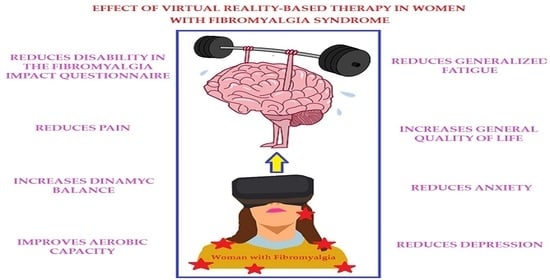
Virtual Reality-Based Therapy Reduces the Disabling Impact of Fibromyalgia Syndrome in Women: Systematic Review with Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Design
2.2. Search Strategy and Data Sources
2.3. Study Selection and Inclusion Criteria
2.4. Data Extraction
2.5. Outcomes
2.6. Risk of Bias and Methodological Quality Assessment
2.7. Statistical Analysis
2.8. Additional Analyses
3. Results
3.1. Study Selection
3.2. Characteristics of the Studies Included in the Review
3.3. Risk of Bias Assessment of the Studies Included in the Review
3.4. Quantitative Synthesis
3.4.1. Impact of FMS Symptoms
3.4.2. Pain
3.4.3. Dynamic Balance
3.4.4. Aerobic Capacity
3.4.5. Fatigue
3.4.6. Quality of Life
3.4.7. Anxiety and Depression
3.5. Qualitative Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Rheum. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomas-Vega, R.; Rodríguez-Almagro, D.; Peinado-Rubia, A.B.; Zagalaz-Anula, N.; Molina, F.; Obrero-Gaitán, E.; Ibáñez-Vera, A.J.; Osuna-Pérez, M.C. Joint Assessment of Equilibrium and Neuromotor Function: A Validation Study in Patients with Fibromyalgia. Diagnostics 2020, 10, 1057. [Google Scholar] [CrossRef]
- Montoro, C.I.; del Paso, G.A.R.; Duschek, S. Alexithymia in fibromyalgia syndrome. Pers. Individ. Differ. 2016, 102, 170–179. [Google Scholar] [CrossRef]
- Peinado-Rubia, A.; Osuna-Pérez, M.C.; Rodríguez-Almagro, D.; Zagalaz-Anula, N.; López-Ruiz, M.C.; Lomas-Vega, R. Impaired Balance in Patients with Fibromyalgia Syndrome: Predictors of the Impact of This Disorder and Balance Confidence. Int. J. Environ. Res. Public Health 2020, 17, 3160. [Google Scholar] [CrossRef]
- Sechi, C.; Lucarelli, L.; Vismara, L. Depressive Symptoms and Quality of Life in a Sample of Italian Women with a Diagnosis of Fibromyalgia: The Role of Attachment Styles. Depress. Res. Treat. 2021, 2021, 5529032. [Google Scholar] [CrossRef]
- Kaleycheva, N.; Cullen, A.E.; Evans, R.; Harris, T.; Nicholson, T.; Chalder, T. The role of lifetime stressors in adult fibromyalgia: Systematic review and meta-analysis of case-control studies. Psychol. Med. 2021, 51, 177–193. [Google Scholar] [CrossRef]
- Gaudreault, N.; Boulay, P. Cardiorespiratory fitness among adults with fibromyalgia. Breathe 2018, 14, e25–e33. [Google Scholar] [CrossRef] [Green Version]
- Sechi, C.; Vismara, L.; Brennstuhl, M.J.; Tarquinio, C.; Lucarelli, L. Adult attachment styles, self-esteem, and quality of life in women with fibromyalgia. Health Psychol. Open 2020, 7, 2055102920947921. [Google Scholar] [CrossRef]
- Offenbaecher, M.; Kohls, N.; Ewert, T.; Sigl, C.; Hieblinger, R.; Toussaint, L.L.; Sirois, F.; Hirsch, J.; Vallejo, M.A.; Kramer, S.; et al. Pain is not the major determinant of quality of life in fibromyalgia: Results from a retrospective “real world” data analysis of fibromyalgia patients. Rheumatol. Int. 2021, 41, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, P. In fibromyalgia, some therapies may provide small improvements in pain and quality of life. Ann. Intern. Med. 2021, 174, JC32. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Walitt, B.; Perrot, S.; Rasker, J.J.; Häuser, W. Fibromyalgia diagnosis and biased assessment: Sex, prevalence and bias. PLoS ONE 2018, 13, e0203755. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, L.P. Worldwide Epidemiology of Fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Okifuji, A.; Hare, B.D. Management of Fibromyalgia Syndrome: Review of Evidence. Pain Ther. 2013, 2, 87–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, C.M.N.; Miyamoto, G.C.; Franco, K.F.M.; Bosmans, J.E. Economic evaluations of educational, physical, and psychological treatments for fibromyalgia. Pain 2021, 162, 2331–2345. [Google Scholar] [CrossRef] [PubMed]
