRef]
- Holt, E. US FDA rules manufacturers to stop selling mesh devices. Lancet 2019, 393, 1686. [Google Scholar] [CrossRef]
- MacCraith, E.; Cunnane, E.M.; Joyce, M.; Forde, J.C.; O’Brien, F.J.; Davis, N.F. Comparison of synthetic mesh erosion and chronic pain rates after surgery for pelvic organ prolapse and stress urinary incontinence: A systematic review. Int. Urogynecol. J. 2021, 32, 573–580. [Google Scholar] [CrossRef]
- Ulrich, D.; Edwards, S.L.; Su, K.; Tan, K.S.; White, J.; Ramshaw, J.A.; Lo, C.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. Human Endometrial Mesenchymal Stem Cells Modulate the Tissue Response and Mechanical Behavior of Polyamide Mesh Implants for Pelvic Organ Prolapse Repair. Tissue Eng. Part A 2013, 20, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Jelovsek, J.E.; Maher, C.; Barber, M.D. Pelvic organ prolapse. Lancet 2007, 369, 1027–1038. [Google Scholar] [CrossRef]
- Mothes, A.R.; Radosa, M.P.; Altendorf-Hofmann, A.; Runnebaum, I.B. Risk index for pelvic organ prolapse based on established individual risk factors. Arch. Gynecol. Obstet. 2016, 293, 617–624. [Google Scholar] [CrossRef]
- Chow, D.; Rodríguez, L.V. Epidemiology and prevalence of pelvic organ prolapse. Curr. Opin. Urol. 2013, 23, 293–298. [Google Scholar] [CrossRef]
- Saunders, K. Recent Advances in Understanding Pelvic-Floor Tissue of Women with and Without Pelvic Organ Prolapse: Considerations for Physical Therapists. Phys. Ther. 2017, 97, 455–463. [Google Scholar] [CrossRef]
- Norton, P.A. Pelvic Floor Disorders: The Role of Fascia and Ligaments. Clin. Obstet. Gynecol. 1993, 36, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.T.; De Lancey, J.O.L. Functional Anatomy of the Pelvic Floor and Lower Urinary Tract. Clin. Obstet. Gynecol. 2004, 47, 3–17. [Google Scholar] [CrossRef]
- Memon, H.U.; Handa, V.L. Vaginal Childbirth and Pelvic Floor Disorders. Women’s Health 2013, 9, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Darzi, S.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. Emerging Nano/Micro-Structured Degradable Polymeric Meshes for Pelvic Floor Reconstruction. Nanomaterials 2020, 10, 1120. [Google Scholar] [CrossRef]
- Rubod, C.; Lecomte-Grosbras, P.; Brieu, M.; Giraudet, G.; Betrouni, N.; Cosson, M. 3D simulation of pelvic system numerical simulation for a better understanding of the contribution of the uterine ligaments. Int. Urogynecol. J. 2013, 24, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Brownridge, P. The nature and consequences of childbirth pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 59, S9–S15. [Google Scholar] [CrossRef]
- Lien, K.-C.; Mooney, B.; DeLancey, J.O.L.; Ashton-Miller, J.A. Levator Ani Muscle Stretch Induced by Simulated Vaginal Birth. Obstet. Gynecol. 2004, 103, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, G.; Delorme, E.; Cosson, M.; Rubod, C. Cystocele and functional anatomy of the pelvic floor: Review and update of the various theories. Int. Urogynecol. J. 2016, 27, 1297–1305. [Google Scholar] [CrossRef]
- Stuepp, L.; Resende, A.P.M.; Oliveira, E.; Castro, R.; Girão, M.J.B.C.; Sartori, M. Pelvic floor muscle training for treatment of pelvic organ prolapse: An assessor-blinded randomized controlled trial. Int. Urogynecol. J. 2011, 22, 1233–1239. [Google Scholar] [CrossRef]
