Individualized Assessment of Exercise Capacity in Response to Acute and Long-Term Enzyme Replacement Therapy in Pediatric Pompe Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Measures
2.1.1. Spirometry
2.1.2. CPET
2.1.3. Six-Minute Walking Test
2.1.4. Motor Function
2.1.5. Evaluation of Alpha-Glucosidase Activity in Dried Blood Spots
2.2. Statistics
3. Results
3.1. Paired Evaluation before and Two Days after Enzyme Administration
3.2. Long-Term Effect of ERT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van der Ploeg, A.T.; Reuser, A.J. Pompe’s disease. Lancet 2008, 372, 1342–1353. [Google Scholar] [CrossRef]
- Van der Ploeg, A.T.; Clemens, P.R.; Corzo, D.; Escolar, D.M.; Florence, J.; Groeneveld, G.J.; Herson, S.; Kishnani, P.S.; Laforet, P.; Lake, S.L.; et al. A Randomized Study of Alglucosidase Alfa in Late-Onset Pompe’s Disease. N. Engl. J. Med. 2010, 362, 1396–1406. [Google Scholar] [CrossRef] [Green Version]
- Kishnani, P.S.; Howell, R.R. Pompe disease in infants and children. Proc. J. Pediatr. 2004, 144, S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Corzo, D.; Nicolino, M.; Byrne, B.; Mandel, H.; Hwu, W.L.; Leslie, N.; Levine, J.; Spencer, C.; McDonald, M.; et al. Recombinant human acid α-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology 2007, 68, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Do, H.V.; Khanna, R.; Gotschall, R. Challenges in treating Pompe disease: An industry perspective. Ann. Transl. Med. 2019, 7, 291. [Google Scholar] [CrossRef]
- Kishnani, P.S.; Goldenberg, P.C.; DeArmey, S.L.; Heller, J.; Benjamin, D.; Young, S.; Bali, D.; Smith, S.A.; Li, J.S.; Mandel, H.; et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol. Genet. Metab. 2010, 99, 26–33. [Google Scholar] [CrossRef] [Green Version]
- ElMallah, M.; Desai, A.; Nading, E.; DeArmey, S.; Kravitz, R.; Kishnani, P. Pulmonary outcome measures in long-term survivors of infantile Pompe disease on enzyme replacement therapy: A case series. Pediatr. Pulmonol. 2020, 55, 674–681. [Google Scholar] [CrossRef]
- Angelini, C.; Semplicini, C.; Ravaglia, S.; Moggio, M.; Comi, G.P.; Musumeci, O.; Pegoraro, E.; Tonin, P.; Filosto, M.; Servidei, S.; et al. New motor outcome function measures in evaluation of Late-Onset Pompe disease before and after enzyme replacement therapy. Muscle Nerve 2012, 45, 831–834. [Google Scholar] [CrossRef]