- Casas-Barragán, A.; Molina, F.; Tapia-Haro, R.M.; García-Ríos, M.C.; Correa-Rodríguez, M.; Aguilar-Ferrándiz, M.E. Association of core body temperature and peripheral blood flow of the hands with pain intensity, pressure pain hypersensitivity, central sensitization, and fibromyalgia symptoms. Ther. Adv. Chronic Dis. 2021, 12, 2040622321997253. [Google Scholar] [CrossRef]
- Rehm, S.; Sachau, J.; Hellriegel, J.; Forstenpointner, J.; Jacobsen, H.B.; Harten, P.; Gierthmühlen, J.; Baron, R. Pain matters for central sensitization: Sensory and psychological parameters in patients with fibromyalgia syndrome. PAIN Rep. 2021, 6, e901. [Google Scholar] [CrossRef]
- Pidal-Miranda, M.; González-Villar, A.J.; Carrillo-De-La-Peña, M.T. Pain Expressions and Inhibitory Control in Patients with Fibromyalgia: Behavioral and Neural Correlates. Front. Behav. Neurosci. 2019, 12, 323. [Google Scholar] [CrossRef]
- Russell, I.J.; Larson, A.A. Neurophysiopathogenesis of Fibromyalgia Syndrome: A Unified Hypothesis. Rheum. Dis. Clin. North Am. 2009, 35, 421–435. [Google Scholar] [CrossRef]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef] [Green Version]
- Uygur-Kucukseymen, E.; Castelo-Branco, L.; Pacheco-Barrios, K.; Luna-Cuadros, M.A.; Cardenas, A.; Giannoni-Luza, S.; Zeng, H.; Gianlorenco, A.C.; Gnoatto-Medeiros, M.; Shaikh, E.S.; et al. Decreased neural inhibitory state in fibromyalgia pain: A cross-sectional study. Neurophysiol. Clin. Neurophysiol. 2020, 50, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, G.; Guymer, E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.; Von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.; Atzeni, F.; Häuser, W.; Flüß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2016, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Del-Moral-García, M.; Obrero-Gaitán, E.; Rodríguez-Almagro, D.; Rodríguez-Huguet, M.; Osuna-Pérez, M.C.; Lomas-Vega, R. Effectiveness of Active Therapy-Based Training to Improve the Balance in Patients with Fibromyalgia: A Systematic Review with Meta-Analysis. J. Clin. Med. 2020, 9, 3771. [Google Scholar] [CrossRef]
- Sosa-Reina, M.D.; Nunez-Nagy, S.; Gallego-Izquierdo, T.; Pecos-Martín, D.; Monserrat, J.; Álvarez-Mon, M. Effectiveness of Therapeutic Exercise in Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. BioMed. Res. Int. 2017, 2017, 2356346. [Google Scholar] [CrossRef]
- Berardi, G.; Senefeld, J.W.; Hunter, S.K.; Bement, M.K.H. Impact of isometric and concentric resistance exercise on pain and fatigue in fibromyalgia. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 121, 1389–1404. [Google Scholar] [CrossRef]
- Izquierdo-Alventosa, R.; Inglés, M.; Cortés-Amador, S.; Gimeno-Mallench, L.; Chirivella-Garrido, J.; Kropotov, J.; Serra-Añó, P. Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3634. [Google Scholar] [CrossRef]
- Caglayan, B.C.; Keskin, A.; Kabul, E.G.; Calik, B.B.; Aslan, U.B.; Karasu, U. Effects of clinical Pilates exercises in individuals with fibromyalgia: A randomized controlled trial. Eur. J. Rheumatol. 2021, 8, 150–155. [Google Scholar] [CrossRef]
- Costa, M.T.S.; Vieira, L.P.; Barbosa, E.D.O.; Oliveira, L.M.; Maillot, P.; Vaghetti, C.A.O.; Carta, M.G.; Machado, S.; Gatica-Rojas, V.; Monteiro-Junior, R.S. Virtual Reality-Based Exercise with Exergames as Medicine in Different Contexts: A Short Review. Clin. Pract. Epidemiol. Ment. Health 2019, 15, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Palacios-Navarro, G.; Hogan, N. Head-Mounted Display-Based Therapies for Adults Post-Stroke: A Systematic Review and Meta-Analysis. Sensors 2021, 21, 1111. [Google Scholar] [CrossRef]