- Siddiqui, N.; Edenfield, A. Clinical challenges in the management of vaginal prolapse. Int. J. Women’s Health 2014, 6, 83–94. [Google Scholar] [CrossRef]
- Mancuso, E.; Downey, C.; Doxford-Hook, E.; Bryant, M.G.; Culmer, P. The use of polymeric meshes for pelvic organ prolapse: Current concepts, challenges, and future perspectives. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Nolfi, A.L.; Brown, B.N.; Liang, R.; Palcsey, S.L.; Bonidie, M.J.; Abramowitch, S.D.; Moalli, P.A. Host response to synthetic mesh in women with mesh complications. Am. J. Obstet. Gynecol. 2016, 215, 206.e1–206.e8. [Google Scholar] [CrossRef] [PubMed]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, Present and Future of Surgical Meshes: A Review. Membranes 2017, 7, 47. [Google Scholar] [CrossRef]
- Barone, W.R.; Abramowitch, S.D.; Moalli, P.A. Host Response to Biomaterials; Host Response to Biomaterials for Pelvic Floor Reconstruction; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 375–423. [Google Scholar]
- Shepherd, J.P.; Feola, A.; Abramowitch, S.D.; Moalli, P.A. Uniaxial biomechanical properties of seven different vaginally implanted meshes for pelvic organ prolapse. Int. Urogynecol. J. 2011, 23, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Pott, P.P.; Schwarz, M.L.R.; Gundling, R.; Nowak, K.; Hohenberger, P.; Roessner, E.D. Mechanical Properties of Mesh Materials Used for Hernia Repair and Soft Tissue Augmentation. PLoS ONE 2012, 7, e46978. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.L.; Damoiseaux, A.; IntHout, J.; Kluivers, K.B.; Withagen, M.I.J. Long-term outcome of vaginal mesh or native tissue in recurrent prolapse: A randomized controlled trial. Int. Urogynecol. J. 2018, 29, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.E.; Hansen, B.L.; Egger, M.J.; Nygaard, I.; Sanchez-Birkhead, A.C.; Hsu, Y.; Clark, L. Changed women: The long-term impact of vaginal mesh complications. Female Pelvic. Med. Reconstr. Surg. 2014, 20, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Margulies, R.U.; Lewicky-Gaupp, C.; Fenner, D.E.; McGuire, E.J.; Clemens, J.Q.; DeLancey, J.O. Complications requiring reoperation following vaginal mesh kit procedures for prolapse. Am. J. Obstet. Gynecol. 2008, 199, 678.e1–678.e4. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, R.M.; Liang, R.; Knight, K.; Carter-Brooks, C.M.; Abramowitch, S.; Moalli, P.A. Impact of polypropylene prolapse mesh on vaginal smooth muscle in rhesus macaque. Am. J. Obstet. Gynecol. 2019, 221, 330.e1–330.e9. [Google Scholar] [CrossRef]
- Liang, R.; Knight, K.M.; Barone, W.; Powers, R.W.; Nolfi, A.; Palcsey, S.; Abramowitch, S.; Moalli, P.A. Extracellular matrix regenerative graft attenuates the negative impact of polypropylene prolapse mesh on vagina in rhesus macaque. Am. J. Obstet. Gynecol. 2017, 216, 153.e–153.e9. [Google Scholar] [CrossRef]
- Feola, A.; Abramowitch, S.; Jallah, Z.; Stein, S.; Barone, W.; Palcsey, S.; Moalli, P. Deterioration in biomechanical properties of the vagina following implantation of a high-stiffness prolapse mesh. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 224–232. [Google Scholar] [CrossRef]
- Gargett, C.E.; Gurung, S.; Darzi, S.; Werkmeister, J.A.; Mukherjee, S. Tissue engineering approaches for treating pelvic organ prolapse using a novel source of stem/stromal cells and new materials. Curr. Opin. Urol. 2019, 29, 450–457. [Google Scholar] [CrossRef]
