Brain Atrophy Mediates the Relationship between Misfolded Proteins Deposition and Cognitive Impairment in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Neurobehavioral Evaluation and Cognitive Severity Definition
2.3. Blood Sampling and Assaying of Plasma Biomarkers: T-tau, Aβ-40, Aβ-42, α-Synuclein, and Neurofilament Light Chain
2.4. Structural MRI Imaging
2.4.1. Image Acquisition
2.4.2. Image Data Processing
2.5. Statistics
2.5.1. Demographic Data
2.5.2. Partial Correlation
2.5.3. Mediation Analysis
- (a)
- The effect of plasma misfolded protein level on brain volume (indirect effect, path a);
- (b)
- The effect of brain volume on neuropsychological assessments by controlling the effect of plasma misfolded proteins (indirect effect, path b);
- (c)
- The mediation effects a × b are defined as the reduction in the relationship between the plasma misfolded proteins and the neuropsychological assessments (total relationship, path c) by including the brain volume in the model (direct path, path c).
3. Results
3.1. Basic Characteristics
3.2. Neuropsychological Assessments
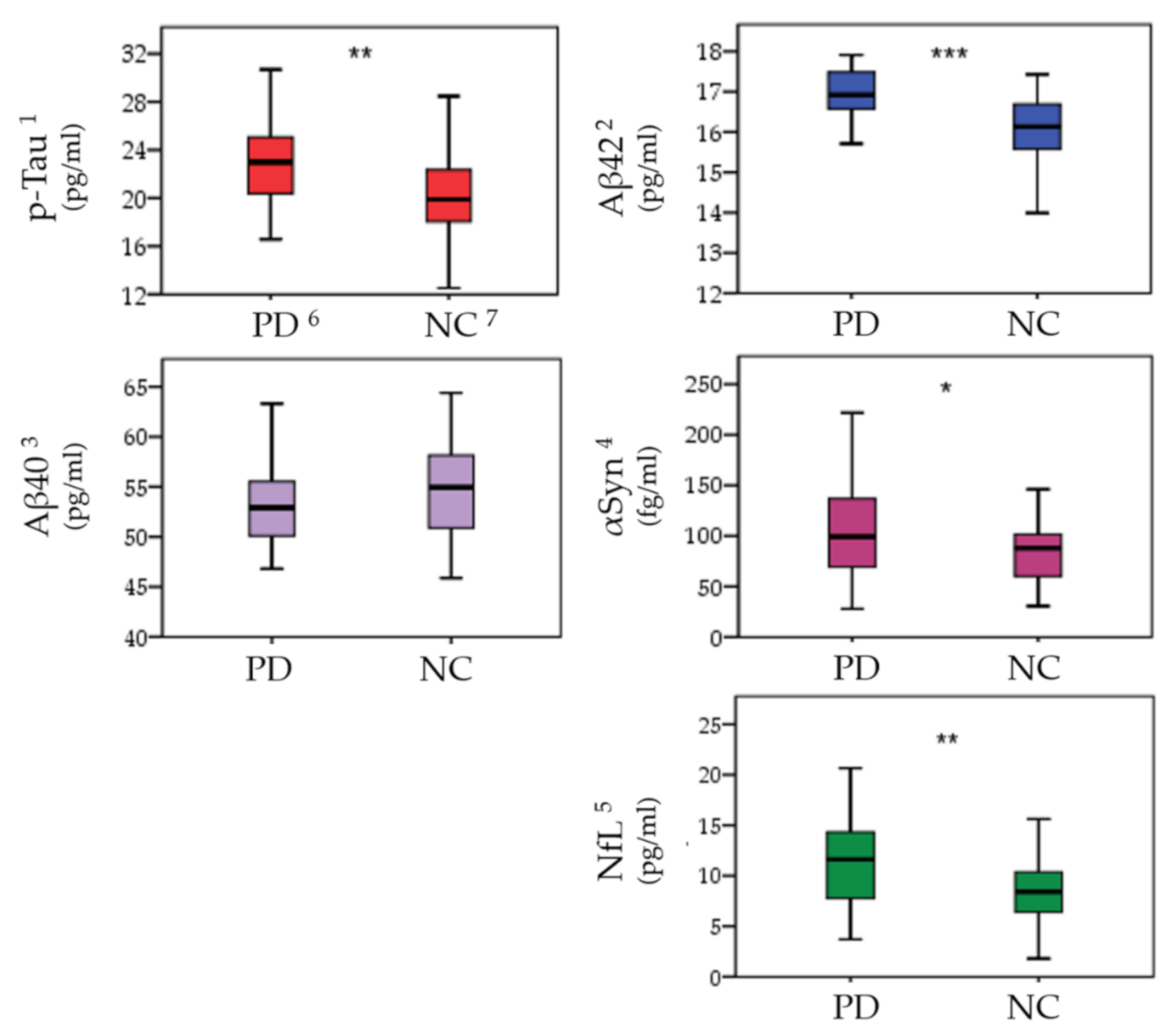
3.3. Plasma Biomarkers
3.4. Brain Volume Analysis
3.5. Correlation Analysis of Plasma Misfolded Proteins, Neuropsychological Assessments, and Brain Atrophy
3.5.1. Misfolded Proteins and ROI Volumes in PD Patients
3.5.2. Neuropsychological Assessments and Brain Volumes in PD Patients
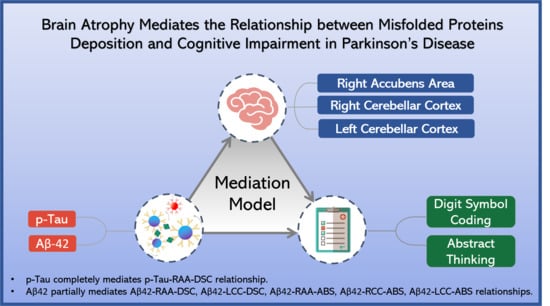
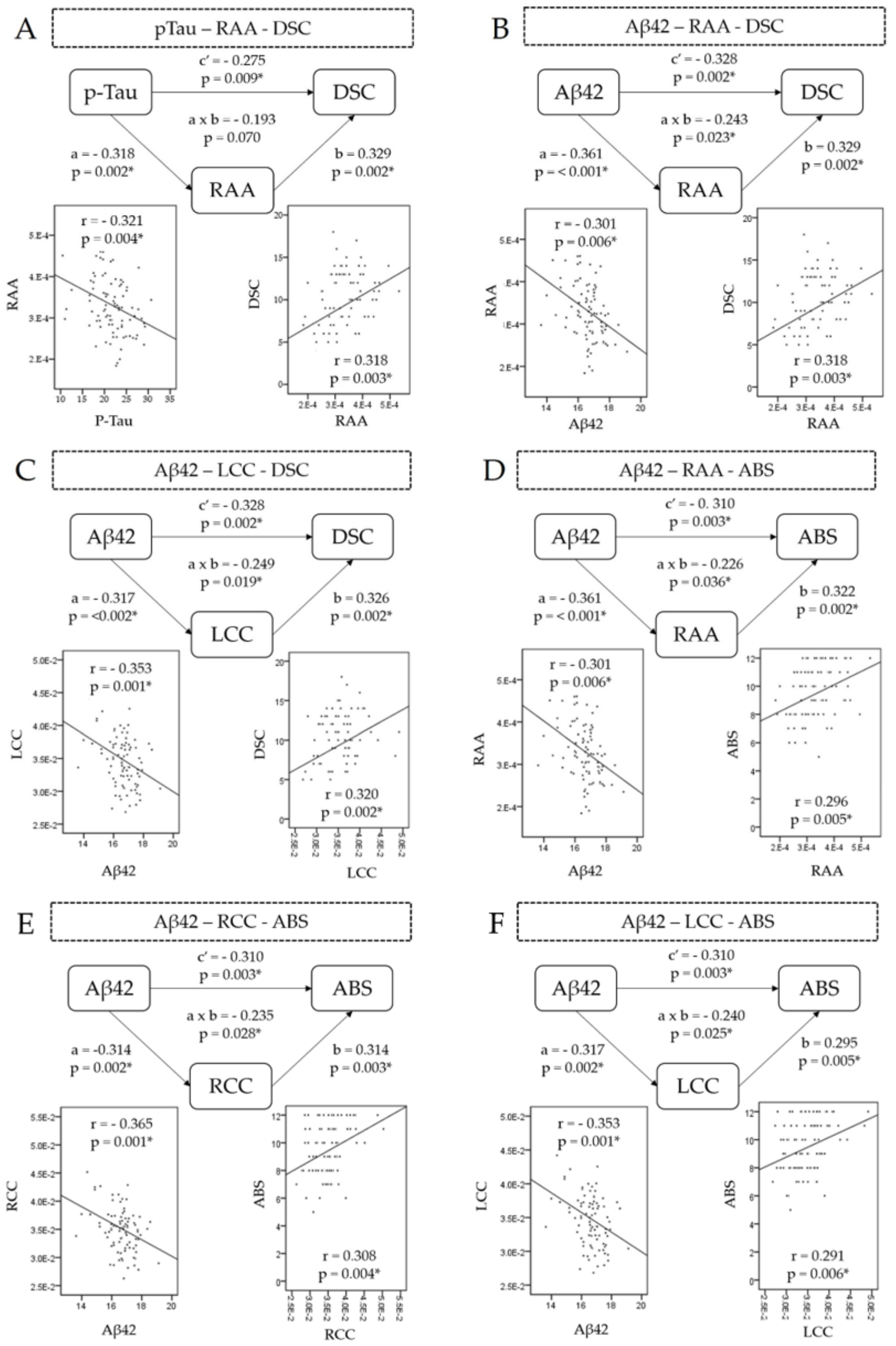
3.6. Mediation Analysis
- p-Tau–digit symbol coding relationship mediator
- Aβ42–digit symbol coding relationship mediator
- Aβ-42–abstract thinking relationship mediator
4. Discussion
4.1. Pathophysiology of Misfolded Protein Deposition and Cortical Atrophy in PD Patients
4.2. Brain Atrophy Involved in Several Neuronal Circuits in Cognitively Impaired PD Patients
4.3. Gray Matter Atrophy Contributions in Misfolded Proteins and Cognitive Impairment Correlation: Mediation Analysis
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebouvier, T.; Chaumette, T.; Paillusson, S.; Duyckaerts, C.; Bruley des Varannes, S.; Neunlist, M.; Derkinderen, P. The second brain and Parkinson’s disease. Eur. J. Neurosci. 2009, 30, 735–741. [Google Scholar] [CrossRef]
- Martínez-Martín, P.; Rodríguez-Blázquez, C.; Mario, A.; Arakaki, T.; Arillo, V.C.; Chaná, P.; Fernández, W.; Garretto, N.; Martínez-Castrillo, J.C.; Rodríguez-Violante, M.; et al. Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism Relat. Disord. 2015, 21, 50–54. [Google Scholar] [CrossRef]