- Yuan, M.; Andrinopoulou, E.; Kruijshaar, M.; Lika, A.; Harlaar, L.; van der Ploeg, A.; Rizopoulos, D.; van der Beek, N. Positive association between physical outcomes and patient-reported outcomes in late-onset Pompe disease: A cross sectional study. Orphanet J. Rare Dis. 2020, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Crescimanno, G.; Modica, R.; Lo Mauro, R.; Musumeci, O.; Toscano, A.; Marrone, O. Role of the cardio-pulmonary exercise test and six-minute walking test in the evaluation of exercise performance in patients with late-onset Pompe disease. Neuromuscul. Disord. 2015, 25, 542–547. [Google Scholar] [CrossRef]
- Bar-Yoseph, R.; Mandel, H.; Mainzer, G.; Gur, M.; Tal, G.; Shalloufeh, G.; Bentur, L. Cardiopulmonary exercise test to quantify enzyme replacement response in pediatric Pompe disease. Pediatr. Pulmonol. 2018, 53, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Quanjer, P.H.; Borsboom, G.J.J.M.; Brunekreef, B.; Zach, M.; Forche, G.; Cotes, J.E.; Sanchis, J.; Paoletti, P. Spirometric reference values for white European children and adolescents: Polgar revisited. Pediatr. Pulmonol. 1995, 19, 135–142. [Google Scholar] [CrossRef]
- Ross, R.M.; Beck, K.C.; Casaburi, R.; Johnson, B.D.; Marciniuk, D.D.; Wagner, P.D.; Weisman, I.M. ATS/ACCP Statement on Cardiopulmonary Exercise Testing (multiple letters). Am. J. Respir. Crit. Care Med. 2003, 167, 1451. [Google Scholar] [CrossRef]
- Mahon, A.; Marjerrison, A.; Lee, J.; Woodruff, M.; Hanna, L. Evaluating the prediction of maximal heart rate in children and adolescents. Res. Q. Exerc. Sport 2010, 81, 466–471. [Google Scholar] [CrossRef]
- Machado, F.; Denadai, B. Validity of maximum heart rate prediction equations for children and adolescents. Arq. Bras. Cardiol. 2011, 97, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Rowland, T.; Hagenbuch, S.; Pober, D.; Garrison, A. Exercise tolerance and thermoregulatory responses during cycling in boys and men. Med. Sci. Sports Exerc. 2008, 40, 282–287. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Sietsema, K.E.; Sun, X.G.; Whipp, B.J. Principles of Exercise Testing and Interpretation, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; MacIntyre, N.R.; McKay, R.T.; Johnson, D.; Wanger, J.S.; Zeballos, R.J.; Bittner, V.; et al. