Pre-Metabolic Syndrome and Incidence of Type 2 Diabetes and Hypertension: From the Korean Genome and Epidemiology Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Definition of MetSyn, New-Onset T2D, and New-Onset Hypertension
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Study Population
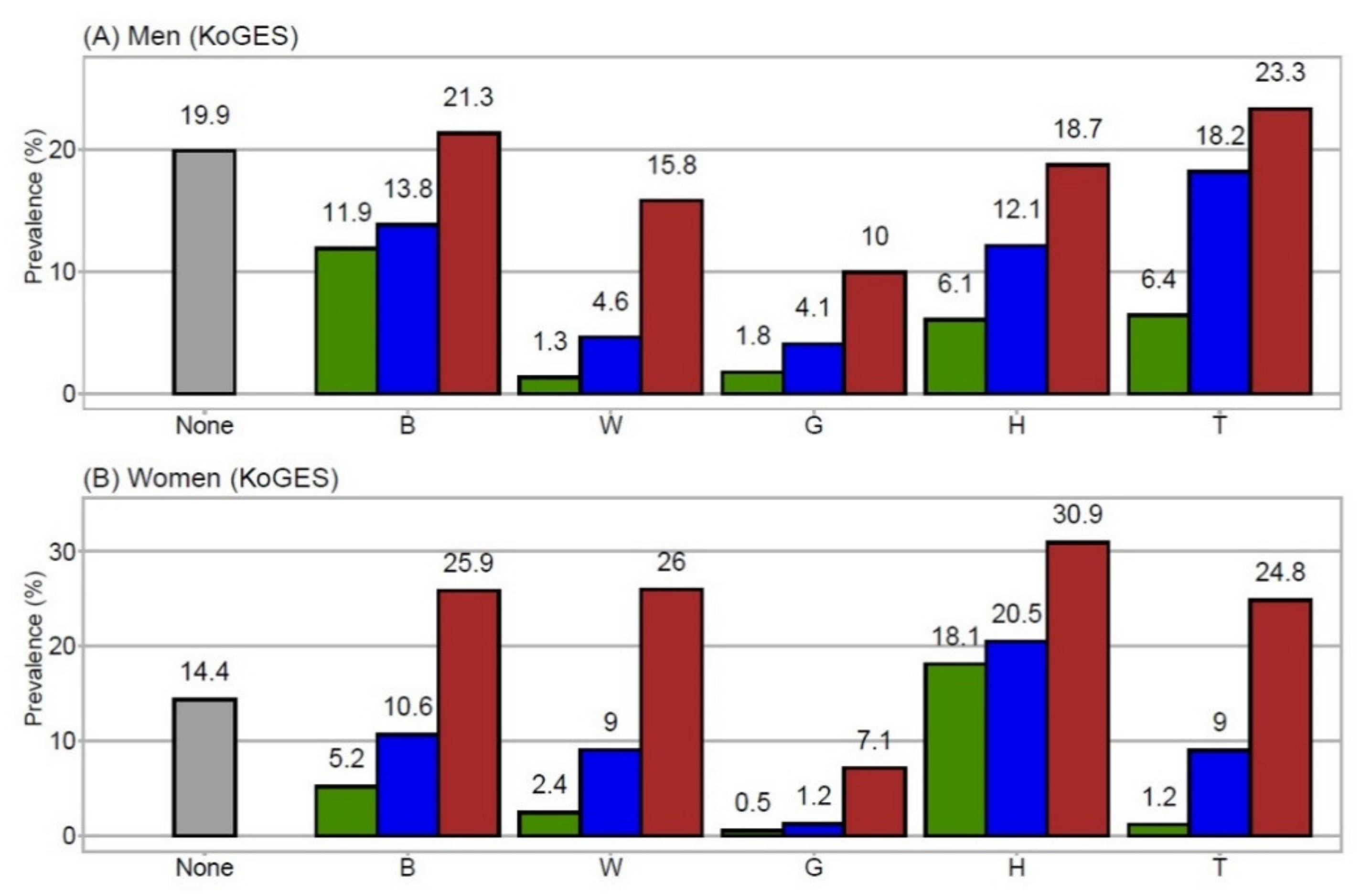
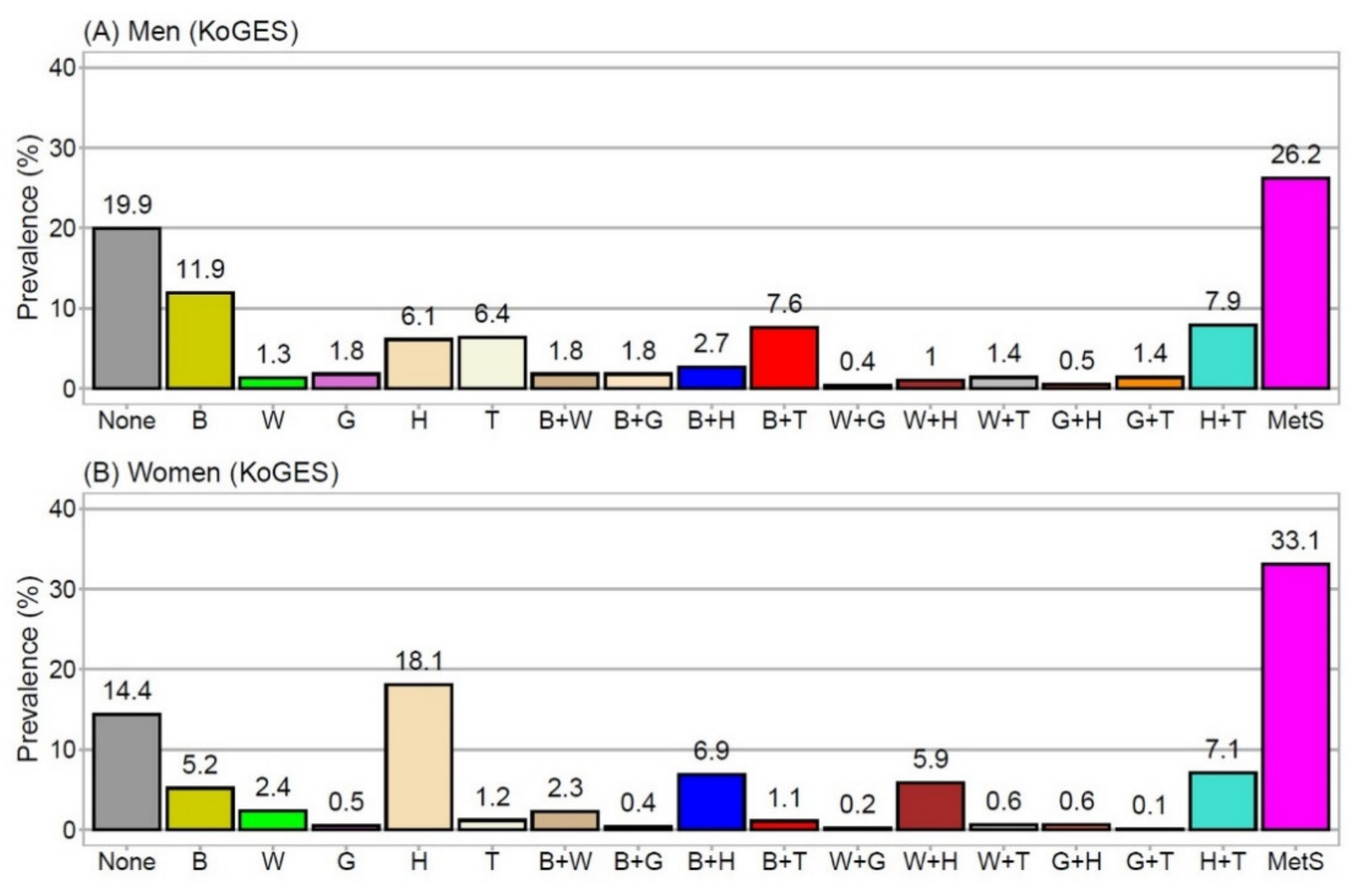
3.2. Prevalence of MetSyn Components
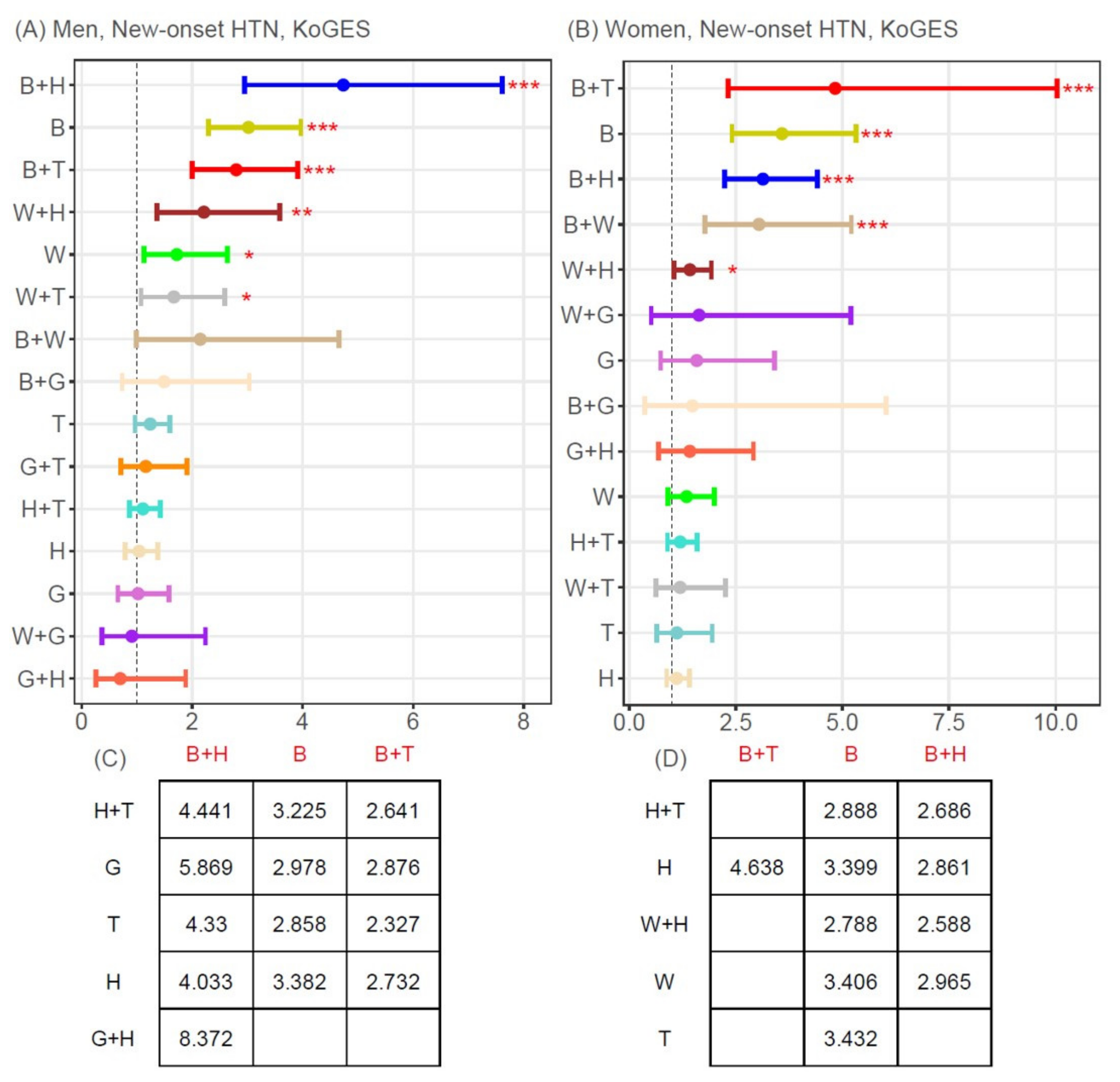
3.3. Associations between MetSyn Components (Single or Two-Component Combinations) and New-Onset T2D or Hypertension
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An american heart association/national heart, lung, and blood institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Lorenzo, C.; Okoloise, M.; Williams, K.; Stern, M.P.; Haffner, S.M. The metabolic syndrome as predictor of type 2 diabetes: The san antonio heart study. Diabetes Care 2003, 26, 3153–3159. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Randhawa, A.K.; Blair, S.N.; Sui, X.; Kuk, J.L. Age- and sex- specific all-cause mortality risk greatest in metabolic syndrome combinations with elevated blood pressure from 7 u.S. Cohorts. PLoS ONE 2019, 14, e0218307. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Jin, X.; Mo, J.; Lin, H.M.; Duan, Y.; Pu, M.; Wolbrette, D.L.; Liao, D. Metabolic syndrome, its preeminent clusters, incident coronary heart disease and all-cause mortality—Results of prospective analysis for the atherosclerosis risk in communities study. J. Intern. Med. 2007, 262, 113–122. [Google Scholar] [CrossRef]