- Meireles, J.; Massano, J. Cognitive impairment and dementia in Parkinson’s disease: Clinical features, diagnosis, and management. Front. Neurol. 2012, 3, 88. [Google Scholar] [CrossRef]
- Lawson, R.A.; Yarnall, A.J.; Johnston, F.; Duncan, G.W.; Khoo, T.K.; Collerton, D.; Taylor, J.P.; Burn, D.J. Cognitive impairment in Parkinson’s disease: Impact on quality of life of carers. Int. J. Geriatr. Psychiatry 2017, 32, 1362–1370. [Google Scholar] [CrossRef]
- Chen, N.C.; Chen, H.L.; Li, S.H.; Chang, Y.H.; Chen, M.H.; Tsai, N.W.; Yu, C.C.; Yang, S.Y.; Lu, C.H.; Lin, W.C. Plasma Levels of α-Synuclein, Aβ-40 and T-tau as Biomarkers to Predict Cognitive Impairment in Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalez, I.; Soto, C. Misfolded protein aggregates: Mechanisms, structures and potential for disease transmission. Semin. Cell Dev. Biol. 2011, 22, 482–487. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.Y.; Horng, H.E.; Yang, C.C.; Chieh, J.J.; Chen, H.H.; Liu, B.H.; Chiu, M.J. Plasma α-synuclein predicts cognitive decline in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 818–824. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations The Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Bjornestad, A.; Tysnes, O.-B.; Larsen, J.P.; Alves, G. Reliability of Three Disability Scales for Detection of Independence Loss in Parkinson’s Disease. Parkinsons Dis. 2016, 2016, 1941034. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-N.; Wang, P.-N.; Liu, H.-C.; Teng, E.L. Cognitive Abilities Screening Instrument, Chinese Version 2.0 (CASI C-2.0): Administration and clinical application. Acta Neurol Taiwan 2012, 21, 180–189. [Google Scholar]
- Lin, C.-J.; Lin, C.-C.; Hung, Y.-Y.; Tsai, M.-C.; Wang, Y.-L.; Tsai, M.-C.; Liu, M.-H.; Lee, Y.-H.; Huang, T.-L. The correlations between results of short-form wechsler adult intelligence Scale-III and demographic/clinical factors in patients with schizophrenia: Preliminary findings. Taiwan J. Psychiatry 2019, 33, 48–50. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Yang, S.Y.; Chiu, M.J.; Lin, C.H.; Horng, H.E.; Yang, C.C.; Chieh, J.J.; Chen, H.H.; Liu, B.H. Development of an ultra-high sensitive immunoassay with plasma biomarker for differentiating Parkinson disease dementia from Parkinson disease using antibody functionalized magnetic nanoparticles. J. Nanobiotechnol. 