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Goemans, N.; Klingels, K.; Van Den Hauwe, M.; Boons, S.; Verstraete, L.; Peeters, C.; Feys, H.; Buyse, G. Six-minute walk test: Reference values and prediction equation in healthy boys aged 5 to12 years. PLoS ONE 2013, 8, e84120. [Google Scholar] [CrossRef] [Green Version]
- Kanburoglu, M.K.; Ozdemir, F.M.; Ozkan, S.; Tunaoglu, F.S. Reference Values of the 6-Minute Walk Test in Healthy Turkish Children and Adolescents Between 11 and 18 Years of Age. Respir. Care 2014, 59, 1369–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundkvist Josenby, A.; Jarnlo, G.-B.; Gummesson, C.; Nordmark, E. Longitudinal Construct Validity of the GMFM-88 Total Score and Goal Total Score and the GMFM-66 Score in a 5-Year Follow-up Study. Phys. Ther. 2009, 89, 342–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.; Kemper, A.; Grosse, S.; Lam, W.; Rose, A.; Ahmad, A.; Gebremariam, A.; Prosser, L. Health and economic outcomes of newborn screening for infantile-onset Pompe disease. Genet. Med. 2021, 23, 758–766. [Google Scholar] [CrossRef]
- Marzorati, M.; Porcelli, S.; Bellistri, G.; Morandi, L.; Grassi, B. Exercise testing in late-onset glycogen storage disease type II patients undergoing enzyme replacement therapy. Neuromuscul. Disord. 2012, 22, S230–S234. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Kazi, Z.B.; Nampoothiri, S.; Jagdeesh, S.; Kabra, M.; Puri, R.D.; Muranjan, M.; Kalaivani, M.; Rehder, C.; Bali, D.; et al. Clinical and Molecular Disease Spectrum and Outcomes in Patients with Infantile-Onset Pompe Disease. J. Pediatr. 2020, 216, 44–50.e5. [Google Scholar] [CrossRef] [PubMed]
- Sechi, A.; Salvadego, D.; Da Ponte, A.; Bertin, N.; Dardis, A.; Cattarossi, S.; Devigili, G.; Reccardini, F.; Bembi, B.; Grassi, B. Investigation on acute effects of enzyme replacement therapy and influence of clinical severity on physiological variables related to exercise tolerance in patients with late onset Pompe disease. Neuromuscul. Disord. 2017, 27, 542–549. [Google Scholar] [CrossRef]
