Diagnostic Accuracy of Ultrasound in the Diagnosis of Small Bowel Obstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
2.2. Patients
2.3. Ultrasound Technique
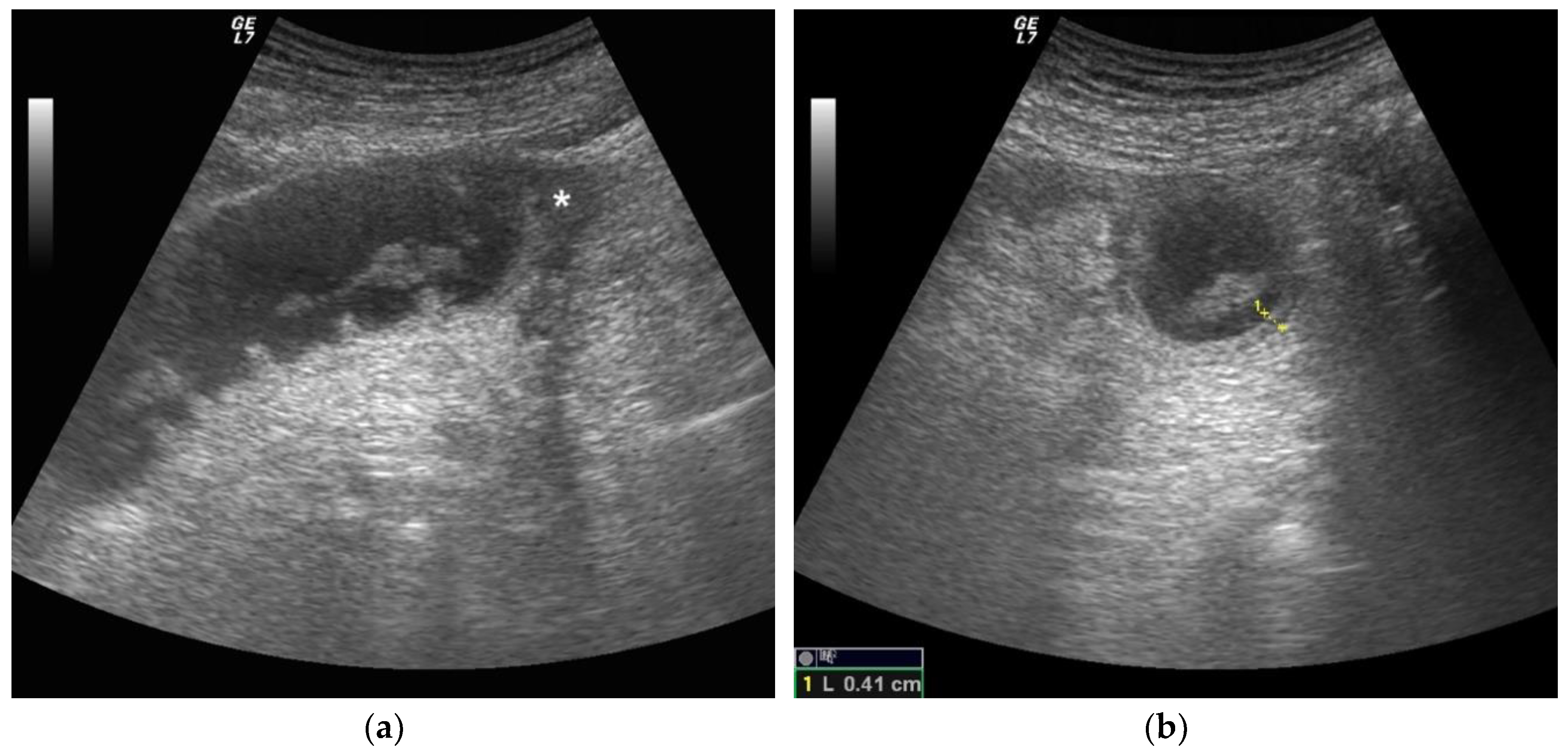
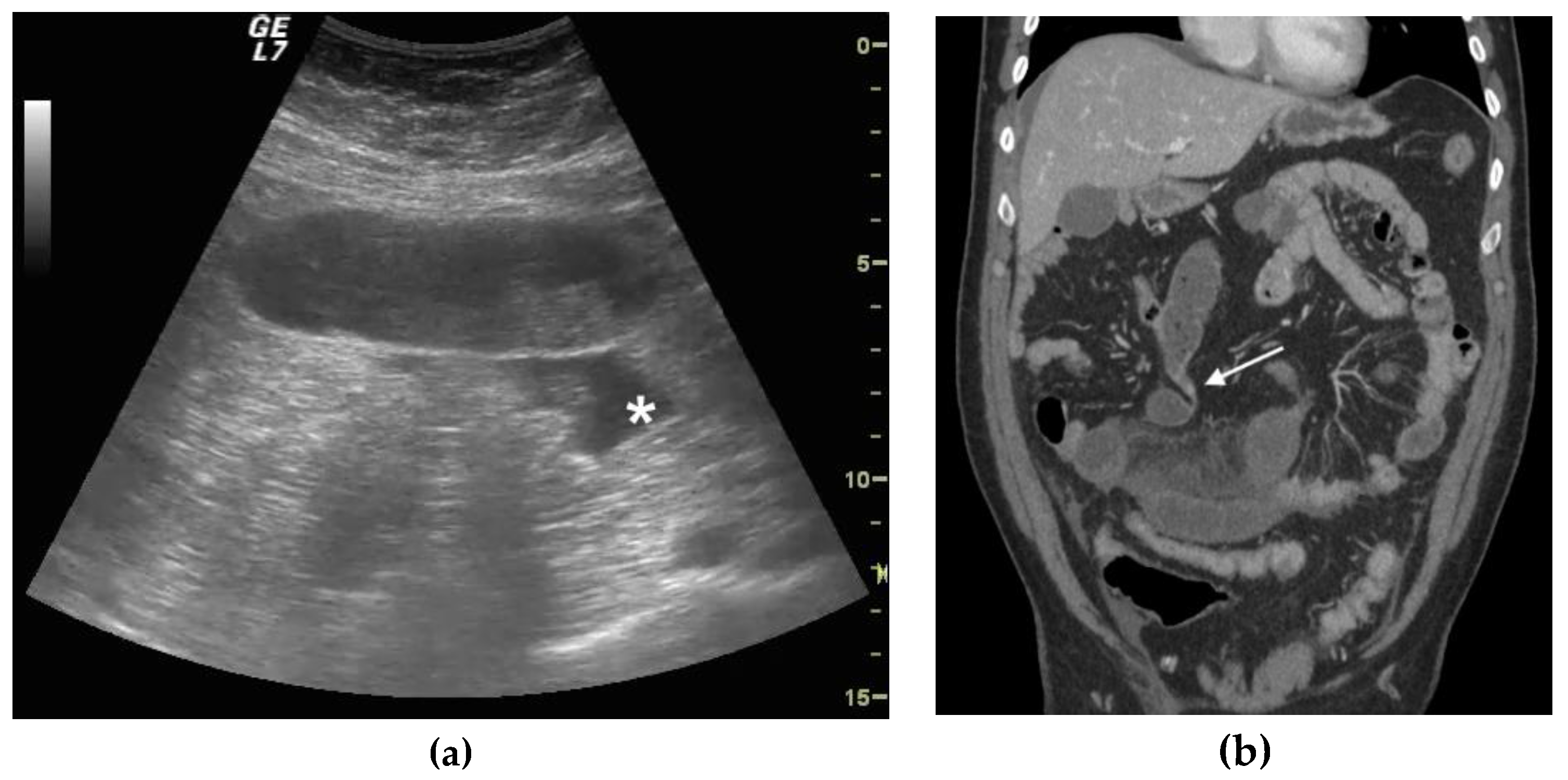
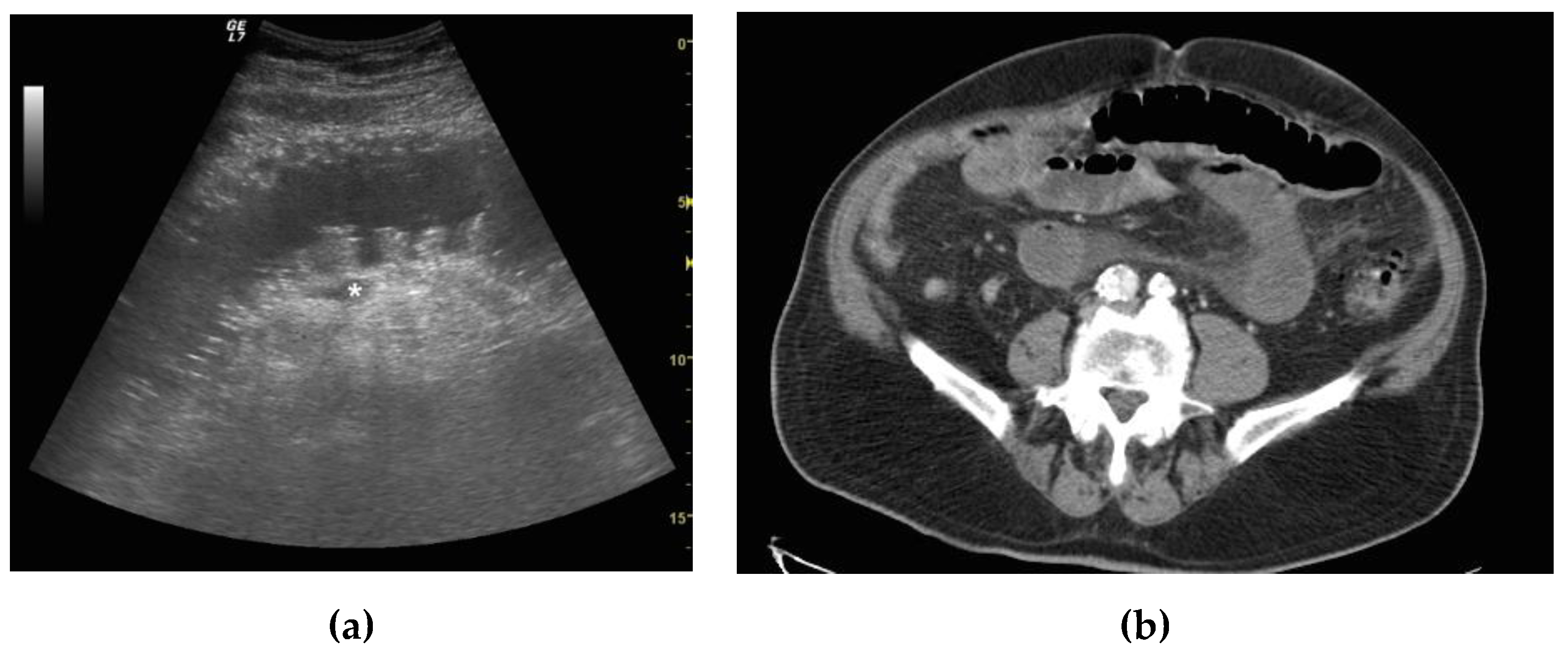
2.4. Ultrasound Diagnosis Criteria and Staging of Small Bowel Obstruction
2.5. CT Criteria and SBO Staging
2.6. Data Analysis and Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, G.; Boman, J.; Shrier, I.; Gordon, P.H. Etiology of small bowel obstruction. Am. J. Surg. 2000, 180, 33–36. [Google Scholar] [CrossRef]
- Skoglar, A.; Gunnarsson, U.; Falk, P. Band adhesions not related to previous abdominal surgery—A retrospective cohort analysis of risk factors. Ann. Med. Surg. 2018, 36, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.R.; Lalani, N. Adult small bowel obstruction. Acad. Emerg. Med. 2013, 20, 528–544. [Google Scholar] [CrossRef]
- Fevang, B.T.; Fevang, J.; Stangeland, L.; Soreide, O.; Svanes, K.; Viste, A. Complications and death after surgical treatment of small bowel obstruction: A 35-year institutional experience. Ann. Surg. 2000, 231, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Hastings, R.S.; Powers, R.D. Abdominal pain in the ed: A 35 year retrospective. Am. J. Emerg. Med. 2011, 29, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Robertson, J.; Koyfman, A. Emergency medicine evaluation and management of small bowel obstruction: Evidence-based recommendations. J. Emerg. Med. 2018, 56, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.B.; Schindler, D.; Kaji, A.H. Bedside ultrasonography for the detection of small bowel obstruction in the emergency department. Emerg. Med. J. 2011, 28, 676–678. [Google Scholar] [CrossRef]
- Sarani, B.; Paspulati, R.M.; Hambley, J.; Efron, D.; Martinez, J.; Perez, A.; Bowles-Cintron, R.; Yi, F.; Hill, S.; Meyer, D.; et al. A multidisciplinary approach to diagnosis and management of bowel obstruction. Curr. Probl. Surg. 2018, 55, 394–438. [Google Scholar] [CrossRef]