2016, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Chiu, M.-J.; Chen, T.-F.; Chang, H.-L.; Liu, B.-H.; Yang, S.-Y. Assay of Plasma Phosphorylated Tau Protein (Threonine 181) and Total Tau Protein in Early-Stage Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Schmansky, N.J.; Rosas, H.D.; Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 2012, 61, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Fossati, S.; Ramos Cejudo, J.; Debure, L.; Pirraglia, E.; Sone, J.Y.; Li, Y.; Chen, J.; Butler, T.; Zetterberg, H.; Blennow, K.; et al. Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer’s disease. Alzheimers Dement (Amst) 2019, 11, 483–492. [Google Scholar] [CrossRef]
- Hanon, O.; Vidal, J.S.; Lehmann, S.; Bombois, S.; Allinquant, B.; Tréluyer, J.M.; Gelé, P.; Delmaire, C.; Blanc, F.; Mangin, J.F.; et al. Plasma amyloid levels within the Alzheimer’s process and correlations with central biomarkers. Alzheimers Dement 2018, 14, 858–868. [Google Scholar] [CrossRef]
- Wills, J.; Jones, J.; Haggerty, T.; Duka, V.; Joyce, J.N.; Sidhu, A. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp. Neurol. 2010, 225, 210–218. [Google Scholar] [CrossRef]
- Coakeley, S.; Strafella, A.P. Imaging tau pathology in Parkinsonisms. NPJ Parkinsons Dis. 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, I.; Hasegawa, M.; Arai, T.; Ikeda, K.; Oshima, K.; Niizato, K.; Aoki, N.; Omi, K.; Higashi, S.; Hosokawa, M.; et al. Tau accumulation in the nucleus accumbens in tangle-predominant dementia. Acta Neuropathol. Commun. 2014, 2, 40. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F.; Wang, D.; Li, C.; Fu, Y.; He, W.; Zhang, J. Tau Pathology in Parkinson’s Disease. Front. Neurol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-f.; Xu, T.-h.; Yan, Y.; Zhou, Y.-r.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Morley, J.E.; Farr, S.A.; Nguyen, A.D.; Xu, F. What is the Physiological Function of Amyloid-Beta Protein? J. Nutr. Health Aging 2019, 23, 225–226. [Google Scholar] [CrossRef]