- Marzorati, M.; Porcelli, S.; Reggiori, B.; Morandi, L.; Grassi, B. Improved exercise tolerance after enzyme replacement therapy in pompe disease. Med. Sci. Sports Exerc. 2012, 44, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Banugaria, S.G.; Prater, S.N.; Patel, T.T.; Dearmey, S.M.; Milleson, C.; Sheets, K.B.; Bali, D.S.; Rehder, C.W.; Raiman, J.A.; Wang, R.A.; et al. Algorithm for the early diagnosis and treatment of patients with cross reactive immunologic material-negative classic infantile pompe disease: A step towards improving the efficacy of ERT. PLoS ONE 2013, 8, e67052. [Google Scholar] [CrossRef]
- Prater, S.N.; Patel, T.T.; Buckley, A.F.; Mandel, H.; Vlodavski, E.; Banugaria, S.G.; Feeney, E.J.; Raben, N.; Kishnani, P.S. Skeletal muscle pathology of infantile Pompe disease during long-term enzyme replacement therapy. Orphanet J. Rare Dis. 2013, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Kuperus, E.; Kruijshaar, M.E.; Wens, S.C.A.; De Vries, J.M.; Favejee, M.M.; Van Der Meijden, J.C.; Rizopoulos, D.; Brusse, E.; Van Doorn, P.A.; Van Der Ploeg, A.T.; et al. Long-term benefit of enzyme replacement therapy in Pompe disease: A 5-year prospective study. Neurology 2017, 89, 2365–2373. [Google Scholar] [CrossRef]
- Chien, Y.H.; Tsai, W.H.; Chang, C.L.; Chiu, P.C.; Chou, Y.Y.; Tsai, F.J.; Wong, S.L.; Lee, N.C.; Hwu, W.L. Earlier and higher dosing of alglucosidase alfa improve outcomes in patients with infantile-onset Pompe disease: Evidence from real-world experiences. Mol. Genet. Metab. Rep. 2020, 23, 100591. [Google Scholar] [CrossRef] [PubMed]
- Case, L.E.; Bjartmar, C.; Morgan, C.; Casey, R.; Charrow, J.; Clancy, J.P.; Dasouki, M.; DeArmey, S.; Nedd, K.; Nevins, M.; et al. Safety and efficacy of alternative alglucosidase alfa regimens in Pompe disease. Neuromuscul. Disord. 2015, 25, 321–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gelder, C.M.; Poelman, E.; Plug, I.; Hoogeveen-Westerveld, M.; van der Beek, N.A.M.E.; Reuser, A.J.J.; van der Ploeg, A.T. Effects of a higher dose of alglucosidase alfa on ventilator-free survival and motor outcome in classic infantile Pompe disease: An open-label single-center study. J. Inherit. Metab. Dis. 2016, 39, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age (years)/sex | 5.5/Female | 6/Male | 11/Male | 10/Male |
