Myocarditis and Pericarditis following COVID-19 Vaccination: Inequalities in Age and Vaccine Types
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Measurement
2.3. Data Analyses
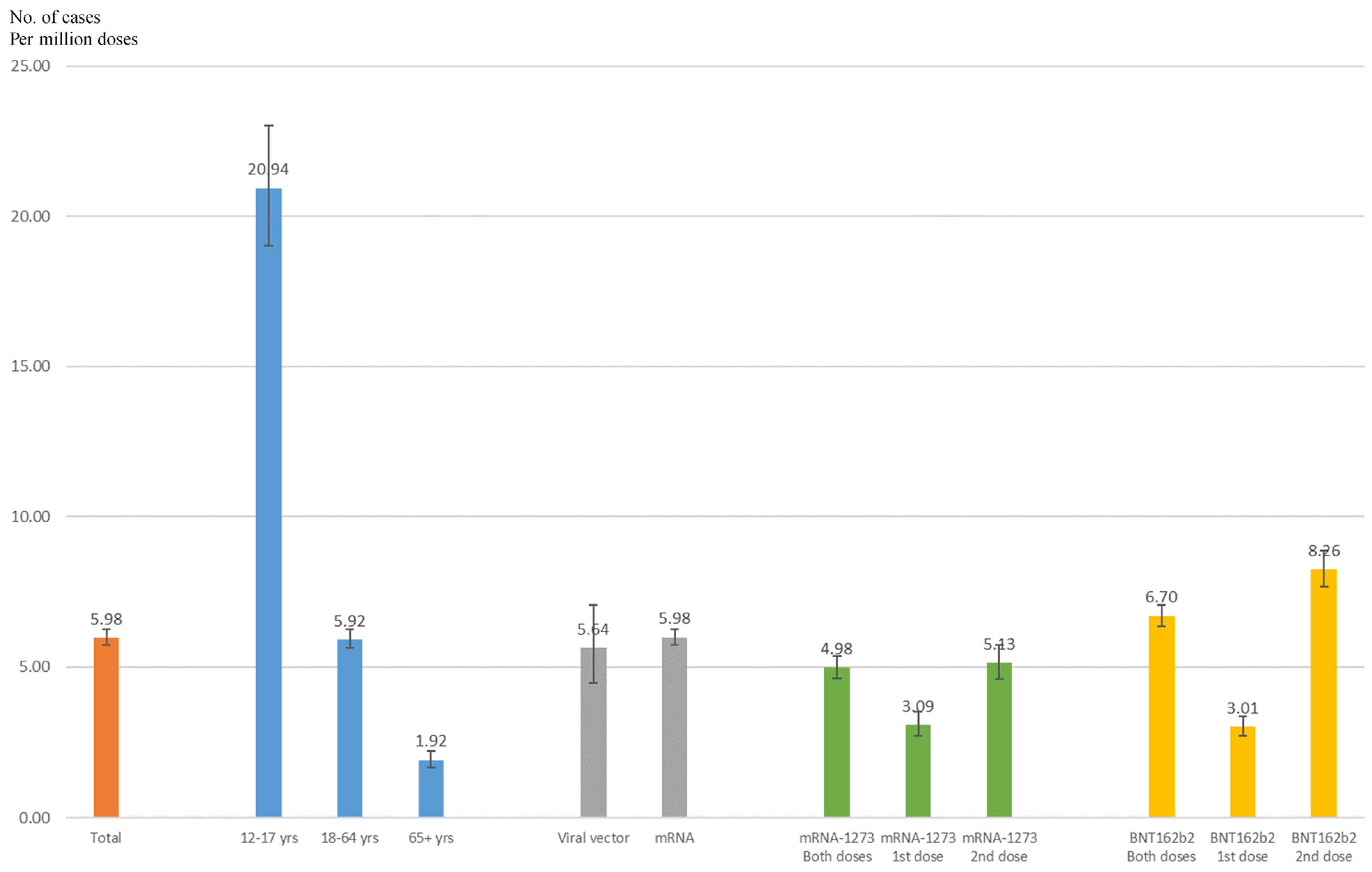
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Drug Administration (FDA). COVID-19 Vaccines. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 31 August 2021).
- Food and Drug Administration (FDA). Emergency Use Authorization for Vaccines Explained. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained (accessed on 31 August 2021).