- Bickell, N.A.; Federman, A.D.; Aufses, A.H., Jr. Influence of time on risk of bowel resection in complete small bowel obstruction. J. Am. Coll. Surg. 2005, 201, 847–854. [Google Scholar] [CrossRef]
- Hwang, U.; Aufses, A.H., Jr.; Bickell, N.A. Factors associated with delays to emergency care for bowel obstruction. Am. J. Surg. 2011, 202, 1–7. [Google Scholar] [CrossRef]
- Maung, A.A.; Johnson, D.C.; Piper, G.L.; Barbosa, R.R.; Rowell, S.E.; Bokhari, F.; Collins, J.N.; Gordon, J.R.; Ra, J.H.; Kerwin, A.J.; et al. Evaluation and management of small-bowel obstruction: An eastern association for the surgery of trauma practice management guideline. J. Trauma Acute Care Surg. 2012, 73, S362–S369. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Pimenta, M.; Guimaraes, L.S. Small bowel obstruction: What to look for. Radiographics 2009, 29, 423–439. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.A.; Meehan, C.P.; Alhajeri, A.N.; Regan, M.C.; O’Keeffe, D.P. The use of mri to demonstrate small bowel obstruction during pregnancy. Br. J. Radiol. 2007, 80, e11–e14. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, A.; Dimbil, U.; Drake, A.; Shokoohi, H. The accuracy of point-of-care ultrasound in detecting small bowel obstruction in emergency department. Emerg. Med. Int. 2018, 2018, 3684081. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.A.; Lahham, S.; Gonzales, M.A.; Nomura, J.T.; Bui, M.K.; Truong, T.A.; Stahlman, B.A.; Fox, J.C.; Kehrl, T. A prospective, multicenter evaluation of point-of-care ultrasound for small-bowel obstruction in the emergency department. Acad. Emerg. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Maglinte, D.D.; Howard, T.J.; Lillemoe, K.D.; Sandrasegaran, K.; Rex, D.K. Small-bowel obstruction: State-of-the-art imaging and its role in clinical management. Clin. Gastroenterol. Hepatol. 2008, 6, 130–139. [Google Scholar] [CrossRef]