| Study duration (years) | 1.2 | 1.3 | 5.3 | 6 |
| Height (cm) | 112.5 | 113 | 139 | 145 |
| Weight (kg) | 21.2 | 26.5 | 37.2 | 44.1 |
| BMI (%) | 16.8 (83) | 20.8 (99) | 19.3 (78) | 21 (92) |
| GAA mutation | R854X/L355P | L355P/D404N | L355P/L355P | L355P/D404N |
| CRIM status | Positive | Positive | Positive | Positive |
| Ethnicity | Muslim | Druze | Druze | Druze |
| Onset | Infantile | Infantile | Infantile | Infantile |
| ERT onset age (months) | 5 | 3 | 7 | 1 |
| Cardiomyopathy at infancy | Yes | Yes | Yes | Yes |
| Current echo | Normal | Normal | Normal | Normal |
| Drop foot | No | Yes | Yes | Yes |
| Gastrostomy | No | Yes | No | No |
| First Evaluation | Second Evaluation (+7 Months) | Third Evaluation (+14 Months) | ||||
|---|---|---|---|---|---|---|
| Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | |
| Age at test (years) | 5.5 | 5.5 | 6.2 | 6.2 | 6.7 | 6.7 |
| Height (cm) | 112.5 | 112.5 | 116.5 | 116.5 | 119 | 119 |
| BMI percentile | 83 | 83 | 73 | 73 | 84 | 84 |
| ERT dose (mg/kg) | 40/EOW | 40/EOW | 40/EOW | 40/EOW | 40/EOW | 40/EOW |
| Enzyme level (μmol/L/h) | 34.85 | 1.22 | N/A | 1.24 | 49.87 | 0.83 |
| FEV1 (L/s) | 1.12 | 1.07 | 1.22 | 1.17 | 1.4 | 1.29 |
| FEV1 (% of the predicted value) | 99 | 94 | 100 | 96 | 109 | 101 |
| FVC (L) | 1.19 | 1.42 | 1.26 | 1.25 | 1.46 | 1.34 |
| FVC (% of the predicted value) | 97 | 115 | 94 | 93 | 103 | 94 |
| Peak HR (bpm) | 198 | 197 | 199 | 195 | 208 | 199 |
| Absolute peak VO2 (mL/min) | 615 | 647 | 698 | 626 | 731 | 695 |
| Specific peak VO2 (mL/kg/min) | 29 | 30.5 | 31.6 | 28.3 | 30 | 28.5 |
| Peak VO2 (% of the predicted value) | 62 | 65 | 68 | 61 | 67 | 64 |
| VE/VCO2 slope | 38.7 | 37.8 | 41.7 | 32 | 42.4 | 33.5 |
| O2 pulse (% of the predicted value) | 64 | 68 | 70 | 64 | 65 | 65 |
| Breathing reserve | L | N | L | N | L | N |
| 6MWD (meters) | 495 | 495 | 576 | 527 | 555 | 534 |
| Walking %, running (%), jumping (%) GMFM-88) | 100 | 100 | 100 | 100 | 100 | 100 |
| Total score (%) (GMFM-88) | 98.5 | 99.6 | 98.4 | 98.8 | 100 | 100 |
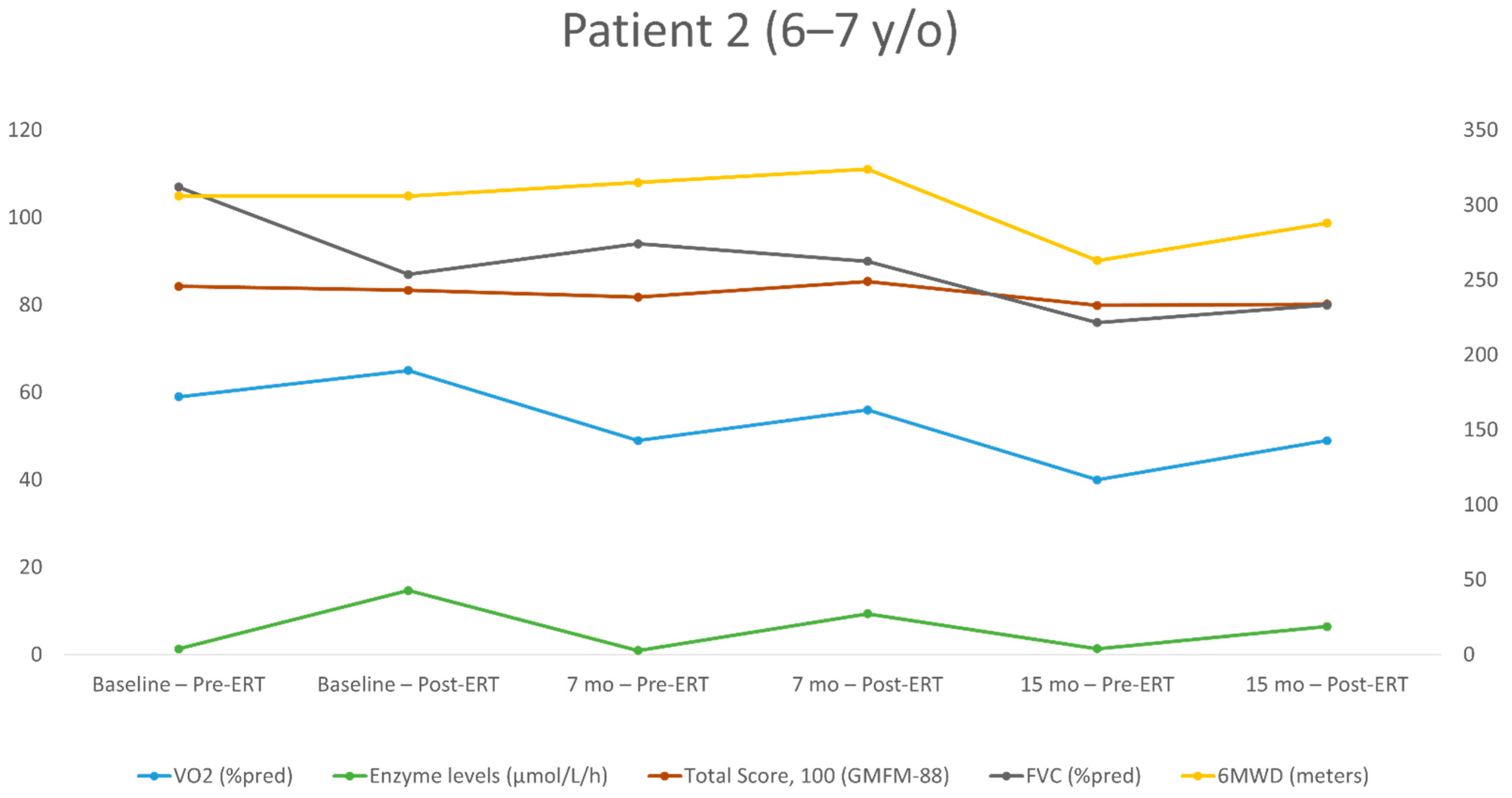
| First Evaluation | Second Evaluation (+7 Months) | Third Evaluation (+15 Months) | ||||
|---|---|---|---|---|---|---|
| Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | |
| Age at test (years) | 6.2 | 6.2 | 6.9 | 6.9 | 7.5 | 7.5 |
| Height (cm) | 113 | 113 | 118 | 118 | 123 | 123 |
| BMI percentile | 99 | 99 | 97 | 97 | 97 | 97 |
| ERT dose (mg/kg) | 20/EOW | 20/EOW | 40/EOW | 40/EOW | 40/EOW | 40/EOW |
| Enzyme level (μmol/L/h) | 14.68 | 1.34 | 9.35 | 0.95 | 6.45 | 1.35 |
| FEV1 (L/s) | 1.03 | 1.29 | 1.16 | 1.2 | 1.12 | 1.06 |
| FEV1 (% of the predicted value) | 87 | 104 | 88 | 91 | 77 | 73 |
| FVC (L) | 1.15 | 1.47 | 1.33 | 1.38 | 1.32 | 1.24 |
| FVC (% of the predicted value) | 87 | 107 | 90 | 94 | 80 | 76 |
| Peak HR (bpm) | 202 | 204 | 190 | 172 | 170 | 185 |
| Absolute peak VO2 (mL/min) | 751 | 721 | 726 | 644 | 736 | 590 |
| Specific peak VO2 (mL/kg/min) | 28.3 | 27.2 | 25.9 | 23 | 23.6 | 18.9 |
| Peak VO2 (% of the predicted value) | 65 | 59 | 56 | 49 | 49 | 40 |
| VE/VCO2 slope | 39.9 | 32.3 | 40 | 42.4 | 38 | 43.2 |
| O2 pulse (% of the predicted value) | 65 | 59 | 60 | 58 | 59 | 43 |
| Breathing reserve | L | N | N | N | N | N |
| 6MWD (meters) | 306 | 306 | 324 | 315 | 288 | 263 |
| Walking (%), running (%), jumping (%) (GMFM-88) | 54.1 | 58.3 | 61.1 | 54.1 | 50 | 50 |
| Total score (%) (GMFM-88) | 83.4 | 84.3 | 85.4 | 81.8 | 80.2 | 79.88 |
| First Evaluation | Second Evaluation (+1 Month) | Third Evaluation (+55 Months) | Fourth Evaluation (+63 Months) | |||||
|---|---|---|---|---|---|---|---|---|
| Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | |
| Age at test (years) | 11.2 | 11.2 | 11.3 | 11.3 | 15.9 | 15.9 | 16.5 | 16.5 |
| Height (cm) | 139 | 139 | 139 | 139 | 165 | 165 | 166 | 166 |
| BMI percentile | 78 | 78 | 78 | 78 | 65 | 65 | 57 | 57 |
| ERT dose (mg/kg) | 20/EOW | 20/EOW | 40/EOW | 40/EOW | 20/W | 20/W | 20/W | 20/W |
| Enzyme level (μmol/L/h) | 8.7 | 0.55 | 9.35 | 2 | N/A | 2.85 | 10.15 | 3.55 |
| FEV1 (L/s) | 1.64 | 1.7 | 1.8 | 1.78 | 2.9 | 2.93 | 2.77 | 2.84 |
| FEV1 (% of the predicted value) | 81 | 85 | 89 | 88 | 80 | 80 | 73 | 75 |
| FVC (L) | 1.72 | 1.78 | 2.03 | 1.93 | 2.96 | 2.98 | 2.8 | 3.08 |
| FVC (% of the predicted value) | 75 | 78 | 88 | 84 | 71 | 71 | 64 | 71 |
| Peak HR (bpm) | 172 | 166 | 189 | 166 | 175 | 130 | 168 | 157 |
| Absolute peak VO2 (mL/min) | 1400 | 1308 | 1610 | 1346 | 1197 | 1117 | 1147 | 1119 |