- Darzi, S.; Werkmeister, J.A.; Deane, J.A.; Gargett, C.E. Identification and Characterization of Human Endometrial Mesenchymal Stem/Stromal Cells and Their Potential for Cellular Therapy. Stem Cells Transl. Med. 2016, 5, 1127–1132. [Google Scholar] [CrossRef]
- Heathman, T.R.; Nienow, A.W.; McCall, M.; Coopman, K.; Kara, B.; Hewitt, C. The translation of cell-based therapies: Clinical landscape and manufacturing challenges. Regen. Med. 2015, 10, 49–64. [Google Scholar] [CrossRef]
- Kode, J.A.; Mukherjee, S.; Joglekar, M.V.; Hardikar, A.A. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009, 11, 377–391. [Google Scholar] [CrossRef]
- Cao, W.; Cao, K.; Cao, J.; Wang, Y.; Shi, Y. Mesenchymal stem cells and adaptive immune responses. Immunol. Lett. 2015, 168, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Davies, L. Mesenchymal stromal cells and the innate immune response. Immunol. Lett. 2015, 168, 140–146. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Paul, K.; Werkmeister, J.A.; Gargett, C.E. Mesenchymal stem cell-based bioengineered constructs: Foreign body response, cross-talk with macrophages and impact of biomaterial design strategies for pelvic floor disorders. Interface Focus 2019, 9, 20180089. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Pagliara, D.; Locatelli, F. Mesenchymal stromal cell therapy: A revolution in Regenerative Medicine? Bone Marrow Transplant. 2011, 47, 164–171. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Ulrich, D.; Muralitharan, R.R.; Gargett, C.E. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin. Biol. Ther. 2013, 13, 1387–1400. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Kelly, R.W.; Fraser, H.M.; Critchley, H.O.D. Endocrine Regulation of Menstruation. Endocr. Rev. 2006, 27, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Nguyen, H.P.T.; Ye, L. Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 2012, 13, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Henriet, P.; Chevronnay, H.P.G.; Marbaix, E. The endocrine and paracrine control of menstruation. Mol. Cell. Endocrinol. 2012, 358, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Cervelló, I.; Martínez-Conejero, J.A.; Horcajadas, J.A.; Pellicer, A.; Simón, C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum. Reprod. 2007, 22, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Schwab, K.E.; Gargett, C.E. Clonogenicity of Human Endometrial Epithelial and Stromal Cells1. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef]
- Schwab, K.E.; Chan, R.; Gargett, C.E. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil. Steril. 2005, 84, 1124–1130. [Google Scholar] [CrossRef]
- Ulrich, D.; Tan, K.S.; Deane, J.; Schwab, K.; Cheong, A.; Rosamilia, A.; Gargett, C.E. Mesenchymal stem/stromal cells in post-menopausal endometrium. Hum. Reprod. 2014, 29, 1895–1905. [Google Scholar] [CrossRef]
- Schwab, K.E.; Gargett, C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007, 22, 2903–2911. [Google Scholar] [CrossRef]
- Gargett, C.E.; Gurung, S. Endometrial Mesenchymal Stem/Stromal Cells, Their Fibroblast Progeny in Endometriosis, and More. Biol. Reprod. 2016, 94, 129. [Google Scholar] [CrossRef]