- Montoro-Cárdenas, D.; Cortés-Pérez, I.; Zagalaz-Anula, N.; Osuna-Pérez, M.C.; Obrero-Gaitán, E.; Lomas-Vega, R. Nintendo Wii Balance Board therapy for postural control in children with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2021, 63, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; Zagalaz-Anula, N.; Montoro-Cárdenas, D.; Lomas-Vega, R.; Obrero-Gaitán, E.; Osuna-Pérez, M. Leap Motion Controller Video Game-Based Therapy for Upper Extremity Motor Recovery in Patients with Central Nervous System Diseases. A Systematic Review with Meta-Analysis. Sensors 2021, 21, 2065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, D.; Liu, Y.; Wang, J.; Xiao, Q. Virtual reality for limb motor function, balance, gait, cognition and daily function of stroke patients: A systematic review and meta-analysis. J. Adv. Nurs. 2021, 77, 3255–3273. [Google Scholar] [CrossRef] [PubMed]
- Ahern, M.M.; Dean, L.V.; Stoddard, C.C.; Agrawal, A.; Kim, K.; Cook, C.E.; Garcia, A.N. The Effectiveness of Virtual Reality in Patients with Spinal Pain: A Systematic Review and Meta-Analysis. Pain Pract. 2020, 20, 656–675. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.; Elkins, M.R. Telephysiotherapy. J. Physiother. 2020, 66, 143–144. [Google Scholar] [CrossRef]
- Darnall, B.D.; Krishnamurthy, P.; Tsuei, J.; Minor, J.D. Self-Administered Skills-Based Virtual Reality Intervention for Chronic Pain: Randomized Controlled Pilot Study. JMIR Form. Res. 2020, 4, e17293. [Google Scholar] [CrossRef]
- Herrero, R.; García-Palacios, A.; Castilla, D.; Molinari, G.; Botella, C. Virtual Reality for the Induction of Positive Emotions in the Treatment of Fibromyalgia: A Pilot Study over Acceptability, Satisfaction, and the Effect of Virtual Reality on Mood. Cyberpsychology Behav. Soc. Netw. 2014, 17, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, J.; Kristensen, L.Q.; Brooks, E.P.; Brooks, A.L. Women with fibromyalgia’s experience with three motion-controlled video game consoles and indicators of symptom severity and performance of activities of daily living. Disabil. Rehabil. Assist. Technol. 2013, 10, 61–66. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, L.G.; Elkins, M.; Maher, C.; Moseley, A.M.; Herbert, R.; Sherrington, C. There was evidence of convergent and construct validity of Physiotherapy Evidence Database quality scale for physiotherapy trials. J. Clin. Epidemiol. 2010, 63, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Elkins, M.R.; Moseley, A.M.; Sherrington, C.; Herbert, R.D.; Maher, C.G. Growth in the Physiotherapy Evidence Database (PEDro) and use of the PEDro scale. Br. J. Sports Med. 2012, 47, 188–189. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.; Harbour, R.; Haugh, M.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [Green Version]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.; Sowden, A.; Stewart, G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis Software Version 3; Biostat: Washington, DC, USA, 2020. [Google Scholar]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The Handbook of Research Synthesis and Meta-Analysis; Russell Sage Foundation: Manhattan, NY, USA, 2009. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Der Simonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Faraone, S.V. Interpreting estimates of treatment effects: Implications for managed care. Pharm. Ther. 2008, 33, 700–711. [Google Scholar]
- Rücker, G.; Schwarzer, G. Beyond the forest plot: The drapery plot. Res. Synth. Methods 2020, 12, 13–19. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Statistical heterogeneity in systematic reviews of clinical trials: A critical appraisal of guidelines and practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef]