| Specific peak VO2 (mL/kg/min) | 37.6 | 35.2 | 43.3 | 36.2 | 20.3 | 18.9 | 19.4 | 19 |
| Peak VO2 (% of the predicted value) | 84 | 79 | 97 | 81 | 43 | 40 | 41 | 40 |
| VE/VCO2 slope | 37.9 | 24.6 | 41.1 | 30.4 | 36.7 | 23.4 | 32.7 | 32.5 |
| O2 pulse (% of the predicted value) | 8.4 | 7.9 | 8.5 | 8.1 | 6.8 | 8.6 | 6.8 | 7.1 |
| Breathing reserve | N | N | L | N | N | N | N | N |
| 6MWD (meters) | 620 | 450 | 570 | 580 | 457 | 495 | 468 | 442 |
| Walking (%), running (%), jumping (%) (GMFM-88) | 98.6 | 97.2 | 100 | 97 | 77.7 | 77.7 | 70.8 | 65.2 |
| Total score (%) (GMFM-88) | 98 | 97.2 | 97.8 | 97.2 | 89.5 | 89.5 | 91.6 | 87.1 |
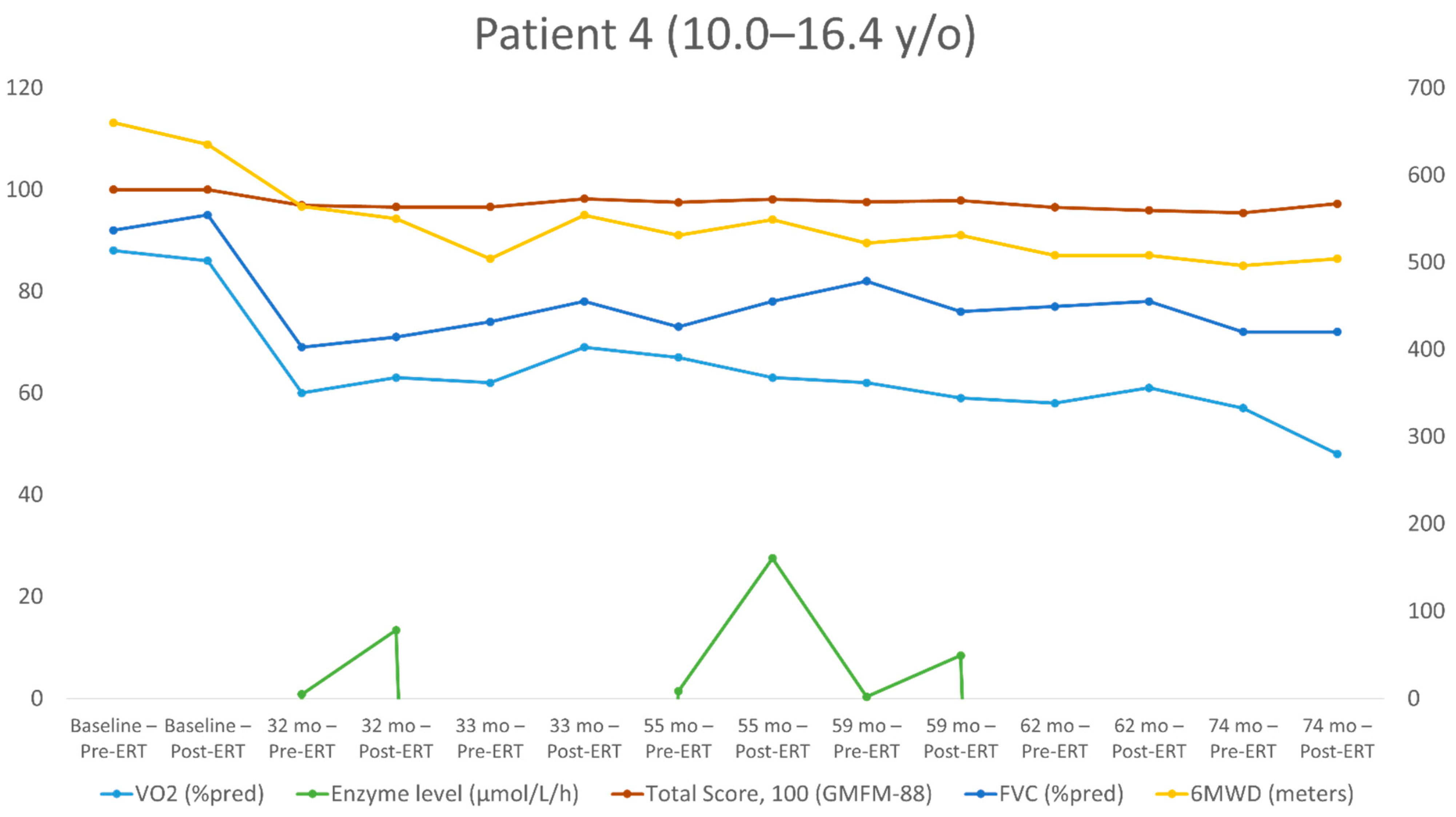
| First Evaluation | Second Evaluation (+32 Months) | Third Evaluation (+33 Months) | Fourth Evaluation (+55 Months) | Fifth Evaluation (+59 Months) | Sixth Evaluation (+62 Months) | Seventh Evaluation (+74 Months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | Post-ERT | Pre-ERT | |
| Age at test (years) | 10 | 10 | 12.8 | 12.8 | 12.9 | 12.9 | 14.7 | 14.7 | 15.1 | 15.1 | 15.4 | 15.4 | 16.4 | 16.4 |
| Height (cm) | 145 | 145 | 167 | 167 | 167 | 167 | 177 | 177 | 177 | 178 | 178 | 178 | 186 | 186 |
| BMI percentiles | 92 | 92 | 94 | 92 | 83 | 83 | 97 | 97 | 98 | 98 | 98 | 98 | 95 | 95 |
| ERT dose (mg/kg) EOW | 20 | 20 | 20 | 20 | 40 | 40 | 40 | 40 | 20 | 20 | 40 | 40 | 40 | 40 |
| Enzyme level (μmol/L/h) | N/A | N/A | 13.4 | 0.85 | N/A | N/A | 27.5 | 1.43 | 8.45 | 0.34 | N/A | N/A | N/A | N/A |
| FEV1 (L/s) | 1.99 | 2.01 | 2.51 | 2.29 | 2.74 | 2.62 | 3.09 | 3.12 | 3.3 | 3.41 | 3.39 | 3.23 | 3.45 | 3.45 |
| FEV1 (% of the predicted value) | 89 | 90 | 74 | 67 | 80 | 77 | 77 | 78 | 80 | 82 | 81 | 77 | 74 | 74 |
| FVC (L) | 2.44 | 2.38 | 2.82 | 2.73 | 3.1 | 2.95 | 3.69 | 3.44 | 3.65 | 4.01 | 3.85 | 3.8 | 3.94 | 3.95 |
| FVC (% of the predicted value) | 95 | 92 | 71 | 69 | 78 | 74 | 78 | 73 | 76 | 82 | 78 | 77 | 72 | 72 |
| Peak HR (bpm) | 190 | 186 | 206 | 200 | 197 | 198 | 203 | 205 | 207 | 204 | 201 | 191 | 198 | 201 |
| Absolute peak VO2 (mL/min) | 1764 | 1733 | 2000 | 1908 | 2165 | 1950 | 2320 | 2440 | 2151 | 2274 | 2261 | 2134 | 1838 | 2163 |
| Specific peak VO2 (mL/kg/min) | 40 | 40.2 | 30.3 | 28.9 | 33.31 | 30 | 26.4 | 27.7 | 22.96 | 23.99 | 23.93 | 22.58 | 19.5 | 23.01 |