- Marott, S.C.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Benn, M. Components of the metabolic syndrome and risk of type 2 diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 3212–3221. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.K.; Han, K.; Kim, M.K.; Koh, E.S.; Kim, E.S.; Nam, G.E.; Hong, O.K.; Kim, B.; Kwon, H.S. Combinations of metabolic syndrome components and the risk of type 2 diabetes mellitus: A nationwide cohort study. Diabetes Res. Clin. Pract. 2020, 165, 108237. [Google Scholar] [CrossRef]
- Guize, L.; Thomas, F.; Pannier, B.; Bean, K.; Jego, B.; Benetos, A. All-cause mortality associated with specific combinations of the metabolic syndrome according to recent definitions. Diabetes Care 2007, 30, 2381–2387. [Google Scholar] [CrossRef] [Green Version]
- Kuk, J.L.; Ardern, C.I. Age and sex differences in the clustering of metabolic syndrome factors: Association with mortality risk. Diabetes Care 2010, 33, 2457–2461. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Chen, X.; Li, L.; Zhou, R.; Huang, J.; Yang, D. Apolipoprotein b/apolipoprotein a1 ratio is a good predictive marker of metabolic syndrome and pre-metabolic syndrome in chinese adolescent women with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2013, 39, 203–209. [Google Scholar] [CrossRef] [PubMed]