- Preda, S.; Govoni, S.; Lanni, C.; Racchi, M.; Mura, E.; Grilli, M.; Marchi, M. Acute beta-amyloid administration disrupts the cholinergic control of dopamine release in the nucleus accumbens. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 1062–1070. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, L.Y.; Tzen, K.Y.; Chen, Y.F.; Chen, T.F.; Lai, Y.M.; Yen, R.F.; Huang, Y.Y.; Shiue, C.Y.; Yang, S.Y.; Chiu, M.J. The Relation between Brain Amyloid Deposition, Cortical Atrophy, and Plasma Biomarkers in Amnesic Mild Cognitive Impairment and Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 175. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, B.C.; Lin, C.H. Integrated Plasma and Neuroimaging Biomarkers Associated with Motor and Cognition Severity in Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, D.G.; Zimmermann, N.; Netto, T.M.; Tukamoto, G.; Ventura, N.; de Castro Bellini Leite, S.; Cabral, R.F.; Fonseca, R.P.; Bahia, P.R.; Gasparetto, E.L. Regional Cerebral Gray Matter Volume in HIV-Positive Patients with Executive Function Deficits. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2016, 26, 450–457. [Google Scholar] [CrossRef]
- Sizemore, R.J.; Seeger-Armbruster, S.; Hughes, S.M.; Parr-Brownlie, L.C. Viral vector-based tools advance knowledge of basal ganglia anatomy and physiology. J. Neurophysiol. 2016, 115, 2124–2146. [Google Scholar] [CrossRef]
- Goto, Y.; Grace, A.A. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008, 31, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Krienen, F.M.; Buckner, R.L. Segregated Fronto-Cerebellar Circuits Revealed by Intrinsic Functional Connectivity. Cereb. Cortex 2009, 19, 2485–2497. [Google Scholar] [CrossRef] [PubMed]
- Bellebaum, C.; Daum, I. Cerebellar involvement in executive control. Cerebellum 2007, 6, 184–192. [Google Scholar] [CrossRef]
- Caspell-Garcia, C.; Simuni, T.; Tosun-Turgut, D.; Wu, I.W.; Zhang, Y.; Nalls, M.; Singleton, A.; Shaw, L.A.; Kang, J.H.; Trojanowski, J.Q.; et al. Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS ONE 2017, 12, e0175674. [Google Scholar] [CrossRef]
- Hanganu, A.; Bedetti, C.; Degroot, C.; Mejia-Constain, B.; Lafontaine, A.L.; Soland, V.; Chouinard, S.; Bruneau, M.A.; Mellah, S.; Belleville, S.; et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain 2014, 137, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S. Clinical implications for dopaminergic and functional neuroimage research in cognitive symptoms of Parkinson’s disease. Mol. Med. 2021, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, I. Mavridis’ atrophy in Parkinson’s disease: “The peak of the iceberg”. J. Transl. Intern. Med. 2014, 2, 124. [Google Scholar] [CrossRef]
