A Feasibility Study of an Extrusion-Based Fabrication Process for Personalized Drugs
Abstract
1. Introduction
2. Method
2.1. Material
2.1.1. HPMC Gel Preparation
2.1.2. IR and SR Mixture Preparation
2.2. Characterization of Material Extrusion Behavior
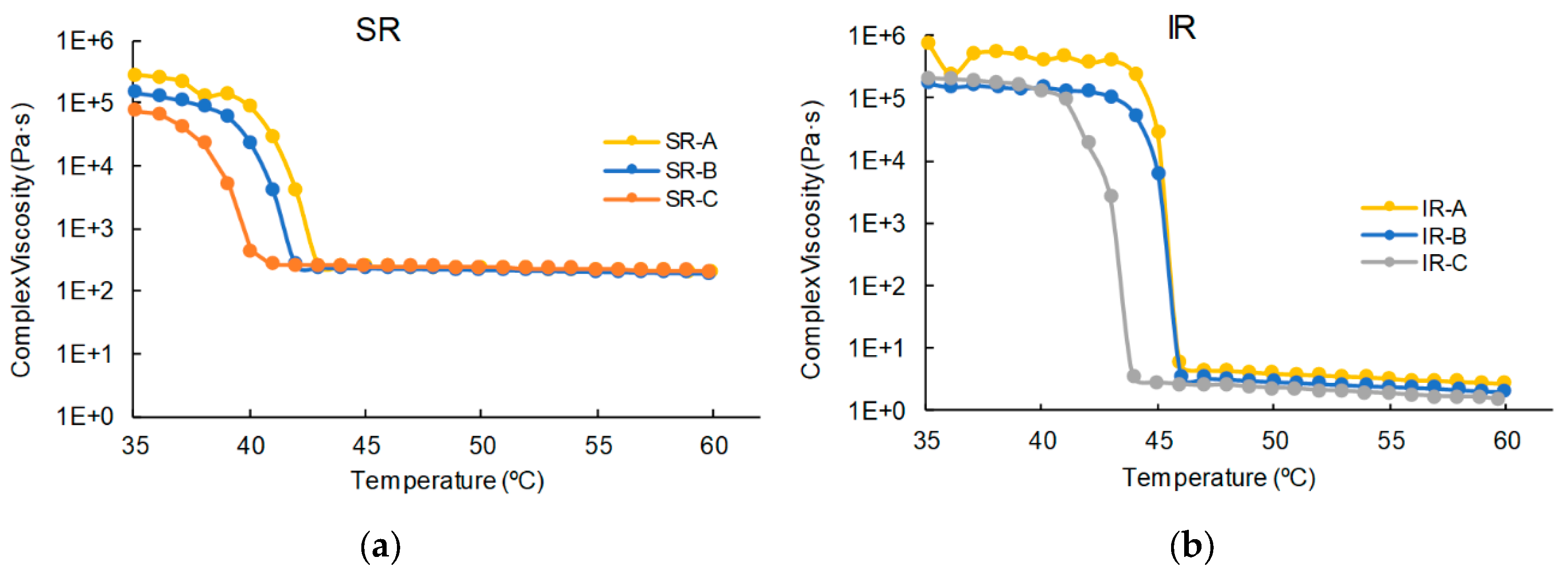
2.2.1. Viscosity Test
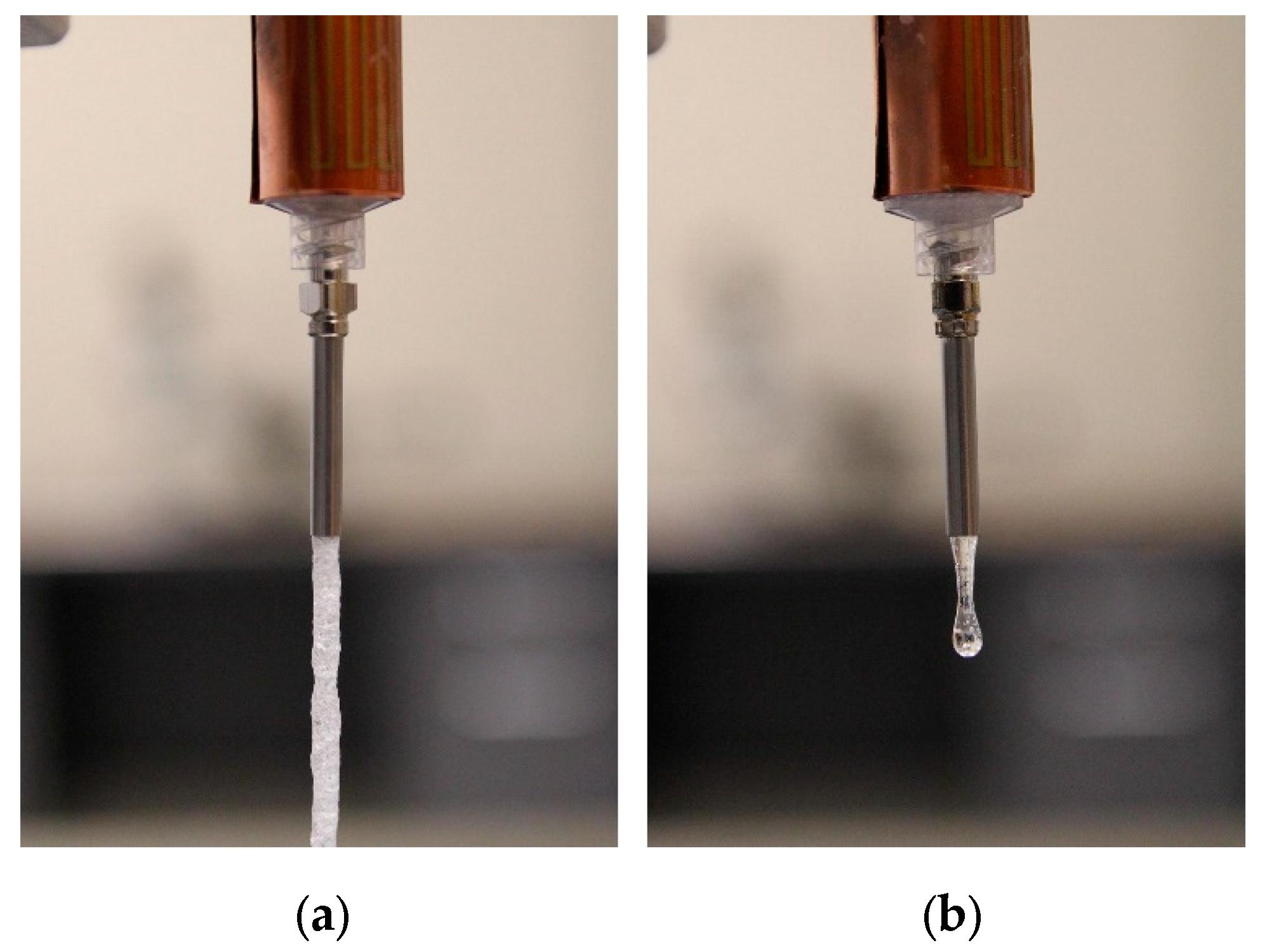
2.2.2. Extrusion Temperature Test
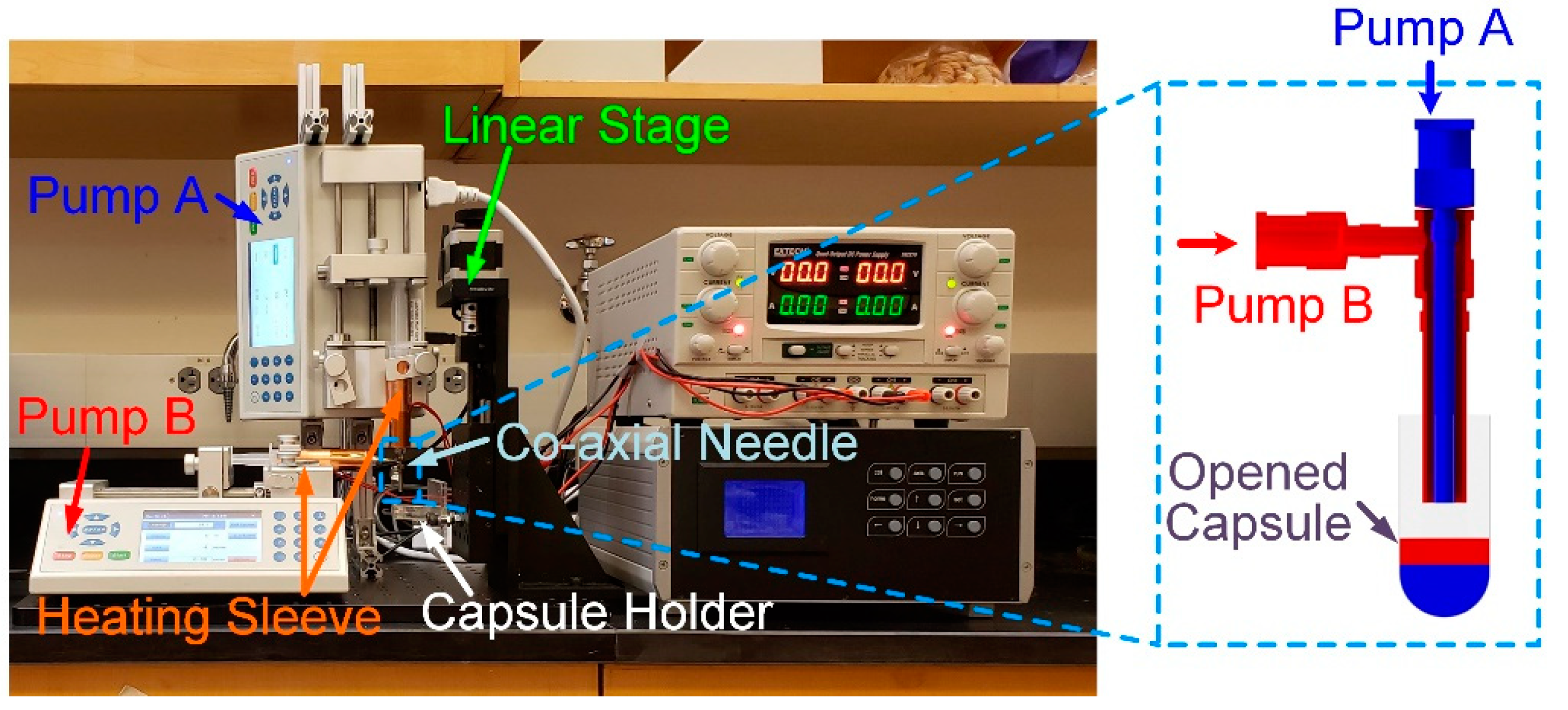
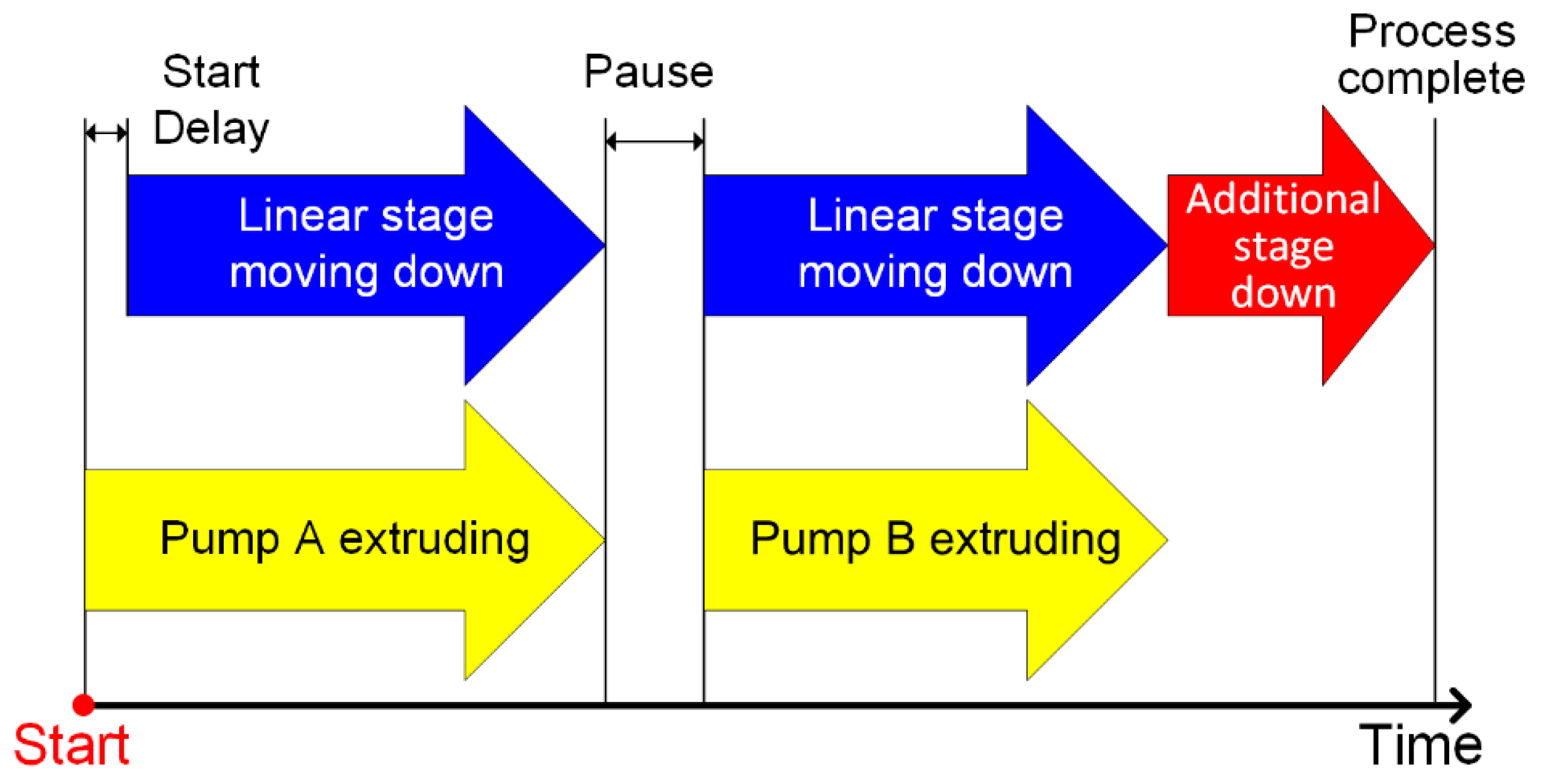
2.3. Fabrication Setup and Experimental Procedures
2.3.1. Fabrication Setup
2.3.2. In-Capsule Extrusion
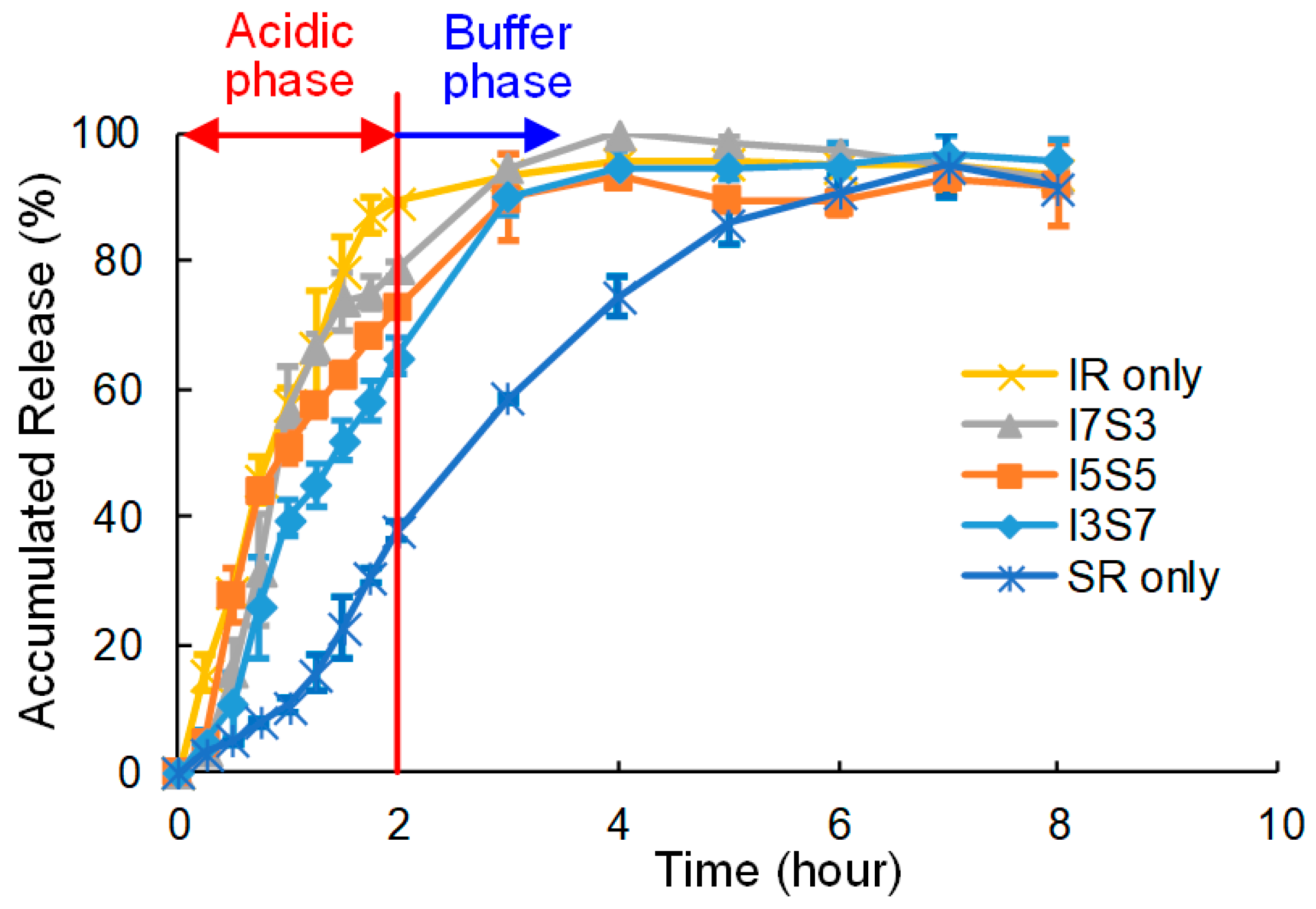
2.3.3. In Vitro Dissolution Test
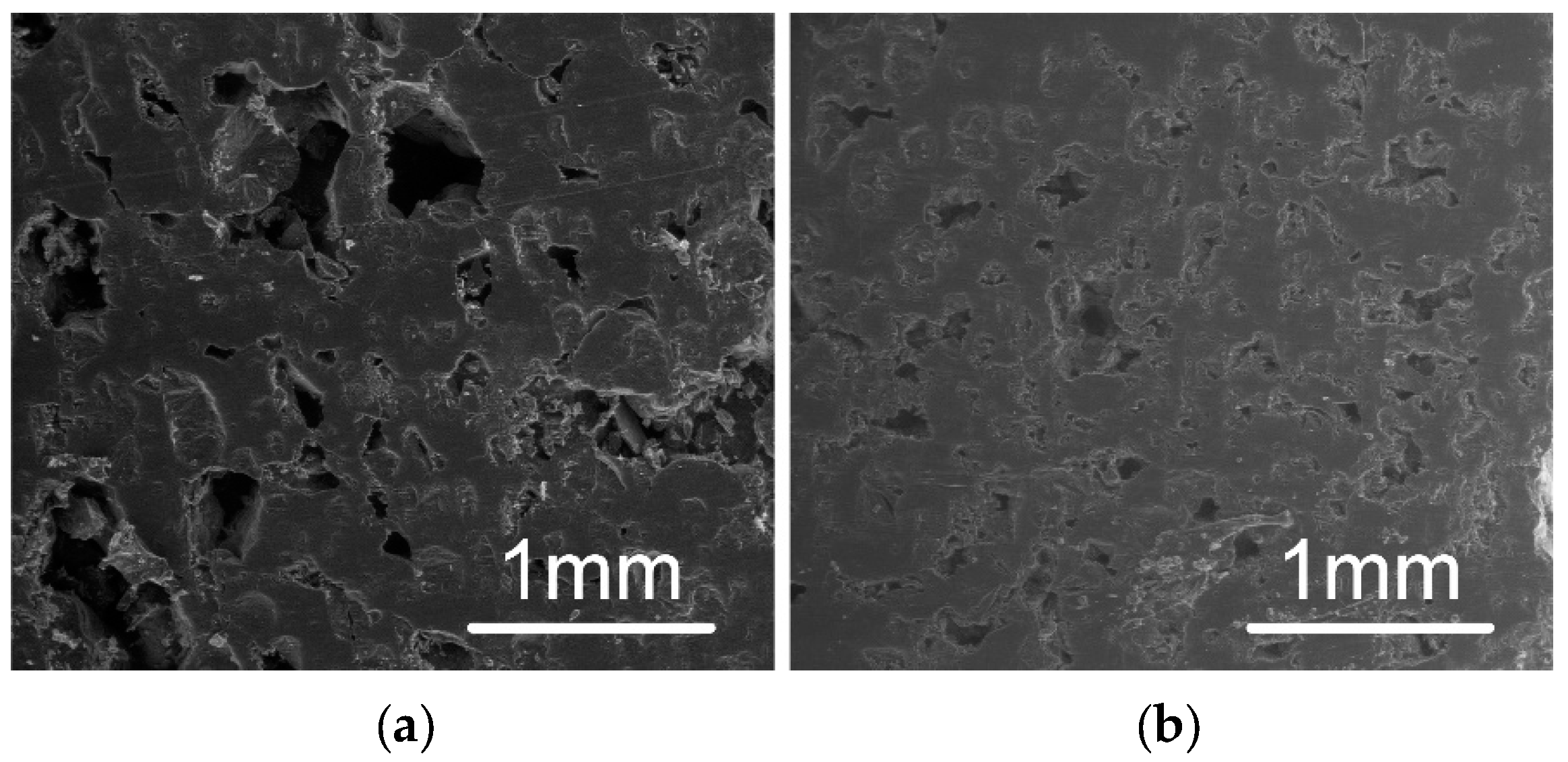
2.3.4. Scanning Electron Microscope (SEM)
3. Results
3.1. Viscosity and Extrusion
3.2. Fabricated Capsules
3.3. Dissolution Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamburg, M.A. The Path to Personalized Medicine. N. Engl. J. Med. 2010, 363, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Shah, T.; Vickery, C.; Smeeth, L.; Hingorani, A.D.; Casas, J.P. Fulfilling the promise of personalized medicine? Systematic review and field synopsis of pharmacogenetic studies. PLoS ONE 2009, 4, e7960. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M. Biomarkers for personalized oncology: Recent advances and future challenges. Metabolism 2015, 64, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, G. Personalized medicine: Revolutionizing drug discovery and patient care. Trends Biotechnol. 2001, 19, 491–496. [Google Scholar] [CrossRef]
- Kalia, M. Personalized oncology: Recent advances and future challenges. Metabolism 2013, 62, S11–S14. [Google Scholar] [CrossRef]
- Ma, Q.; Lu, A.Y.H. Pharmacogenetics, pharmacogenomics, and individualized medicine. Pharmacol. Rev. 2011, 63, 437–459. [Google Scholar] [CrossRef]
- Lesko, L.J. Personalized medicine: Elusive dream or imminent reality? Clin. Pharmacol. Ther. 2007, 81, 807–816. [Google Scholar] [CrossRef]
- Mathur, S.; Mathur, S.; Sutton, J.; Sutton, J. Personalized medicine could transform healthcare (Review). Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef]
- Kantae, V.; Krekels, E.H.J.; Esdonk, M.J.V.; Lindenburg, P.; Harms, A.C.; Knibbe, C.A.J.; Van der Graaf, P.H.; Hankemeier, T. Integration of pharmacometabolomics with pharmacokinetics and pharmacodynamics: Towards personalized drug therapy. Metabolomics 2017, 13, 9. [Google Scholar] [CrossRef]