- De las Fuentes, L.; Brown, A.L.; Mathews, S.J.; Waggoner, A.D.; Soto, P.F.; Gropler, R.J.; Dávila-Román, V.G. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur. Heart J. 2007, 28, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidigal Fde, C.; Ribeiro, A.Q.; Babio, N.; Salas-Salvadó, J.; Bressan, J. Prevalence of metabolic syndrome and pre-metabolic syndrome in health professionals: Latinmets brazil study. Diabetol. Metab. Syndr. 2015, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, K.; Oku, M.; Hayashi, S.; Inujima, A.; Shibahara, N.; Chen, L.; Igarashi, Y.; Tobe, K.; Saito, S.; Kadowaki, M.; et al. Identifying pre-disease signals before metabolic syndrome in mice by dynamical network biomarkers. Sci. Rep. 2019, 9, 8767. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G. Cohort profile: The korean genome and epidemiology study (koges) consortium. Int. J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef]
- Nichols, G.A.; Moler, E.J. Diabetes incidence for all possible combinations of metabolic syndrome components. Diabetes Res. Clin. Pract. 2010, 90, 115–121. [Google Scholar] [CrossRef]
- Kurotani, K.; Miyamoto, T.; Kochi, T.; Eguchi, M.; Imai, T.; Nishihara, A.; Tomita, K.; Uehara, A.; Yamamoto, M.; Murakami, T.; et al. Metabolic syndrome components and diabetes incidence according to the presence or absence of impaired fasting glucose: The japan epidemiology collaboration on occupational health study. J. Epidemiol. 2017, 27, 408–412. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Zhang, Y.L.; Hernandez-Ono, A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch. Med. Res. 2005, 36, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Fu, J.; Koonen, D.P.; Kuivenhoven, J.A.; Snieder, H.; Hofker, M.H. Are hypertriglyceridemia and low hdl causal factors in the development of insulin resistance? Atherosclerosis 2014, 233, 130–138. [Google Scholar] [CrossRef]
- Lu, K.; Ding, R.; Wang, L.; Wu, S.; Chen, J.; Hu, D. Association between prevalence of hypertension and components of metabolic syndrome: The data from kailuan community. Clin. Exp. Hypertens. 2015, 37, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Yang, Z.; Wang, S.; Sun, F.; Zhan, S. The associations of metabolic syndrome with incident hypertension, type 2 diabetes mellitus and chronic kidney disease: A cohort study. Endocrine 2018, 60, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Shariq, O.A.; McKenzie, T.J. Obesity-related hypertension: A review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. 2020, 9, 80–93. [Google Scholar] [CrossRef] [PubMed]

| Men | Women | p-Value | |
|---|---|---|---|
| Number | 4037 | 4400 | |
| Age (y) | 51.6 ± 8.7 | 52.5 ± 9.0 | <0.001 |
| Body mass index (kg/m2) | 24.2 ± 2.9 | 24.9 ± 3.3 | <0.001 |
| Waist circumference (cm) | 83.6 ± 7.7 | 81.7 ± 9.6 | <0.001 |
| Systolic blood pressure (mmHg) | 122.4 ± 17.0 | 121.3 ± 19.8 | 0.004 |
| Diastolic blood pressure (mmHg) | 82.2 ± 11.1 | 79.1 ± 12.0 | <0.001 |
| Fasting glucose (mg/dL) | 89.8 ± 22.8 | 84.7 ± 18.1 | <0.001 |