- Mak, E.; Bergsland, N.; Dwyer, M.G.; Zivadinov, R.; Kandiah, N. Subcortical atrophy is associated with cognitive impairment in mild Parkinson disease: A combined investigation of volumetric changes, cortical thickness, and vertex-based shape analysis. AJNR Am. J. Neuroradiol. 2014, 35, 2257–2264. [Google Scholar] [CrossRef]
- Hepp, D.H.; Vergoossen, D.L.E.; Huisman, E.; Lemstra, A.W.; Bank, N.B.; Berendse, H.W.; Rozemuller, A.J.; Foncke, E.M.J.; van de Berg, W.D.J. Distribution and Load of Amyloid-β Pathology in Parkinson Disease and Dementia with Lewy Bodies. J. Neuropathol. Exp. Neurol. 2016, 75, 936–945. [Google Scholar] [CrossRef]
- Ghazi Saidi, L. Visuospatial and Executive Deficits in Parkinson’s Disease: A Review. Acta Sci. Neurol. 2020, 3, 08–26. [Google Scholar] [CrossRef]
- Koziol, L.F.; Budding, D.E.; Chidekel, D. From movement to thought: Executive function, embodied cognition, and the cerebellum. Cerebellum 2012, 11, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Milardi, D.; Quartarone, A.; Bramanti, A.; Anastasi, G.; Bertino, S.; Basile, G.A.; Buonasera, P.; Pilone, G.; Celeste, G.; Rizzo, G.; et al. The Cortico-Basal Ganglia-Cerebellar Network: Past, Present and Future Perspectives. Front. Syst. Neurosci. 2019, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Bostan, A.C.; Strick, P.L. The basal ganglia and the cerebellum: Nodes in an integrated network. Nat. Rev. Neurosci. 2018, 19, 338–350. [Google Scholar] [CrossRef]
- Bassil, F.; Brown, H.J.; Pattabhiraman, S.; Iwasyk, J.E.; Maghames, C.M.; Meymand, E.S.; Cox, T.O.; Riddle, D.M.; Zhang, B.; Trojanowski, J.Q.; et al. Amyloid-Beta (Aβ) Plaques Promote Seeding and Spreading of Alpha-Synuclein and Tau in a Mouse Model of Lewy Body Disorders with Aβ Pathology. Neuron 2020, 105, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.R.; Maass, A.; Pressman, P.; Stiver, J.; Schonhaut, D.R.; Baker, S.L.; Kramer, J.; Rabinovici, G.D.; Jagust, W.J. Associations Between Tau, β-Amyloid, and Cognition in Parkinson Disease. JAMA Neurol. 2018, 75, 227–235. [Google Scholar] [CrossRef]
- Irwin, D.J.; Hurtig, H.I. The Contribution of Tau, Amyloid-Beta and Alpha-Synuclein Pathology to Dementia in Lewy Body Disorders. J. Alzheimers Dis. Parkinsonism 2018, 8, 444. [Google Scholar] [CrossRef]