- Siddaway, A.P.; Wood, A.M.; Hedges, L.V. How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses. Annu. Rev. Psychol. 2019, 70, 747–770. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.S.; Carvalho, L.C.; Menezes, F.D.S.; Frazin, A.; Gomes, E.D.C.; Iunes, D.H. Effects of Exergames in Women with Fibromyalgia: A Randomized Controlled Study. Games Health J. 2020, 9, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Collado-Mateo, D.; Dominguez-Muñoz, F.J.; Adsuar, J.C.; Garcia-Gordillo, M.A.; Gusi, N. Effects of Exergames on Quality of Life, Pain, and Disease Effect in Women with Fibromyalgia: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 1725–1731. [Google Scholar] [CrossRef]
- Villafaina, S.; Collado-Mateo, D.; Fuentes, J.P.; Rohlfs-Domínguez, P.; Gusi, N. Effects of Exergames on Brain Dynamics in Women with Fibromyalgia: A Randomized Controlled Trial. J. Clin. Med. 2019, 8, 1015. [Google Scholar] [CrossRef] [Green Version]
- Collado-Mateo, D.; Dominguez-Muñoz, F.J.; Adsuar, J.C.; Merellano-Navarro, E.; Gusi, N. Exergames for women with fibromyalgia: A randomised controlled trial to evaluate the effects on mobility skills, balance and fear of falling. PeerJ 2017, 5, e3211. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Palacios, A.; Herrero, R.; Vizcaíno, Y.; Belmonte, M.A.; Castilla, D.; Molinari, G.; Baños, R.M.; Botella, C. Integrating Virtual Reality with Activity Management for the Treatment of Fibromyalgia. Clin. J. Pain 2015, 31, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Gulsen, C.; Soke, F.; Eldemir, K.; Apaydin, Y.; Ozkul, C.; Guclu-Gunduz, A.; Akcali, D.T. Effect of fully immersive virtual reality treatment combined with exercise in fibromyalgia patients: A randomized controlled trial. Assist. Technol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Leon-Llamas, J.; Villafaina, S.; Murillo-Garcia, A.; Dominguez-Muñoz, F.; Gusi, N. Effects of 24-Week Exergame Intervention on the Gray Matter Volume of Different Brain Structures in Women with Fibromyalgia: A Single-Blind, Randomized Controlled Trial. J. Clin. Med. 2020, 9, 2436. [Google Scholar] [CrossRef] [PubMed]
- Martín-Martínez, J.P.; Villafaina, S.; Collado-Mateo, D.; Pérez-Gómez, J.; Gusi, N. Effects of 24-week exergame intervention on physical function under single- and dual-task conditions in fibromyalgia: A randomized controlled trial. Scand. J. Med. Sci. Sports 2019, 29, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Kahveci, A.; Muci, B.; Günendi, Z.; Karataş, G.K. The Effect of Virtual Reality Exercises on Pain, Functionality, Cardiopulmonary Capacity, and Quality of Life in Fibromyalgia Syndrome: A Randomized Controlled Study. Games Health J. 2021, 10, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Villafaina, S.; Collado-Mateo, D.; Domínguez-Muñoz, F.J.; Fuentes-García, J.P.; Gusi, N. Benefits of 24-Week Exergame Intervention on Health-Related Quality of Life and Pain in Women with Fibromyalgia: A Single-Blind, Randomized Controlled Trial. Games Health J. 2019, 8, 380–386. [Google Scholar] [CrossRef]
- Villafaina, S.; Borrega-Mouquinho, Y.; Fuentes-García, J.P.; Collado-Mateo, D.; Gusi, N. Effect of Exergame Training and Detraining on Lower-Body Strength, Agility, and Cardiorespiratory Fitness in Women with Fibromyalgia: Single-Blinded Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 17, 161. [Google Scholar] [CrossRef] [Green Version]
- Cipolletta, S.; Tomaino, S.C.M.; Magno, E.L.; Faccio, E. Illness Experiences and Attitudes towards Medication in Online Communities for People with Fibromyalgia. Int. J. Environ. Res. Public Health 2020, 17, 8683. [Google Scholar] [CrossRef]