- Spitzer, T.L.; Rojas, A.; Zelenko, Z.; Aghajanova, L.; Erikson, D.W.; Barragan, F.; Meyer, M.; Tamaresis, J.S.; Hamilton, A.E.; Irwin, J.C.; et al. Perivascular Human Endometrial Mesenchymal Stem Cells Express Pathways Relevant to Self-Renewal, Lineage Specification, and Functional Phenotype1. Biol. Reprod. 2012, 86, 58. [Google Scholar] [CrossRef]
- Masuda, H.; Anwar, S.S.; Bühring, H.-J.; Rao, J.R.; Gargett, C.E. A Novel Marker of Human Endometrial Mesenchymal Stem-Like Cells. Cell Transplant. 2012, 21, 2201–2214. [Google Scholar] [CrossRef]
- Sivasubramaniyan, K.; Harichandan, A.; Schumann, S.; Sobiesiak, M.; Lengerke, C.; Maurer, A.; Kalbacher, H.; Bühring, H.-J. Prospective Isolation of Mesenchymal Stem Cells from Human Bone Marrow Using Novel Antibodies Directed Against Sushi Domain Containing 2. Stem Cells Dev. 2013, 22, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.-H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Gurung, S.; Werkmeister, J.A.; Gargett, C.E. Inhibition of Transforming Growth Factor-β Receptor signaling promotes culture expansion of undifferentiated human Endometrial Mesenchymal Stem/stromal Cells. Sci. Rep. 2015, 5, 15042. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Williams, S.; Deane, J.; Werkmeister, J.A.; Gargett, C.E. The Transcriptome of Human Endometrial Mesenchymal Stem Cells Under TGFβR Inhibition Reveals Improved Potential for Cell-Based Therapies. Front. Cell Dev. Biol. 2018, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Lucciola, R.; Vrljicak, P.; Gurung, S.; Filby, C.; Darzi, S.; Muter, J.; Ott, S.; Brosens, J.J.; Gargett, C.E. Impact of Sustained Transforming Growth Factor-β Receptor Inhibition on Chromatin Accessibility and Gene Expression in Cultured Human Endometrial MSC. Front. Cell Dev. Biol. 2020, 8, 567610. [Google Scholar] [CrossRef]
- Samsonraj, R.; Rai, B.; Sathiyanathan, P.; Puan, K.J.; Rötzschke, O.; Hui, J.H.; Raghunath, M.; Stanton, L.W.; Nurcombe, V.; Cool, S.M. Establishing Criteria for Human Mesenchymal Stem Cell Potency. Stem Cells 2015, 33, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Masuda, H. Adult stem cells in the endometrium. Mol. Hum. Reprod. 2010, 16, 818–834. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Paul, K.; Cousins, F.; Werkmeister, J.A.; Gargett, C.E. Electrospun Nanofiber Meshes With Endometrial MSCs Modulate Foreign Body Response by Increased Angiogenesis, Matrix Synthesis, and Anti-Inflammatory Gene Expression in Mice: Implication in Pelvic Floor. Front. Pharmacol. 2020, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Edwards, S.L.; Tan, K.S.; White, J.; Kandel, S.; Ramshaw, J.A.; Gargett, C.E.; Werkmeister, J.A. Induction of endometrial mesenchymal stem cells into tissue-forming cells suitable for fascial repair. Acta Biomater. 2014, 10, 5012–5020. [Google Scholar] [CrossRef]
- Edwards, S.; Ulrich, D.; White, J.; Su, K.; Rosamilia, A.; Ramshaw, J.; Gargett, C.; Werkmeister, J. Temporal changes in the biomechanical properties of endometrial mesenchymal stem cell seeded scaffolds in a rat model. Acta Biomater. 2015, 13, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Letouzey, V.; Tan, K.S.; Deane, J.; Ulrich, D.; Gurung, S.; Ong, Y.R.; Gargett, C.E. Isolation and Characterisation of Mesenchymal Stem/Stromal Cells in the Ovine Endometrium. PLoS ONE 2015, 10, e0127531. [Google Scholar] [CrossRef]
