Luciferase-Based Detection of Antibodies for the Diagnosis of HPV-Associated Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Human Serum Samples
2.2. DNA Constructs for Luciferase-Tagged Antigens
2.3. LIPS Analysis
2.4. Detection of HPV E6 Antibodies by LIPSTICKS
2.5. Data Analysis
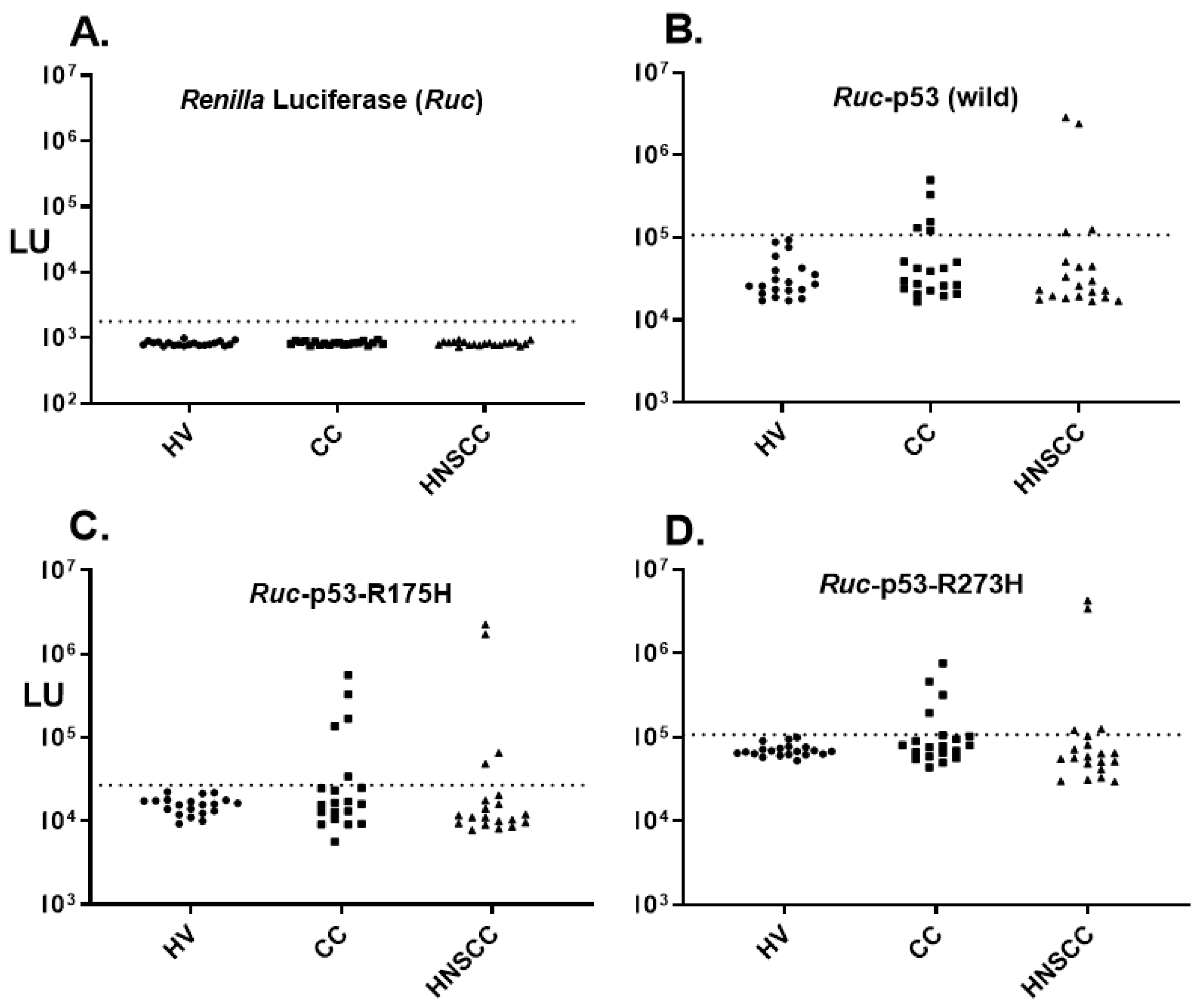
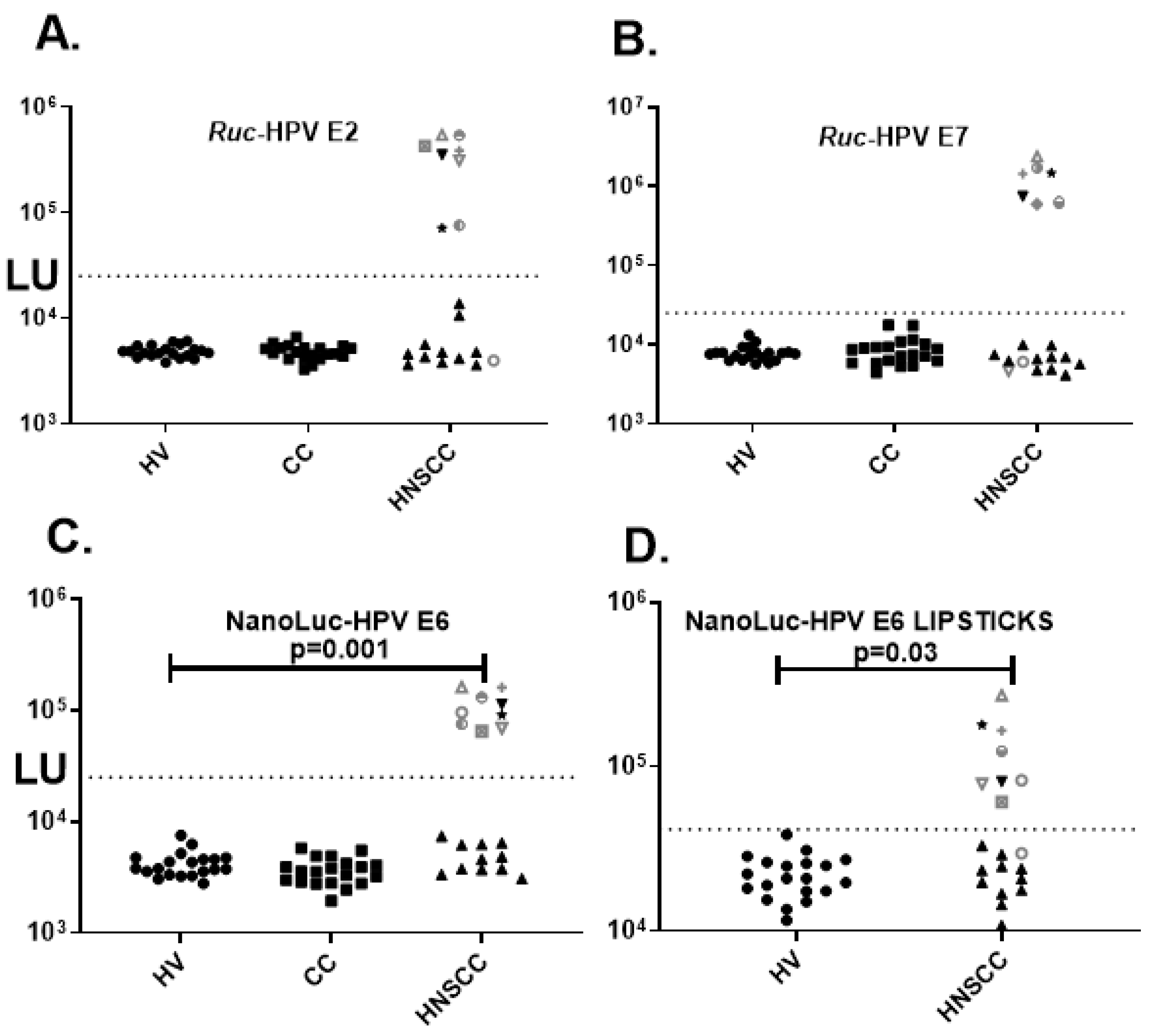
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Arantes, L.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Rubini, C.; De Michele, F.; Balercia, P.; Girotto, R.; Troiano, G.; Lo Muzio, L.; Santarelli, A. American Joint Committee on Cancer staging system 7th edition versus 8th edition: Any improvement for patients with squamous cell carcinoma of the tongue? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.P.; de Melo, J.B.; Carreira, I.M. Head and neck cancer: Searching for genomic and epigenetic biomarkers in body fluids-the state of art. Mol. Cytogenet. 2019, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.T.; Low, J.; Lim, S.G.; Chung, M.C. Serum autoantibodies as biomarkers for early cancer detection. FEBS J. 2009, 276, 6880–6904. [Google Scholar] [CrossRef] [PubMed]
- Soussi, T. p53 Antibodies in the sera of patients with various types of cancer: A review. Cancer Res. 2000, 60, 1777–1788. [Google Scholar]
- Zumbach, K.; Hoffmann, M.; Kahn, T.; Bosch, F.; Gottschlich, S.; Gorogh, T.; Rudert, H.; Pawlita, M. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in patients with head-and-neck squamous-cell carcinoma. Int. J. Cancer 2000, 85, 815–818. [Google Scholar] [CrossRef]
- Anderson, K.S.; Gerber, J.E.; D’Souza, G.; Pai, S.I.; Cheng, J.N.; Alam, R.; Kesiraju, S.; Chowell, D.; Gross, N.D.; Haddad, R.; et al. Biologic predictors of serologic responses to HPV in oropharyngeal cancer: The HOTSPOT study. Oral Oncol. 2015, 51, 751–758. [Google Scholar] [CrossRef]