- Cao, C.-F.; Ma, K.-L.; Li, Q.-L.; Luan, F.-J.; Wang, Q.-B.; Zhang, M.-H.; Viswanath, O.; Myrcik, D.; Varrassi, G.; Wang, H.-Q. Balneotherapy for Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1493. [Google Scholar] [CrossRef]
- Masquelier, E.; D’Haeyere, J. Physical activity in the treatment of fibromyalgia. Jt. Bone Spine 2021, 88, 105202. [Google Scholar] [CrossRef]
- Busch, A.J.; Webber, S.; Richards, R.S.; Bidonde, J.; Schachter, C.L.; Schafer, L.; Danyliw, A.; Sawant, A.; Bello-Haas, V.D.; Rader, T.; et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2013, 2013, CD010884. [Google Scholar] [CrossRef]
- Bennett, R.M.; Friend, R.; Jones, K.D.; Ward, R.; Han, B.K.; Ross, R.L. The Revised Fibromyalgia Impact Questionnaire (FIQR): Validation and psychometric properties. Arthritis Res. Ther. 2009, 11, R120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumaa, M.; Youssef, A.R. Is Virtual Reality Effective in Orthopedic Rehabilitation? A Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 1304–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadpour, N.; Randall, H.; Choksi, H.; Gao, A.; Vaughan, C.; Poronnik, P. Virtual Reality interventions for acute and chronic pain management. Int. J. Biochem. Cell Biol. 2019, 114, 105568. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Yeo, E.; Moghaddampour, P.; Chau, B.; Humbert, S. Virtual and augmented reality in the treatment of phantom limb pain: A literature review. NeuroRehabilitation 2017, 40, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Zusman, M. Forebrain-mediated sensitization of central pain pathways: ‘non-specific’ pain and a new image for MT. Man. Ther. 2002, 7, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Goldman-Rakic, P.S. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos. Trans. R. Soc. B Biol. Sci. 1996, 351, 1445–1453. [Google Scholar] [CrossRef]
- Koçyiğit, B.F.; Akaltun, M.S. Kinesiophobia Levels in Fibromyalgia Syndrome and the Relationship Between Pain, Disease Activity, Depression. Arch. Rheumatol. 2020, 35, 214–219. [Google Scholar] [CrossRef]
- Núñez-Fuentes, D.; Obrero-Gaitán, E.; Zagalaz-Anula, N.; Ibáñez-Vera, A.; Achalandabaso-Ochoa, A.; López-Ruiz, M.; Rodríguez-Almagro, D.; Lomas-Vega, R. Alteration of Postural Balance in Patients with Fibromyalgia Syndrome—A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 127. [Google Scholar] [CrossRef]
- Cagnie, B.; Coppieters, I.; Denecker, S.; Six, J.; Danneels, L.; Meeus, M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin. Arthritis Rheum. 2014, 44, 68–75. [Google Scholar] [CrossRef]
- Cheng, C.-A.; Chiu, Y.-W.; Wu, D.; Kuan, Y.-C.; Chen, S.-N.; Tam, K.-W. Effectiveness of Tai Chi on fibromyalgia patients: A meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 46, 1–8. [Google Scholar] [CrossRef]
- Walitt, B.; Klose, P.; Üçeyler, N.; Phillips, T.; Häuser, W. Antipsychotics for fibromyalgia in adults. Cochrane Database Syst. Rev. 2016, CD011804. [Google Scholar] [CrossRef] [PubMed]
- Dailey, D.L.; Law, L.A.F.; Vance, C.G.T.; Rakel, B.A.; Merriwether, E.N.; Darghosian, L.; Golchha, M.; Geasland, K.M.; Spitz, R.; Crofford, L.J.; et al. Perceived function and physical performance are associated with pain and fatigue in women with fibromyalgia. Arthritis Res. 2016, 18, 68. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, A.; Papastavrou, E.; Avraamides, M.N.; Charalambous, A. Virtual Reality and Symptoms Management of Anxiety, Depression, Fatigue, and Pain: A Systematic Review. SAGE Open Nurs. 2020, 6, 2377960820936163. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.G.; Zimmerman, M.B.; Dailey, D.L.; Rakel, B.A.; Geasland, K.M.; Chimenti, R.L.; Williams, J.M.; Golchha, M.; Crofford, L.J.; Sluka, K.A. Reduction in movement-evoked pain and fatigue during initial 30-minute transcutaneous electrical nerve stimulation treatment predicts transcutaneous electrical nerve stimulation responders in women with fibromyalgia. Pain 2020, 162, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Montana, J.I.; Matamala-Gomez, M.; Maisto, M.; Mavrodiev, P.A.; Cavalera, C.M.; Diana, B.; Mantovani, F.; Realdon, O. The Benefits of emotion Regulation Interventions in Virtual Reality for the Improvement of Wellbeing in Adults and Older Adults: A Systematic Review. J. Clin. Med. 2020, 9, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcos-Carmona, I.M.; Castro-Sánchez, A.M.; Matarán-Peñarrocha, G.A.; Gutiérrez-Rubio, A.B.; Ramos-González, E.; Moreno-Lorenzo, C. Effects of aerobic exercise program and relaxation techniques on anxiety, quality of sleep, depression, and quality of life in patients with fibromyalgia: A randomized controlled trial. Med. Clin. 2011, 137, 398–401. [Google Scholar] [CrossRef]
- Van Abbema, R.; Van Wilgen, C.P.; Van Der Schans, C.P.; Van Ittersum, M.W. Patients with more severe symptoms benefit the most from an intensive multimodal programme in patients with fibromyalgia. Disabil. Rehabil. 2010, 33, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Freeman, D.; Reeve, S.; Robinson, A.; Ehlers, A.; Clark, D.; Spanlang, B.; Slater, M. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychol. Med. 2017, 47, 2393–2400. [Google Scholar] [CrossRef]
- Fodor, L.A.; Coteț, C.D.; Cuijpers, P.; Szamoskozi, T.; David, D.; Cristea, I.A. The effectiveness of virtual reality based interventions for symptoms of anxiety and depression: A meta-analysis. Sci. Rep. 2018, 8, 10323. [Google Scholar] [CrossRef] [Green Version]
- Masaoka, Y.; Homma, I. The source generator of respiratory-related anxiety potential in the human brain. Neurosci. Lett. 2000, 283, 21–24. [Google Scholar] [CrossRef]
- Gormsen, L.; Rosenberg, R.; Bach, F.; Jensen, T.S. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur. J. Pain 2010, 14, 127.e1–127.e8. [Google Scholar] [CrossRef]
- Thieme, K.; Turk, D.C.; Flor, H. Comorbid Depression and Anxiety in Fibromyalgia Syndrome: Relationship to Somatic and Psychosocial Variables. Psychosom. Med. 2004, 66, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Montoro, C.I.; Duschek, S.; Schuepbach, D.; Gandarillas, M.; del Paso, G.A.R. Cerebral blood flow variability in fibromyalgia syndrome: Relationships with emotional, clinical and functional variables. PLoS ONE 2018, 13, e0204267. [Google Scholar] [CrossRef]
- Rodriguez, A.; Tembl, J.; Mesa-Gresa, P.; Muñoz, M.; Montoya, P.; Rey, B. Altered cerebral blood flow velocity features in fibromyalgia patients in resting-state conditions. PLoS ONE 2017, 12, e0180253. [Google Scholar] [CrossRef] [Green Version]
- Galvez-Sánchez, C.M.; del Paso, G.A.R.; Duschek, S. Cognitive Impairments in Fibromyalgia Syndrome: Associations with Positive and Negative Affect, Alexithymia, Pain Catastrophizing and Self-Esteem. Front. Psychol. 2018, 9, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Liew, C.; Leon, G.; Neese, M.; Cronan, T.A. You get used to it, or do you: Symptom length predicts less fibromyalgia physical impairment, but only for those with above-average self-efficacy. Psychol. Health Med. 2018, 24, 207–220. [Google Scholar] [CrossRef]









| Databases | Search Strategy |
|---|---|
| PubMed Medline | (fibromyalgia[mh] OR fibromyalgia[tiab] OR fibromyalgia syndrome[tiab] OR fibromyalgia*[tiab] OR chronic, fatigue syndrome[tiab]) AND (virtual reality[mh] OR virtual reality[tiab] OR virtual reality exposure therapy[mh] OR virtual reality exposure therapy[tiab] OR exergam*) |