- Thomson, K.; Nachlis, H. Emergency Use Authorizations During the COVID-19 Pandemic: Lessons From Hydroxychloroquine for Vaccine Authorization and Approval. JAMA 2020, 324, 1282–1283. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.; Jones, A.; Robinson, E. Public Trust and Willingness to Vaccinate Against COVID-19 in the US From 14 October 2020 to 29 March 2021. JAMA 2021, 325, 2397–2399. [Google Scholar] [CrossRef] [PubMed]
- Black, S.B.; Law, B.; Chen, R.T.; Dekker, C.L.; Sturkenboom, M.; Huang, W.T.; Gurwith, M.; Poland, G. The critical role of background rates of possible adverse events in the assessment of COVID-19 vaccine safety. Vaccine 2021, 39, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.D.; Kung, C.S.; Perez, D.L. Helping the Public Understand Adverse Events Associated With COVID-19 Vaccinations: Lessons Learned From Functional Neurological Disorder. JAMA Neurol. 2021, 78, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Kreps, S.; Prasad, S.; Brownstein, J.S.; Hswen, Y.; Garibaldi, B.T.; Zhang, B.; Kriner, D.L. Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. Open 2020, 3, e2025594. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 3, e2025594. [Google Scholar] [CrossRef]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Pieroni, M.; Rapezzi, C.; Olivotto, I. The spectrum of myocarditis: From pathology to the clinics. Virchows Arch. Int. J. Pathol. 2019, 475, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Bouriche, F.; Toro, A.; Negre, V.; Yvorra, S. Acute Pericarditis: Aetiologic Diagnosis and Practical Aspect of the Management. Curr. Probl. Cardiol. 2021, 46, 100769. [Google Scholar] [CrossRef] [PubMed]
- Oakley, C.M. Myocarditis, pericarditis and other pericardial diseases. Heart Br. Card. Soc. 2000, 84, 449–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.; Roper, M.H.; Sperling, L.; Schieber, R.A.; Heffelfinger, J.D.; Casey, C.G.; Miller, J.W.; Santibanez, S.; Herwaldt, B.; Hightower, P.; et al. Myocarditis, pericarditis, and dilated cardiomyopathy after smallpox vaccination among civilians in the United States, January–October 2003. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 46 (Suppl. 3), S242–S250. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Mehta, J.L. COVID-19 Vaccine and Myocarditis. Am. J. Cardiol. 2021, 157, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vaccine Adverse Event Reporting System (VAERS). Available online: https://vaers.hhs.gov/index.html (accessed on 31 August 2021).
- Centers for Disease Control and Prevention (CDC). COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 31 August 2021).
- Van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.; Lindquist, M.; Orre, R.; Egberts, A.C. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gubernot, D.; Jazwa, A.; Niu, M.; Baumblatt, J.; Gee, J.; Moro, P.; Duffy, J.; Harrington, T.; McNeil, M.M.; Broder, K.; et al. U.S. Population-Based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines. Vaccine 2021, 39, 3666–3677. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Boylan, M.; Roddy, J.; Lim, N.; Morgan, R.; McAdam, B.; Kiernan, F. Recovery of a critically ill patient with COVID-19 myocarditis. Ir. J. Med Sci. 2021, 1–5. [Google Scholar] [CrossRef]
| All Reports to the VAERS | Myocarditis/Pericarditis Reports | |||
|---|---|---|---|---|
| No | Yes | |||
| % | % | % | p | |
| Individual | ||||
| Age | <0.0001 | |||
| 12–17 | 3.94 | 3.86 | 21.22 | |
| 18–24 | 6.26 | 6.18 | 23.77 | |
| 25–44 | 32.95 | 32.98 | 27.80 | |
| 45–64 | 34.67 | 34.74 | 18.42 | |
| 65+ | 22.18 | 22.24 | 8.79 | |
| Sex | <0.0001 | |||
| Female | 71.38 | 71.58 | 26.88 | |
| Male | 28.62 | 28.42 | 73.12 | |
| Region | <0.0001 | |||