- Anderson, K.S.; Wong, J.; D’Souza, G.; Riemer, A.B.; Lorch, J.; Haddad, R.; Pai, S.I.; Longtine, J.; McClean, M.; LaBaer, J.; et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br. J. Cancer 2011, 104, 1896–1905. [Google Scholar] [CrossRef]
- Morrison, B.J.; Labo, N.; Miley, W.J.; Whitby, D. Serodiagnosis for tumor viruses. Semin. Oncol. 2015, 42, 191–206. [Google Scholar] [CrossRef]
- Inan, H.; Wang, S.; Inci, F.; Baday, M.; Zangar, R.; Kesiraju, S.; Anderson, K.S.; Cunningham, B.T.; Demirci, U. Isolation, Detection, and Quantification of Cancer Biomarkers in HPV-Associated Malignancies. Sci. Rep. 2017, 7, 3322. [Google Scholar] [CrossRef]
- Beachler, D.C.; Viscidi, R.; Sugar, E.A.; Minkoff, H.; Strickler, H.D.; Cranston, R.D.; Wiley, D.J.; Jacobson, L.P.; Weber, K.M.; Margolick, J.B.; et al. A longitudinal study of human papillomavirus 16 L1, e6, and e7 seropositivity and oral human papillomavirus 16 infection. Sex. Transm. Dis. 2015, 42, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Johansson, M.; Waterboer, T.; Kaaks, R.; Chang-Claude, J.; Drogen, D.; Tjonneland, A.; Overvad, K.; Quiros, J.R.; Gonzalez, C.A.; et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J. Clin. Oncol. 2013, 31, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Ferreiro-Iglesias, A.; Nygard, M.; Bender, N.; Schroeder, L.; Hildesheim, A.; Robbins, H.A.; Pawlita, M.; Langseth, H.; Schlecht, N.F.; et al. Timing of HPV16-E6 antibody seroconversion before OPSCC: Findings from the HPVC3 consortium. Ann. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Lebovitz, E.E.; Notkins, A.L. Luciferase immunoprecipitation systems for measuring antibodies in autoimmune and infectious diseases. Transl. Res. 2015, 165, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.; Johnson, S.A.; Peterson, B.A.; Natarajan, V.; Salgado, M.; Dewar, R.L.; Burbelo, P.D.; Doria-Rose, N.A.; Graf, E.H.; Greenwald, J.H.; et al. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 2012, 119, 4645–4655. [Google Scholar] [CrossRef] [PubMed]