- Fiorenzato, E.; Biundo, R.; Cecchin, D.; Frigo, A.C.; Kim, J.; Weis, L.; Strafella, A.P.; Antonini, A. Brain Amyloid Contribution to Cognitive Dysfunction in Early-Stage Parkinson’s Disease: The PPMI Dataset. J. Alzheimers Dis. 2018, 66, 229–237. [Google Scholar] [CrossRef] [PubMed]

| Clinical Demographics | PD 1 Patients (n = 54) | Controls (n = 37) | p |
|---|---|---|---|
| Age (year, mean ± SD) | 60.69 ± 8.61 | 57.65 ± 8.02 | 0.093 |
| Sex (M/F) | 33/21 | 17/20 | 0.199 |
| Education (year) | 11.38 ± 4.35 | 13.78 ± 3.74 | <0.001 * |
| Disease duration (year) | 4.97 ± 3.32 | ||
| UPDRS 2 I | 2.70 ± 2.11 | ||
| UPDRS II | 11.63 ± 8.04 | ||
| UPDRS III | 26.94 ± 17.53 | ||
| UPDRS 176 | 41.28 ± 25.99 | ||
| Modified H&Y 3 | 2.19 ± 1.22 | ||
| SE-ADL 4 | 65.02 ± 28.61 | ||
| MMSE 5 | 25.65 ± 3.97 | 29.16 ± 1.01 | <0.001 * |
| Neuropsychological Assessments | PD 2 Patients (n = 54) | Controls (n = 37) | p |
|---|---|---|---|
| Attention | |||
| Digit span | 10.25 ± 2.78 | 11.54 ± 1.89 | 0.174 |
| Attention | 7.23 ± 0.73 | 7.73 ± 0.65 | 0.076 |
| Orientation | 17.04 ± 2.36 | 17.65 ± 0.86 | 0.570 |
| Executive | |||
| Digit symbol coding | 7.15 ± 3.63 | 12.22 ± 2.38 | <0.001 * |
| Arithmetic | 8.88 ± 2.90 | 11.08 ± 3.18 | 0.008 * |
| Abstract Thinking | 8.62 ± 2.06 | 10.62 ± 1.42 | <0.001 * |
| Memory | |||
| Short-term memory | 9.30 ± 2.30 | 10.63 ± 2.28 | 0.163 |
| Long-term memory | 9.80 ± 1.07 | 10.00 ± 0.00 | 0.876 |
| Information | 9.88 ± 3.28 | 12.27 ± 3.20 | 0.012 * |
| Speech and Language | |||
| Comprehension | 9.71 ± 3.11 | 12.46 ± 3.05 | 0.001 * |
| Language | 9.44 ± 1.06 | 9.84 ± 0.53 | 0.120 |
| Semantic fluency | 7.48 ± 2.39 | 8.54 ± 1.80 | 0.025 * |
| Visuospatial | |||
| Picture completion | 8.40 ± 3.26 | 11.08 ± 2.98 | 0.001 * |
| Block design | 7.75 ± 3.43 | 11.03 ± 2.83 | <0.001 * |
| Drawing | 8.37 ± 2.51 | 9.84 ± 0.55 | 0.037 * |
| CASI 1 | 84.33 ± 16.00 | 93.70 ± 4.66 | 0.028 * |
| PD Brain Regions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cerebellar Cortex | Caudate Nucleus | Cerebellar Cortex | Caudate Nucleus | Accumbens 1 | ||||||
| Hemisphere | L 2 | L | R 3 | R | R | |||||
| r | p | r | p | r | p | r | p | r | p | |
| Misfolded proteins | ||||||||||
| p-Tau | −0.311 | 0.005 * | −0.320 | 0.004 * | −0.342 | 0.002 * | −0.321 | 0.004 * | ||
| Aβ−42 | −0.353 | 0.001 * | −0.397 | <0.001 * | −0.365 | 0.001 * | −0.422 | <0.001 * | −0.301 | 0.006 * |
| Neuropsychological assessments | ||||||||||
| MMSE 4 | 0.413 | <0.001 * | 0.290 | <0.001 * | 0.362 | <0.001* | 0.409 | <0.001 * | ||