- Darzi, S.; Deane, J.; Nold, C.A.; Edwards, S.E.; Gough, D.; Mukherjee, S.; Gurung, S.; Tan, K.S.; Vashi, A.V.; Werkmeister, J.A.; et al. Endometrial Mesenchymal Stem/Stromal Cells Modulate the Macrophage Response to Implanted Polyamide/Gelatin Composite Mesh in Immunocompromised and Immunocompetent Mice. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Gurung, S.; Deane, J.; Darzi, S.; Werkmeister, J.A.; Gargett, C.E. In Vivo Survival of Human Endometrial Mesenchymal Stem Cells Transplanted Under the Kidney Capsule of Immunocompromised Mice. Stem Cells Dev. 2018, 27, 35–43. [Google Scholar] [CrossRef]
- Emmerson, S.; Mukherjee, S.; Melendez-Munoz, J.; Cousins, F.; Edwards, S.; Karjalainen, P.; Ng, M.; Tan, K.; Darzi, S.; Bhakoo, K.; et al. Composite mesh design for delivery of autologous mesenchymal stem cells influences mesh integration, exposure and biocompatibility in an ovine model of pelvic organ prolapse. Biomaterials 2019, 225, 119495. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Rosamilia, A.; Kadam, V.; Truong, Y.; Werkmeister, J.A.; Gargett, C.E. Blended Nanostructured Degradable Mesh with Endometrial Mesenchymal Stem Cells Promotes Tissue Integration and Anti-Inflammatory Response in Vivo for Pelvic Floor Application. Biomacromolecules 2019, 20, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Darzi, S.; McPhee, G.; Del Borgo, M.P.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. 3D bioprinted endometrial stem cells on melt electrospun poly ε-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 2019, 97, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Ulrich, D.; Sturm, M.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. Comparing the Effect of TGF-β Receptor Inhibition on Human Perivascular Mesenchymal Stromal Cells Derived from Endometrium, Bone Marrow and Adipose Tissues. J. Pers. Med. 2020, 10, 261. [Google Scholar] [CrossRef]
- Paul, K.; Darzi, S.; Del Borgo, M.P.; Cousins, F.L.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. Vaginal delivery of tissue engineered endometrial mesenchymal stem/stromal cells in an aloe vera-alginate hydrogel alleviates maternal simulated birth injury. Appl. Mater. Today 2021, 22, 100890. [Google Scholar] [CrossRef]
- Han, X.; Meng, X.; Yin, Z.; Rogers, A.; Zhong, J.; Rillema, P.; Jackson, J.A.; Ichim, T.E.; Minev, B.; Carrier, E.; et al. Inhibition of intracranial glioma growth by endometrial regenerative cells. Cell Cycle 2009, 8, 606–610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MacNeil, S.; Mangir, N.; Roman, S.; Mironska, E. Tissue engineering for the pelvic floor. Curr. Opin. Urol. 2019, 29, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Edwards, S.L.; White, J.F.; Supit, T.; Ramshaw, J.A.M.; Lo, C.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. A Preclinical Evaluation of Alternative Synthetic Biomaterials for Fascial Defect Repair Using a Rat Abdominal Hernia Model. PLoS ONE 2012, 7, e50044. [Google Scholar] [CrossRef] [PubMed]
- Vashaghian, M.; Zaat, S.J.; Smit, T.H.; Roovers, J.-P. Biomimetic implants for pelvic floor repair. Neurourol. Urodyn. 2018, 37, 566–580. [Google Scholar] [CrossRef]