| Total cholesterol (mg/dL) | 191.8 ± 35.6 | 191.3 ± 35 | 0.568 |
| Triglycerides (mg/dL) | 176.6 ± 118.1 | 146.2 ± 83.7 | <0.001 |
| HDL-cholesterol (mg/dL) | 43.6 ± 10.0 | 45.8 ± 10.0 | <0.001 |
| Hypertension, n (%) | 529 (13.1) | 721 (16.4) | <0.001 |
| Diabetes, n (%) | 217 (5.4) | 138 (3.1) | <0.001 |
| Smoking, n (%) | 1780 (44.1) | 105 (2.4) | <0.001 |
| Alcohol intake, n (%) | 18.6 ± 28.4 | 1.4 ± 6.2 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, A.-R.; Kwon, Y.-J.; Kim, J.-K. Pre-Metabolic Syndrome and Incidence of Type 2 Diabetes and Hypertension: From the Korean Genome and Epidemiology Study. J. Pers. Med. 2021, 11, 700. https://doi.org/10.3390/jpm11080700
Cho A-R, Kwon Y-J, Kim J-K. Pre-Metabolic Syndrome and Incidence of Type 2 Diabetes and Hypertension: From the Korean Genome and Epidemiology Study. Journal of Personalized Medicine. 2021; 11(8):700. https://doi.org/10.3390/jpm11080700
Chicago/Turabian StyleCho, A-Ra, Yu-Jin Kwon, and Jong-Koo Kim. 2021. "Pre-Metabolic Syndrome and Incidence of Type 2 Diabetes and Hypertension: From the Korean Genome and Epidemiology Study" Journal of Personalized Medicine 11, no. 8: 700. https://doi.org/10.3390/jpm11080700
APA StyleCho, A.-R., Kwon, Y.-J., & Kim, J.-K. (2021). Pre-Metabolic Syndrome and Incidence of Type 2 Diabetes and Hypertension: From the Korean Genome and Epidemiology Study. Journal of Personalized Medicine, 11(8), 700. https://doi.org/10.3390/jpm11080700