- Cohen, J.S. Ways to minimize adverse drug reactions: Individualized doses and common sense are key. Postgrad. Med. 1999, 106, 163–172. [Google Scholar] [CrossRef]
- Tamargo, J.; le Heuzey, J.-Y.; Mabo, P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur. J. Clin. Pharm. 2015, 71, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Jiang, W.; Zhang, X.; Lionberger, R.; Makhlouf, F.; Schuirmann, D.J.; Muldowney, L.; Chen, M.L.; Davit, B.; Conner, D.; et al. Novel bioequivalence approach for narrow therapeutic index drugs. Clin. Pharmacol. Ther. 2015, 97, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Burns, M. Management of Narrow Therapeutic Index Drugs. J. Thromb. Thrombolysis 1999, 7, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kathuria, H.; Tan, J.J.Y.; Kang, L. 3D printed drug delivery and testing systems—A passing fad or the future? Adv. Drug Deliv. Rev. 2018, 132, 139–168. [Google Scholar] [CrossRef]
- Liang, K.; Brambilla, D.; Leroux, J.-C. Is 3d printing of pharmaceuticals a disruptor or enabler? Adv. Mater. 2018, 31, 1805680. [Google Scholar] [CrossRef]
- Boukouvala, F.; Niotis, V.; Ramachandran, R.; Muzzio, F.J.; Ierapetritou, M.G. An integrated approach for dynamic flowsheet modeling and sensitivity analysis of a continuous tablet manufacturing process. Comput. Chem. Eng. 2012, 42, 30–47. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef]
- Visser, J.C.; Woerdenbag, H.J.; Hanff, L.M.; Frijlink, H.W. Personalized medicine in pediatrics: The clinical potential of orodispersible films. AAPS PharmSciTech 2017, 18, 267–272. [Google Scholar] [CrossRef]
- Wening, K.; Breitkreutz, J. Oral drug delivery in personalized medicine: Unmet needs and novel approaches. Int. J. Pharm. 2011, 404, 1–9. [Google Scholar] [CrossRef]
- Sandler, N.; Määttänen, A.; Ihalainen, P.; Kronberg, L.; Meierjohann, A.; Viitala, T.; Peltonen, J. Inkjet printing of drug substances and use of porous substrates-towards individualized dosing. J. Pharm. Sci. 2011, 100, 3386–3395. [Google Scholar] [CrossRef]
- Ozeki, Y.; Ando, M.; Watanabe, Y.; Danjo, K. Evaluation of novel one-step dry-coated tablets as a platform for delayed-release tablets. J. Control. Release 2004, 95, 51–60. [Google Scholar] [CrossRef]