- Mukherjee, S.; Venugopal, J.R.; Ravichandran, R.; Ramalingam, M.; Raghunath, M.; Ramakrishna, S. Nanofiber Technology for Controlling Stem Cell Functions and Tissue Engineering. Micro Nanotechnol. Eng. Stem Cells Tissues 2013, 1, 27–51. [Google Scholar] [CrossRef]
- He, H.; Wu, X.; Wang, Y.; Zhu, C.; Tong, X.; Yang, M.; Yang, L.; Huang, W.; Wu, F.; Zong, H.; et al. Preclinical animal study and human clinical trial data of co-electrospun poly(L-lactide-co-caprolactone) and fibrinogen mesh for anterior pelvic floor reconstruction. Int. J. Nanomed. 2016, 11, 389–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Regueros, S.R.; Albersen, M.; Manodoro, S.; Zia, S.; Osman, N.I.; Bullock, A.; Chapple, C.; Deprest, J.; MacNeil, S. AcuteIn VivoResponse to an Alternative Implant for Urogynecology. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Shores, L.S.; Votaw, N.L.; Collier, J.H. Biomaterial strategies for generating therapeutic immune responses. Adv. Drug Deliv. Rev. 2017, 114, 3–18. [Google Scholar] [CrossRef]
- Paul, N.E.; Skazik, C.; Harwardt, M.; Bartneck, M.; Denecke, B.; Klee, D.; Salber, J.; Zwadlo-Klarwasser, G. Topographical control of human macrophages by a regularly microstructured polyvinylidene fluoride surface. Biomaterials 2008, 29, 4056–4064. [Google Scholar] [CrossRef]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef]
- Sridharan, I.; Ma, Y.; Kim, T.; Kobak, W.; Rotmensch, J.; Wang, R. Structural and mechanical profiles of native collagen fibers in vaginal wall connective tissues. Biomaterials 2012, 33, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Couri, B.M.; Lenis, A.T.; Borazjani, A.; Paraiso, M.F.R.; Damaser, M.S. Animal models of female pelvic organ prolapse: Lessons learned. Expert Rev. Obstet. Gynecol. 2012, 7, 249–260. [Google Scholar] [CrossRef]
- Emmerson, S.; Young, N.; Rosamilia, A.; Parkinson, L.; Edwards, S.L.; Vashi, A.V.; Davies-Tuck, M.; White, J.; Elgass, K.; Lo, C.; et al. Ovine multiparity is associated with diminished vaginal muscularis, increased elastic fibres and vaginal wall weakness: Implication for pelvic organ prolapse. Sci. Rep. 2017, 7, srep45709. [Google Scholar] [CrossRef]
- Mori da Cunha, M.; Mackova, K.; Hympanova, L.; Bortolini, M.; Deprest, J. Animal models for pelvic organ pro-lapse: Systematic review. Int. Urogynecol. J. 2021, 32, 1331–1344. [Google Scholar] [CrossRef]
- Ashton-Miller, J.A.; DeLancey, J.O.L. Functional Anatomy of the Female Pelvic Floor. Ann. N. Y. Acad. Sci. 2007, 1101, 266–296. [Google Scholar] [CrossRef]
- Ulrich, D.; Edwards, S.L.; Letouzey, V.; Su, K.; White, J.F.; Rosamilia, A.; Gargett, C.E.; Werkmeister, J.A. Regional Variation in Tissue Composition and Biomechanical Properties of Postmenopausal Ovine and Human Vagina. PLoS ONE 2014, 9, e104972. [Google Scholar] [CrossRef] [PubMed]
- Young, N.; Rosamilia, A.; Arkwright, J.; Lee, J.; Davies-Tuck, M.; Melendez, J.; Werkmeister, J.; Gargett, C.E. Vaginal wall weakness in parous ewes: A potential preclinical model of pelvic organ prolapse. Int. Urogynecol. J. 2016, 28, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Hida, N.; Nishiyama, N.; Miyoshi, S.; Kira, S.; Segawa, K.; Uyama, T.; Mori, T.; Miyado, K.; Ikegami, Y.; Cui, C.; et al. Novel Cardiac Precursor-Like Cells from Human Menstrual Blood-Derived Mesenchymal Cells. Stem Cells 2008, 26, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hu, X.; Yu, H.; Xu, Y.; Wang, L.; Chen, H.; Chen, H.; Wu, R.; Zhang, Z.; Xiang, C.; et al. Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. J. Cell. Mol. Med. 2013, 17, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, S.; Khanmohammadi, M.; Zarnani, A.H.; Talebi, S.; Edalatkhah, H.; Eghtesad, S.; Nikokar, I.; Kazemnejad, S. Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. J. Tissue Eng. Regen. Med. 2015, 9, E124–E134. [Google Scholar] [CrossRef]