| Peak VO2 (% of the predicted value) | 86 | 88 | 63 | 60 | 69 | 62 | 63 | 67 | 59 | 62 | 61 | 58 | 48 | 57 |
| VE/VCO2 slope | 36 | 31.1 | 30.5 | 29.5 | 26.8 | 28.5 | 29.3 | 27.3 | 31.8 | 30.6 | 28.8 | 30 | 27.9 | 28.4 |
| O2 pulse (% of the predicted value) | 91 | 95 | 61 | 60 | 70 | 63 | 69 | 71 | 56 | 60 | 60 | 60 | 48 | 56 |
| Breathing reserve | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 6MWD (meters) | 635 | 660 | 550 | 564 | 554 | 504 | 549 | 531 | 531 | 522 | 508 | 508 | 504 | 496 |
| Walking (%), running (%), jumping (%) (GMFM-88) | 100 | 100 | 95.8 | 94.4 | 98.6 | 97.2 | 98.6 | 93 | 94.4 | 93 | 90.2 | 90.2 | 91.6 | 87.5 |
| Total Score %-GMFM-88 | 100 | 100 | 96.58 | 96.9 | 98.2 | 96.58 | 98.1 | 97.5 | 97.84 | 97.56 | 95.9 | 96.5 | 97.2 | 95.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bar-Yoseph, R.; Tal, G.; Dumin, E.; Hanna, M.; Mainzer, G.; Zucker-Toledano, M.; Shallufi, G.; Jahshan, M.; Mandel, H.; Bentur, L. Individualized Assessment of Exercise Capacity in Response to Acute and Long-Term Enzyme Replacement Therapy in Pediatric Pompe Disease. J. Pers. Med. 2021, 11, 1105. https://doi.org/10.3390/jpm11111105
Bar-Yoseph R, Tal G, Dumin E, Hanna M, Mainzer G, Zucker-Toledano M, Shallufi G, Jahshan M, Mandel H, Bentur L. Individualized Assessment of Exercise Capacity in Response to Acute and Long-Term Enzyme Replacement Therapy in Pediatric Pompe Disease. Journal of Personalized Medicine. 2021; 11(11):1105. https://doi.org/10.3390/jpm11111105
Chicago/Turabian StyleBar-Yoseph, Ronen, Galit Tal, Elena Dumin, Moneera Hanna, Gur Mainzer, Merav Zucker-Toledano, George Shallufi, Mira Jahshan, Hanna Mandel, and Lea Bentur. 2021. "Individualized Assessment of Exercise Capacity in Response to Acute and Long-Term Enzyme Replacement Therapy in Pediatric Pompe Disease" Journal of Personalized Medicine 11, no. 11: 1105. https://doi.org/10.3390/jpm11111105
APA StyleBar-Yoseph, R., Tal, G., Dumin, E., Hanna, M., Mainzer, G., Zucker-Toledano, M., Shallufi, G., Jahshan, M., Mandel, H., & Bentur, L. (2021). Individualized Assessment of Exercise Capacity in Response to Acute and Long-Term Enzyme Replacement Therapy in Pediatric Pompe Disease. Journal of Personalized Medicine, 11(11), 1105. https://doi.org/10.3390/jpm11111105