| Web of Science | TOPIC: (*fibromyalgia* OR *chronic, fatigue syndrome*) AND TOPIC: (*virtual reality* OR *exergame*) |
| SCOPUS | (TITLE-ABS-KEY (“fibromyalgia” OR “fibromyalgia syndrome” OR “chronic fatigue syndrome”) AND TITLE-ABS-KEY (“virtual reality” OR “exercises” OR “videogames”)) |
| PEDro | Fibromyalgia AND virtual reality Fibromyalgia AND exergames |
| CINAHL Complete | AB (fibromyalgia OR fibromyalgia syndrome OR chronic fatigue syndrome) AND AB (virtual reality OR exergames OR videogames) |
| Experimental Group | Control Group | Outcomes | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Characteristics | Intervention Characteristics | Sample Characteristics | |||||||||||||||||
| Authorship and Date | Country | K | N | Ne | Age | BMI | Evol. Years | Type | Weeks | Ses/Week | Min | Nc | Age | BMI | Evol. Years | Type Control | Variable | Test | Follow-Up |
| Collado-Mateo, D. et al., 2017a [62] | Spain | 6 | 76 | 41 | 52.52 | 25.79 | 9.6 | ni VRBT | 8 | 2 | 60 | 35 | 52.47 | 27.75 | 11.02 | NI | FMS Impact | FIQ | Inm. Effect |
| Quality of Life | EuroQol-5D | ||||||||||||||||||
| Fatigue | FIQ-Fatigue | ||||||||||||||||||
| Pain | FIQ-Pain | ||||||||||||||||||
| Anx/Dep | VAS | ||||||||||||||||||
| Collado-Mateo, D. et al., 2017b [64] | Spain | 1 | 76 | 41 | 52.43 | 25.79 | 10.36 | ni VRBT | 8 | 2 | 60 | 35 | 52.58 | 27.75 | 12.48 | NI | Dynamic Balance | TGUGT | Inm. Effect |
| García-Palacios, A. et al., 2015 [65] | Spain | 4 | 59 | 30 | 50.48 | NR | 9.32 | ni VRBT | 3 | 2 | 60 | 29 | 50.48 | NR | 9.32 | NI | FMS Impact | FIQ | Inm. Effect |
| Quality of Life | QLI-SP | ||||||||||||||||||
| Pain | BPI | ||||||||||||||||||
| Depression | BDI-II | ||||||||||||||||||
| Gulsen, C. et al., 2020 [66] | Turkey | 5 | 16 | 8 | 46.5 | 26.81 | 4 | iVRBT + CTBTE | 8 | 2 | 80 | 8 | 38.5 | 22.85 | 4 | CTBTE | FMS Impact | FIQ | Inm. Effect |
| Pain | VAS | ||||||||||||||||||
| Fatigue | FSS | ||||||||||||||||||
| Aerobic capacity | 6-MWT | ||||||||||||||||||
| Quality of Life | SF-36 | ||||||||||||||||||
| León-Llamas, J.L. et al., 2020 [67] | Spain | 1/ QS | 50 | 25 | 54 | 27 | 8.5 | ni VRBT | 24 | 2 | 60 | 25 | 53 | 28.5 | 11 | NI | Aerobic capacity | PVO2 | Inm. Effect |
| Martín-Martínez, J.P. et al., 2019 [68] | Spain | 1 | 55 | 28 | 54.04 | 27.36 | 19.2 | ni VRBT | 24 | 2 | 60 | 27 | 53.41 | 28.84 | 16.76 | NI | Dynamic Balance | TGUGT | Inm. Effect |
| Polat, M. et al., 2021 [69] | Turkey | 7 | 40 | 20 | 42.6 | 26.6 | 1.5 | ni VRBT + CTBTE | 8 | 3 | 35 | 20 | 47 | 27.9 | 1.4 | CTBTE | FMS Impact | FIQ | Inm. Effect |
| Aerobic capacity | 6-MWT | ||||||||||||||||||
| Pain | VAS | ||||||||||||||||||
| Fatigue | FSS | ||||||||||||||||||
| Quality of Life | EQ-5D-5L | ||||||||||||||||||
| Anx/Dep | HADS-A/-D | ||||||||||||||||||
| Silva de Carvalho, M. et al., 2020 [61] | Brasil | 4 | 21 | 11 | 55.64 | 30.28 | 9.91 | ni VRBT | 7 | 3 | 60 | 10 | 47.7 | 26.09 | 14.65 | ST | FMS Impact | FIQ | Inm. Effect |
| Aerobic capacity | 6-MWT | ||||||||||||||||||
| Fatigue | FIQ-Fatigue | ||||||||||||||||||
| Pain | FIQ-Pain | ||||||||||||||||||
| Villafaina, S. et al., 2019a [63] | Spain | 2 | 55 | 28 | 54.04 | 27.36 | 19.2 | ni VRBT | 24 | 2 | 60 | 27 | 53.41 | 28.84 | 16.74 | NI | Pain | VAS | Inm. Effect |
| Quality of Life | EQ-5D-5L | ||||||||||||||||||
| Villafaina, S. et al., 2019b [70] | Spain | 3 | 37 | 22 | 54.27 | 27.1 | NR | ni VRBT | 24 | 2 | 60 | 15 | 53.44 | 28.19 | NR | NI | FMS Impact | FIQ | Inm. Effect |
| Dynamic Balance | TGUGT | ||||||||||||||||||
| Aerobic capacity | 6-MWT | ||||||||||||||||||
| Villafaina, S. et al., 2019c [71] | Spain | QS | 50 | 25 | 52 | NR | 16 | ni VRBT | 24 | 2 | 60 | 25 | 54 | NR | 16 | NI | Brain Dynamics | EEG Signals | Inm. Effect |