- Fathi-Kazerooni, M.; Tavoosidana, G.; Taghizadeh-Jahed, M.; Khanjani, S.; Golshahi, H.; Gargett, C.E.; Edalatkhah, H.; Kazemnejad, S. Comparative restoration of acute liver failure by menstrual blood stem cells compared with bone marrow stem cells in mice model. Cytotherapy 2017, 19, 1474–1490. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, C.; Chen, L.; Wang, X.; Xiang, B.; Wu, X.; Guo, Y.; Mou, X.; Yuan, L.; Chen, B.; et al. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl. Med. 2017, 6, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Li, P.; Tan, J. Human Menstrual Blood-Derived Stromal Cells Promote Recovery of Premature Ovarian Insufficiency Via Regulating the ECM-Dependent FAK/AKT Signaling. Stem Cell Rev. Rep. 2018, 15, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Manshadi, M.D.; Navid, S.; Hoshino, Y.; Daneshi, E.; Noory, P.; Abbasi, M. The effects of human menstrual blood stem cells-derived granulosa cells on ovarian follicle formation in a rat model of premature ovarian failure. Microsc. Res. Tech. 2019, 82, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Zafardoust, S.; Kazemnejad, S.; Darzi, M.; Fathi-Kazerooni, M.; Rastegari, H.; Mohammadzadeh, A. Improvement of Pregnancy Rate and Live Birth Rate in Poor Ovarian Responders by Intraovarian Administration of Autologous Menstrual Blood Derived- Mesenchymal Stromal Cells: Phase I/II Clinical Trial. Stem Cell Rev. Rep. 2020, 16, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Chen, L.; Wang, X.; Zhao, Y.; Wang, Y.; Xiang, C. Transplantation of Menstrual Blood-Derived Mesenchymal Stem Cells Promotes the Repair of LPS-Induced Acute Lung Injury. Int. J. Mol. Sci. 2017, 18, 689. [Google Scholar] [CrossRef] [PubMed]
- Farzamfar, S.; Salehi, M.; Ehterami, A.; Naseri-Nosar, M.; Vaez, A.; Zarnani, A.H.; Sahrapeyma, H.; Shokri, M.-R.; Aleahmad, M. Promotion of excisional wound repair by a menstrual blood-derived stem cell-seeded decellularized human amniotic membrane. Biomed. Eng. Lett. 2018, 8, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Kaneko, Y.; Maki, M.; Yu, S.-J.; Ali, M.; Allickson, J.G.; Sanberg, C.D.; Kuzmin-Nichols, N.; Sanberg, P.R. Menstrual Blood Cells Display Stem Cell–Like Phenotypic Markers and Exert Neuroprotection Following Transplantation in Experimental Stroke. Stem Cells Dev. 2010, 19, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Uyama, T.; Miyado, K.; Terai, M.; Kyo, S.; Kiyono, T. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol. Biol. Cell 2007, 18, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. MSCs: The Sentinel and Safe-Guards of Injury. J. Cell. Physiol. 2016, 231, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, Z.; Yang, Y.; Zhang, M.; Wu, J.; Miao, Y. Diagnostic value of pelvic floor ultrasonography for diagnosis of pelvic organ prolapse: A systematic review. Int. Urogynecol. J. 2019, 31, 15–33. [Google Scholar] [CrossRef]