- Hosseini, A.; Körber, M.; Bodmeier, R. Direct compression of cushion-layered ethyl cellulose-coated extended release pellets into rapidly disintegrating tablets without changes in the release profile. Int. J. Pharm. 2013, 457, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Yamamoto, K.; Nishihata, T. Overall mechanism behind matrix sustained release (SR) tablets prepared with hydroxypropyl methylcellulose 2910. J. Control. Release 1995, 35, 59–66. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3d printing of medicines: Engineering novel oral devices with unique design and drug release characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Genina, N.; Fors, D.; Vakili, H.; Ihalainen, P.; Pohjala, L.; Ehlers, H.; Kassamakoc, I.; Haeggström, E.; Vuorela, P.; Peltonen, J.; et al. Tailoring controlled-release oral dosage forms by combining inkjet and flexographic printing techniques. Eur. J. Pharm. Sci. 2012, 47, 615–623. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.W.; Katstra, W.E.; Palazzolo, R.D.; Giritlioglu, B.; Teung, P.; Cima, M.J. Multimechanism oral dosage forms fabricated by three dimensional printingE. J. Control. Release 2000, 66, 11–17. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Piñeiro, G.D.; Montero, J.M.G.; Diaz, M.J.L.; Barcia, M.G.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Watanabe, Y.; Takayama, K.; Endo, H.; Matsumoto, M. Effect of hydroxypropylmethylcellulose (HPMC) on the release profiles and bioavailability of a poorly water-soluble drug from tablets prepared using macrogol and HPMC. Int. J. Pharm. 2000, 202, 173–178. [Google Scholar] [CrossRef]
- Lin, H.-R.; Chen, Y.-T.; Wu, Y.-C.; Lin, Y.-J. Glycol chitin/PAA hydrogel composite incorporated bio-functionalized PLGA microspheres intended for sustained release of anticancer drug through intratumoral injection. J. Biomater. Sci. Polym. Ed. 2018, 29, 1839–1858. [Google Scholar] [CrossRef]
- Pawar, H.; Douroumis, D.; Boateng, J.S. Preparation and optimization of PMAA–chitosan–PEG nanoparticles for oral drug delivery. Colloids Surf. B Biointerfaces 2012, 90, 102–108. [Google Scholar] [CrossRef]
- Ban, C.; Jo, M.; Lim, S.; Choi, Y.J. Control of the gastrointestinal digestion of solid lipid nanoparticles using PEGylated emulsifiers. Food Chem. 2018, 239, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Abd-Allah, F.I.; Dawaba, H.M.; Ahmed, A.M.S. Preparation, characterization, and stability studies of piroxicam- loaded microemulsions in topical formulations. Drug Discov. 2010, 4, 267–275. [Google Scholar]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (peg)–poly(n-isopropylacrylamide) (pnipaam) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef]
- Torrado, S.; Cadorniga, R.; Torrado, J.J. Effect of drug release rate on bioavailability of different aspirin tablets. Int. J. Pharm. 1996, 133, 65–70. [Google Scholar] [CrossRef]
- Barry, E.; Borer, L.L. Experiments with aspirin. J. Chem. Educ. 2000, 77, 354. [Google Scholar] [CrossRef]
- Yu, I.; Chen, R.; Grindrod, S. Fabrication of gellan gum tubular structure using coaxial needles: A study on wall thickness and encapsulation. In Proceedings of the ASME 2018 13th International Manufacturing Science and Engineering Conference, College Station, TX, USA, 18–22 June 2018. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]



| IR Component | IR-A | IR-B | IR-C |
|---|---|---|---|
| HPMC2910 | 5 | 10 | 15 |
| PEG | 85 | 80 | 75 |
| ASA | 10 | 10 | 10 |
| SR Component | SR-A | SR-B | SR-C |
|---|---|---|---|
| HPMC2208 | 10 | 15 | 20 |
| PEG | 75 | 70 | 65 |
| PAA | 5 | 5 | 5 |
| ASA | 10 | 10 | 10 |
| Ratio (volume/volume) | IR-C | SR-C |
|---|---|---|
| SR only | 0.0 | 0.9 |
| I3S7 (3:7) | 0.27 | 0.63 |
| I5S5 (5:5) | 0.45 | 0.45 |
| I7S3 (7:3) | 0.63 | 0.27 |
| IR only | 0.9 | 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, I.; Chen, R.K. A Feasibility Study of an Extrusion-Based Fabrication Process for Personalized Drugs. J. Pers. Med. 2020, 10, 16. https://doi.org/10.3390/jpm10010016
Yu I, Chen RK. A Feasibility Study of an Extrusion-Based Fabrication Process for Personalized Drugs. Journal of Personalized Medicine. 2020; 10(1):16. https://doi.org/10.3390/jpm10010016
Chicago/Turabian StyleYu, Ilhan, and Roland K. Chen. 2020. "A Feasibility Study of an Extrusion-Based Fabrication Process for Personalized Drugs" Journal of Personalized Medicine 10, no. 1: 16. https://doi.org/10.3390/jpm10010016
APA StyleYu, I., & Chen, R. K. (2020). A Feasibility Study of an Extrusion-Based Fabrication Process for Personalized Drugs. Journal of Personalized Medicine, 10(1), 16. https://doi.org/10.3390/jpm10010016