| Items | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authorship | ||||||||||||
| Collado-Mateo, D. et al., 2017a [62] | N | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Collado-Mateo, D. et al., 2017b [64] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| García-Palacios, A. et al., 2015 [65] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Gulsen, C. et al., 2020 [66] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| León-Llamas, J.L. et al., 2020 [67] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Martín-Martínez, J.P. et al., 2019 [68] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Polat, M. et al., 2021 [69] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Silva de Carvalho, M. et al., 2020 [61] | Y | Y | N | Y | N | N | Y | N | Y | Y | Y | 6 |
| Villafaina, S. et al., 2019a [63] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Villafaina, S. et al., 2019b [70] | Y | Y | N | Y | N | N | Y | N | Y | Y | Y | 6 |
| Villafaina, S. et al., 2019c [71] | Y | Y | N | N | N | N | Y | Y | Y | Y | Y | 6 |
| Outcomes | Summary of Findings | Quality of Evidence (Grade) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled Effect Het | Publication Bias | ||||||||||||||
| K | N | Ns | SMD | 95% CI | I2 (p for Q-test) | Funnel Plot (p for Egger) | Trim and Fill | Risk of Bias | Incons | Indirect | Imprec | Pub. Bias | Quality | ||
| Adj SMD | % of Var | ||||||||||||||
| Impact of FMS Symptoms | 6 | 249 | 41.5 | −0.62 | −0.93 to −0.31 | 5.2% (p = 0.4) | Sym (p = 0.9) | −0.62 | 0% | Medium | No | No | Yes | No | Moderate |
| Pain | 6 | 267 | 44.5 | −0.45 | −0.69 to −0.21 | 0% (p = 0.52) | Asym (p = 0.2) | −0.72 | 28% | Medium | No | No | Yes | Yes | Low |
| Dynamic Balance | 3 | 168 | 56 | −0.76 | −1.12 to −0.39 | 4.4% (p = 0.35) | Sym (p = 0.52) | −0.75 | 0% | Medium | No | No | Yes | No | Low |
| Aerobic Capacity | 5 | 164 | 32.8 | 0.32 | 0.004 to 0.63 | 0% (p = 0.57) | Asym (p = 0.31) | 0.36 | 12% | Medium | No | No | Yes | Yes | Low |
| Fatigue | 4 | 153 | 38.5 | −0.58 | −1.02 to −0.14 | 5.4% (p = 0.37) | Asym (p = 0.09) | −0.48 | 20% | Medium | No | No | Yes | Yes | Low |
| Quality of Life | 5 | 246 | 49.2 | 0.55 | 0.3 to 0.81 | 0% (p = 0.73) | Sym (p = 0.9) | 0.52 | 0% | Medium | No | No | Yes | No | Moderate |
| Anxiety | 3 | 137 | 45.7 | −0.47 | −0.91 to −0.03 | 0% (p = 0.32) | Asym (p =0.2) | −0.57 | 22% | Medium | No | No | Yes | Yes | Very-Low |
| Depression | 4 | 196 | 49 | −0.46 | −0.76 to −0.16 | 4.6% (p = 0.4) | Asym (p =0.14) | −0.52 | 13% | Medium | No | No | Yes | Yes | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Pérez, I.; Zagalaz-Anula, N.; Ibancos-Losada, M.d.R.; Nieto-Escámez, F.A.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Reduces the Disabling Impact of Fibromyalgia Syndrome in Women: Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Pers. Med. 2021, 11, 1167. https://doi.org/10.3390/jpm11111167
Cortés-Pérez I, Zagalaz-Anula N, Ibancos-Losada MdR, Nieto-Escámez FA, Obrero-Gaitán E, Osuna-Pérez MC. Virtual Reality-Based Therapy Reduces the Disabling Impact of Fibromyalgia Syndrome in Women: Systematic Review with Meta-Analysis of Randomized Controlled Trials. Journal of Personalized Medicine. 2021; 11(11):1167. https://doi.org/10.3390/jpm11111167
Chicago/Turabian StyleCortés-Pérez, Irene, Noelia Zagalaz-Anula, María del Rocío Ibancos-Losada, Francisco Antonio Nieto-Escámez, Esteban Obrero-Gaitán, and María Catalina Osuna-Pérez. 2021. "Virtual Reality-Based Therapy Reduces the Disabling Impact of Fibromyalgia Syndrome in Women: Systematic Review with Meta-Analysis of Randomized Controlled Trials" Journal of Personalized Medicine 11, no. 11: 1167. https://doi.org/10.3390/jpm11111167