| Northeast | 19.49 | 19.47 | 23.82 | |
| Midwest | 24.13 | 24.15 | 18.69 | |
| South | 31.71 | 31.73 | 27.75 | |
| West | 24.67 | 24.65 | 29.74 | |
| Vaccine | ||||
| Type | <0.0001 | |||
| Viral vector: Ad26.COV2.S (Janssen) | 10.21 | 10.24 | 3.69 | |
| mRNA: mRNA-1273 (Moderna) | 44.20 | 44.25 | 33.22 | |
| mRNA: BNT162b2 (Pfizer–BioNTech) | 45.59 | 45.51 | 63.09 | |
| Dose | <0.0001 | |||
| 1st | 65.77 | 65.90 | 36.53 | |
| 2nd | 34.23 | 34.10 | 63.47 | |
| Adverse events | ||||
| Serious | 8.17 | 7.89 | 69.42 | <0.0001 |
| Death | 1.29 | 1.30 | 0.94 | 0.1502 |
| Life-threatening illness | 1.67 | 1.60 | 16.12 | <0.0001 |
| Hospitalization | 5.90 | 5.62 | 66.82 | <0.0001 |
| Disability | 1.48 | 1.47 | 3.53 | <0.0001 |
| ER visit | 13.62 | 13.46 | 48.73 | <0.0001 |
| Office visit | 19.92 | 19.85 | 33.98 | <0.0001 |
| Onset interval | <0.0001 | |||
| 0 | 44.70 | 44.87 | 8.52 | |
| 1 | 21.34 | 21.36 | 15.98 | |
| 2–7 | 17.47 | 17.34 | 45.44 | |
| 8+ | 16.49 | 16.43 | 30.06 | |
| Symptoms | ||||
| Chest pain | 2.78 | 2.52 | 58.67 | <0.0001 |
| Dyspnoea | 5.45 | 5.38 | 19.93 | <0.0001 |
| Pyrexia | 15.74 | 15.74 | 16.45 | 0.3714 |
| Chest discomfort | 2.20 | 2.16 | 11.12 | <0.0001 |
| Pain | 14.05 | 14.07 | 11.03 | <0.0001 |
| Fatigue | 15.56 | 15.59 | 9.90 | <0.0001 |
| Chills | 14.36 | 14.39 | 8.58 | <0.0001 |
| Headache | 19.26 | 19.30 | 8.58 | <0.0001 |
| Nausea | 11.24 | 11.27 | 5.84 | <0.0001 |
| Pain in extremity | 9.51 | 9.53 | 5.18 | <0.0001 |
| Myalgia | 5.98 | 5.99 | 4.81 | 0.0218 |
| Palpitations | 1.99 | 1.98 | 4.76 | <0.0001 |
| Dizziness | 11.55 | 11.58 | 4.71 | <0.0001 |
| Vomiting | 4.28 | 4.28 | 4.38 | 0.8214 |
| Malaise | 2.91 | 2.91 | 3.72 | 0.0259 |
| Arthralgia | 6.06 | 6.08 | 3.53 | <0.0001 |
| Viral Vector | mRNA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ad26.COV2.S (Janssen) | mRNA-1273 (Moderna) | BNT162b2 (Pfizer–BioNTech) | |||||||
| Cases | ROR | 95% CI | Cases | ROR | 95% CI | Cases | ROR | 95% CI | |
| Overall | 78 | 1.39 | (0.99–1.97) | 703 | 2.91 | (2.21–3.83) | 1335 | 5.37 | (4.10–7.04) |
| Age | |||||||||
| 12–17 | NA | NA | 426 | 8.19 | (4.37–15.36) | ||||
| 18–24 | 14 | 0.37 | (0.18–0.80) | 197 | 2.25 | (1.28–3.95) | 271 | 2.58 | (1.48–4.52) |
| 25–44 | 28 | 0.89 | (0.41–1.96) | 238 | 1.73 | (0.85–3.50) | 299 | 2.09 | (1.04–4.22) |
| 45–64 | 24 | 2.20 | (0.95–5.11) | 158 | 3.21 | (1.50–6.84) | 192 | 3.95 | (1.86–8.41) |
| 65+ | 8 | 2.64 | (0.96–7.29) | 80 | 2.28 | (1.05–4.94) | 90 | 3.59 | (1.66–7.75) |
| Sex | |||||||||
| Female | 27 | 1.27 | (0.70–2.28) | 202 | 1.80 | (1.12–2.88) | 333 | 3.03 | (1.91–4.81) |
| Male | 49 | 1.21 | (0.78–1.88) | 489 | 3.71 | (2.61–5.28) | 985 | 6.43 | (4.54–9.10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yuan, J.; Lv, G.; Brown, J.; Jiang, X.; Lu, Z.K. Myocarditis and Pericarditis following COVID-19 Vaccination: Inequalities in Age and Vaccine Types. J. Pers. Med. 2021, 11, 1106. https://doi.org/10.3390/jpm11111106
Li M, Yuan J, Lv G, Brown J, Jiang X, Lu ZK. Myocarditis and Pericarditis following COVID-19 Vaccination: Inequalities in Age and Vaccine Types. Journal of Personalized Medicine. 2021; 11(11):1106. https://doi.org/10.3390/jpm11111106
Chicago/Turabian StyleLi, Minghui, Jing Yuan, Gang Lv, Jacob Brown, Xiangxiang Jiang, and Zhiqiang Kevin Lu. 2021. "Myocarditis and Pericarditis following COVID-19 Vaccination: Inequalities in Age and Vaccine Types" Journal of Personalized Medicine 11, no. 11: 1106. https://doi.org/10.3390/jpm11111106
APA StyleLi, M., Yuan, J., Lv, G., Brown, J., Jiang, X., & Lu, Z. K. (2021). Myocarditis and Pericarditis following COVID-19 Vaccination: Inequalities in Age and Vaccine Types. Journal of Personalized Medicine, 11(11), 1106. https://doi.org/10.3390/jpm11111106