- Gottlieb, M.; Peksa, G.D.; Pandurangadu, A.V.; Nakitende, D.; Takhar, S.; Seethala, R.R. Utilization of ultrasound for the evaluation of small bowel obstruction: A systematic review and meta-analysis. Am. J. Emerg. Med. 2018, 36, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Hefny, A.F.; Corr, P.; Abu-Zidan, F.M. The role of ultrasound in the management of intestinal obstruction. J. Emerg. Trauma Shock 2012, 5, 84–86. [Google Scholar]
- Kameda, T.; Taniguchi, N. Overview of point-of-care abdominal ultrasound in emergency and critical care. J. Intensive Care 2016, 4, 53. [Google Scholar] [CrossRef]
- Unluer, E.E.; Yavasi, O.; Eroglu, O.; Yilmaz, C.; Akarca, F.K. Ultrasonography by emergency medicine and radiology residents for the diagnosis of small bowel obstruction. Eur. J. Emerg. Med. 2010, 17, 260–264. [Google Scholar] [CrossRef]
- Van Randen, A.; Lameris, W.; van Es, H.W.; van Heesewijk, H.P.; van Ramshorst, B.; Ten Hove, W.; Bouma, W.H.; van Leeuwen, M.S.; van Keulen, E.M.; Bossuyt, P.M.; et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur. Radiol. 2011, 21, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; April, M.D. What is the diagnostic performance of ultrasonography to diagnose small bowel obstruction? Ann. Emerg. Med. 2018, 72, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.A.; Guerrini, S.; Cioffi Squitieri, N.; Cagini, L.; Macarini, L.; Coppolino, F.; Giganti, M.; Volterrani, L. The role of us examination in the management of acute abdomen. Crit. Ultrasound J. 2013, 5 (Suppl. 1), S6. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Mateer, J.R.; Condon, R.E. Prospective evaluation of abdominal sonography for the diagnosis of bowel obstruction. Ann. Surg. 1996, 223, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kralik, R.; Trnovsky, P.; Kopacova, M. Transabdominal ultrasonography of the small bowel. Gastroenterol. Res. Pract 2013, 2013, 896704. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, G.R.; Benko, A.; Fournier, L.; Peron, J.M.; Morel, E.; Chiche, L. Small bowel obstruction: Role and contribution of sonography. Eur. Radiol. 1997, 7, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Hollerweger, A.; Wustner, M.; Dirks, K. Bowel obstruction: Sonographic evaluation. Ultraschall Med. 2015, 36, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Grassi, R.; Romano, S.; D’Amario, F.; Giorgio Rossi, A.; Romano, L.; Pinto, F.; Di Mizio, R. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur. J. Radiol. 2004, 50, 5–14. [Google Scholar] [CrossRef]
- Frasure, S.E.; Hildreth, A.F.; Seethala, R.; Kimberly, H.H. Accuracy of abdominal ultrasound for the diagnosis of small bowel obstruction in the emergency department. World J. Emerg. Med. 2018, 9, 267–271. [Google Scholar] [CrossRef]
- Paulson, E.K.; Thompson, W.M. Review of small-bowel obstruction: The diagnosis and when to worry. Radiology 2015, 275, 332–342. [Google Scholar] [CrossRef]
- Chuong, A.M.; Corno, L.; Beaussier, H.; Boulay-Coletta, I.; Millet, I.; Hodel, J.; Taourel, P.; Chatellier, G.; Zins, M. Assessment of bowel wall enhancement for the diagnosis of intestinal ischemia in patients with small bowel obstruction: Value of adding unenhanced ct to contrast-enhanced ct. Radiology 2016, 280, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Frasure, S.E.; Hildreth, A.; Takhar, S.; Stone, M.B. Emergency department patients with small bowel obstruction: What is the anticipated clinical course? World J. Emerg. Med. 2016, 7, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, H.; Delirrooyfard, A.; Moatamedfar, A.; Sohani, S.; Sohani, M. A new point of care ultrasound in disposition of patients with small bowel obstruction in emergency department. Int. J. Pharm. Res. Allied Sci. 2016, 5, 200–207. [Google Scholar]
- Dickman, E.; Tessaro, M.O.; Arroyo, A.C.; Haines, L.E.; Marshall, J.P. Clinician-performed abdominal sonography. Eur. J. Trauma Emerg. Surg. 2015, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]