| Arithmetic | 0.416 | <0.001 * | 0.362 | 0.001* | ||||||
| DSC 5 | 0.320 | 0.002 * | 0.318 | 0.003 * | ||||||
| ABS6 | 0.291 | 0.006 * | 0.308 | 0.004 * | 0.296 | 0.005 * | ||||
| Clinical | Brain | Proteins | Path a | Path b | Path a × b | Path c′ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pcoef 1 | z | p | Pcoef | z | p | Pcoef | z | p | Pcoef | z | p | |||
| DSC2 | RAA 4 | p-Tau | −0.318 | −0.316 | 0.002 | 0.329 | 3.245 | 0.002 | −0.193 | −1.837 | 0.070 | −0.275 | −2.664 | 0.009 |
| RAA | Aβ-42 5 | −0.361 | −3.649 | <0.001 | 0.329 | 3.245 | 0.002 | −0.243 | −2.310 | 0.023 | −0.328 | −3.237 | 0.002 | |
| LCC | Aβ-42 | −0.317 | −3.155 | 0.002 | 0.326 | 3.217 | 0.002 | −0.249 | −2.394 | 0.019 | −0.328 | −3.237 | 0.002 | |
| ABS3 | RAA | Aβ-42 | −0.361 | −3.649 | <0.001 | 0.322 | 3.170 | 0.002 | −0.226 | −2.127 | 0.036 | −0.310 | −3.041 | 0.003 |
| RCC | Aβ-42 | −0.314 | −3.125 | 0.002 | 0.314 | 3.090 | 0.003 | −0.235 | −2.243 | 0.028 | −0.310 | −3.041 | 0.003 | |
| LCC | Aβ-42 | −0.317 | −3.155 | 0.002 | 0.295 | 2.878 | 0.005 | −0.240 | −2.278 | 0.025 | −0.310 | −3.041 | 0.003 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.-C.; Lu, C.-Y.; Chen, M.-H.; Chen, Y.-S.; Lu, C.-H.; Lin, Y.-Y.; Chou, K.-H.; Lin, W.-C. Brain Atrophy Mediates the Relationship between Misfolded Proteins Deposition and Cognitive Impairment in Parkinson’s Disease. J. Pers. Med. 2021, 11, 702. https://doi.org/10.3390/jpm11080702
Yu C-C, Lu C-Y, Chen M-H, Chen Y-S, Lu C-H, Lin Y-Y, Chou K-H, Lin W-C. Brain Atrophy Mediates the Relationship between Misfolded Proteins Deposition and Cognitive Impairment in Parkinson’s Disease. Journal of Personalized Medicine. 2021; 11(8):702. https://doi.org/10.3390/jpm11080702
Chicago/Turabian StyleYu, Chiun-Chieh, Chia-Yin Lu, Meng-Hsiang Chen, Yueh-Sheng Chen, Cheng-Hsien Lu, Yi-Yun Lin, Kun-Hsien Chou, and Wei-Che Lin. 2021. "Brain Atrophy Mediates the Relationship between Misfolded Proteins Deposition and Cognitive Impairment in Parkinson’s Disease" Journal of Personalized Medicine 11, no. 8: 702. https://doi.org/10.3390/jpm11080702
APA StyleYu, C.-C., Lu, C.-Y., Chen, M.-H., Chen, Y.-S., Lu, C.-H., Lin, Y.-Y., Chou, K.-H., & Lin, W.-C. (2021). Brain Atrophy Mediates the Relationship between Misfolded Proteins Deposition and Cognitive Impairment in Parkinson’s Disease. Journal of Personalized Medicine, 11(8), 702. https://doi.org/10.3390/jpm11080702