- Eisenberg, V.H.; Steinberg, M.; Weiner, Z.; Alcalay, M.; Itskovitz-Eldor, J.; Schiff, E.; Lowenstein, L. Three-dimensional transperineal ultrasound for imaging mesh implants following sacrocolpopexy. Ultrasound Obstet. Gynecol. 2014, 43, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Organ printing: From bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011, 22, 667–673. [Google Scholar] [CrossRef] [PubMed]



| Source/Model | Cell Type/Scaffold | Properties | Reference |
|---|---|---|---|
| Human | Freshly isolated stromal cells |
| Chan et al., 2004 [52] |
| Human | Freshly isolated CD146+PDGFRβ+(CD140b+CD146+) stromal cells |
| Schwab et al., 2007 [55] |
| Human | SUSD2+ (W5C5+) eMSC |
| Masuda et al., 2012 [58] |
| Human | SUSD2+ eMSC/Polyamide (PA) nanomesh cross-linked with gelatin (PAG) |
| Su et al., 2014 [67] |
| Human/Rat (Subcutaneous wound) | SUSD2+ eMSC/Polyamide (PA) nanomesh cross-linked with gelatin (PAG) | Compared to mesh without eMSC:
| Ulrich et al., 2014 [11] Edwards et al., 2015 [68] |
| Human | SUSD2+ eMSC |
| Gurung et al., 2015 [61] |
| Sheep | CD271+CD45f− ovine eMSC |
| Letouzey et al., 2015 [69] |
| Human/NSG and C57Bl6 mice (wound repair) | mCherry+ SUSD2+ eMSC/PAG mesh |
| Darzi et al., 2018 [70] |
| Human/NSG mice | mCherry−labelledSUSD2+ eMSC |
| Gurung et al., 2018 [71] |
| Human | A83-01 treated SUSD2+ eMSC |
| Gurung et al., 2018 [62] |
| Sheep | Autologous ovine CD271+ eMSC/PAG mesh |
| Emmerson et al., 2019 [72] |
| Human/NSG mice (wound repair) | SUSD2+ eMSC/PLCL nanomesh |
| Mukherjee et al., 2019 [73] Mukherjee et al., 2020 [66] |
| Human/NSG mice (wound repair) | mCherry-labelled SUSD2+ eMSC/3D on 3D printed PCL nanomesh |
| Paul et al., 2019 [74] |
| Human | A83-01 treated SUSD2+ eMSC |
| Lucciola et al., 2020 [63] |
| Human | A83-01-treated SUSD2+ |
| Gurung et al., 2020 [75] |
| Human/rat (vaginal birth injury) | SUSD2+ eMSC/AV+ALG hydrogel |
| Paul et al., 2021 [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennes, D.M.Z.B.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. Endometrial SUSD2+ Mesenchymal Stem/Stromal Cells in Tissue Engineering: Advances in Novel Cellular Constructs for Pelvic Organ Prolapse. J. Pers. Med. 2021, 11, 840. https://doi.org/10.3390/jpm11090840
Hennes DMZB, Rosamilia A, Werkmeister JA, Gargett CE, Mukherjee S. Endometrial SUSD2+ Mesenchymal Stem/Stromal Cells in Tissue Engineering: Advances in Novel Cellular Constructs for Pelvic Organ Prolapse. Journal of Personalized Medicine. 2021; 11(9):840. https://doi.org/10.3390/jpm11090840
Chicago/Turabian StyleHennes, David M. Z. B., Anna Rosamilia, Jerome A. Werkmeister, Caroline E. Gargett, and Shayanti Mukherjee. 2021. "Endometrial SUSD2+ Mesenchymal Stem/Stromal Cells in Tissue Engineering: Advances in Novel Cellular Constructs for Pelvic Organ Prolapse" Journal of Personalized Medicine 11, no. 9: 840. https://doi.org/10.3390/jpm11090840
APA StyleHennes, D. M. Z. B., Rosamilia, A., Werkmeister, J. A., Gargett, C. E., & Mukherjee, S. (2021). Endometrial SUSD2+ Mesenchymal Stem/Stromal Cells in Tissue Engineering: Advances in Novel Cellular Constructs for Pelvic Organ Prolapse. Journal of Personalized Medicine, 11(9), 840. https://doi.org/10.3390/jpm11090840