| Simple | Complicated | Decompensated | |
|---|---|---|---|
| Bowel loops diameter | Increased | Increased | Increased |
| Parietal thickness | Normal | Normal or increased | Increased |
| Valvulae conniventes | Not thickened | Not thickened | Thickened |
| Peristalsis | Present and/or hyperkinetic | Decreased | Absent |
| Free fluid | Absent | Present | Present |
| Total | TP | TN | FP | FN | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR− (95%CI) | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| 43 | 24 | 16 | 1 | 2 | 92.31% (74.87–99.05%) | 94.12% (71.31–99.85%) | 15.69 (2.34–105.41) | 0.08 (00.2–0.31) | 96% | 88.89% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburrini, S.; Lugarà, M.; Iaselli, F.; Saturnino, P.P.; Liguori, C.; Carbone, R.; Vecchione, D.; Abete, R.; Tammaro, P.; Marano, I. Diagnostic Accuracy of Ultrasound in the Diagnosis of Small Bowel Obstruction. Diagnostics 2019, 9, 88. https://doi.org/10.3390/diagnostics9030088
Tamburrini S, Lugarà M, Iaselli F, Saturnino PP, Liguori C, Carbone R, Vecchione D, Abete R, Tammaro P, Marano I. Diagnostic Accuracy of Ultrasound in the Diagnosis of Small Bowel Obstruction. Diagnostics. 2019; 9(3):88. https://doi.org/10.3390/diagnostics9030088
Chicago/Turabian StyleTamburrini, Stefania, Marina Lugarà, Francesco Iaselli, Pietro Paolo Saturnino, Carlo Liguori, Roberto Carbone, Daniela Vecchione, Roberta Abete, Pasquale Tammaro, and Ines Marano. 2019. "Diagnostic Accuracy of Ultrasound in the Diagnosis of Small Bowel Obstruction" Diagnostics 9, no. 3: 88. https://doi.org/10.3390/diagnostics9030088
APA StyleTamburrini, S., Lugarà, M., Iaselli, F., Saturnino, P. P., Liguori, C., Carbone, R., Vecchione, D., Abete, R., Tammaro, P., & Marano, I. (2019). Diagnostic Accuracy of Ultrasound in the Diagnosis of Small Bowel Obstruction. Diagnostics, 9(3), 88. https://doi.org/10.3390/diagnostics9030088