- Ching, K.H.; Burbelo, P.D.; Tipton, C.; Wei, C.; Petri, M.; Sanz, I.; Iadarola, M.J. Two major autoantibody clusters in systemic lupus erythematosus. PLoS ONE 2012, 7, e32001. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Browne, S.K.; Sampaio, E.P.; Giaccone, G.; Zaman, R.; Kristosturyan, E.; Rajan, A.; Ding, L.; Ching, K.H.; Berman, A.; et al. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood 2010, 116, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.K.; Burbelo, P.D.; Chetchotisakd, P.; Suputtamongkol, Y.; Kiertiburanakul, S.; Shaw, P.A.; Kirk, J.L.; Jutivorakool, K.; Zaman, R.; Ding, L.; et al. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 2012, 367, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Burbelo, P.D.; Egli-Spichtig, D.; Perwad, F.; Romero, C.J.; Ichikawa, S.; Farrow, E.; Econs, M.J.; Guthrie, L.C.; Collins, M.T.; et al. Autoimmune hyperphosphatemic tumoral calcinosis in a patient with FGF23 autoantibodies. J. Clin. Investig. 2018, 128, 5368–5373. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Dubovi, E.J.; Simmonds, P.; Medina, J.L.; Henriquez, J.A.; Mishra, N.; Wagner, J.; Tokarz, R.; Cullen, J.M.; Iadarola, M.J.; et al. Serology-enabled discovery of genetically diverse hepaciviruses in a new host. J. Virol. 2012, 86, 6171–6178. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Ching, K.H.; Esper, F.; Iadarola, M.J.; Delwart, E.; Lipkin, W.I.; Kapoor, A. Serological studies confirm the novel astrovirus HMOAstV-C as a highly prevalent human infectious agent. PLoS ONE 2011, 6, e22576. [Google Scholar] [CrossRef] [PubMed]
- Alagaili, A.N.; Briese, T.; Mishra, N.; Kapoor, V.; Sameroff, S.C.; Burbelo, P.D.; de Wit, E.; Munster, V.J.; Hensley, L.E.; Zalmout, I.S.; et al. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio 2014, 5, e00884-14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Fan, H.; Lan, T.; Yang, X.L.; Shi, W.F.; Zhang, W.; Zhu, Y.; Zhang, Y.W.; Xie, Q.M.; Mani, S.; et al. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 2018, 556, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Gunti, S.; Keller, J.M.; Morse, C.G.; Deeks, S.G.; Lionakis, M.S.; Kapoor, A.; Li, Q.; Cohen, J.I.; Notkins, A.L.; et al. Ultrarapid measurement of diagnostic antibodies by magnetic capture of immune complexes. Sci. Rep. 2017, 7, 3818. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Goldman, R.; Mattson, T.L. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Di Canzio, J.; Zurakowski, D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 1998, 221, 35–41. [Google Scholar] [CrossRef]
- Negm, O.H.; Hamed, M.R.; Schoen, R.E.; Whelan, R.L.; Steele, R.J.; Scholefield, J.; Dilnot, E.M.; Shantha Kumara, H.M.; Robertson, J.F.; Sewell, H.F. Human blood autoantibodies in the detection of colorectal cancer. PLoS ONE 2016, 11, e0156971. [Google Scholar] [CrossRef]
- Kunizaki, M.; Sawai, T.; Takeshita, H.; Tominaga, T.; Hidaka, S.; To, K.; Miyazaki, T.; Hamamoto, R.; Nanashima, A.; Nagayasu, T. Clinical value of serum p53 antibody in the diagnosis and prognosis of colorectal cancer. Anticancer Res. 2016, 36, 4171–4175. [Google Scholar]
- Zumbach, K.; Kisseljov, F.; Sacharova, O.; Shaichaev, G.; Semjonova, L.; Pavlova, L.; Pawlita, M. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in cervical-carcinoma patients from Russia. Int. J. Cancer 2000, 85, 313–318. [Google Scholar] [CrossRef]
- Holzinger, D.; Wichmann, G.; Baboci, L.; Michel, A.; Hofler, D.; Wiesenfarth, M.; Schroeder, L.; Boscolo-Rizzo, P.; Herold-Mende, C.; Dyckhoff, G.; et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int. J. Cancer 2017, 140, 2748–2757. [Google Scholar] [CrossRef]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burbelo, P.D.; Chaturvedi, A.; Notkins, A.L.; Gunti, S. Luciferase-Based Detection of Antibodies for the Diagnosis of HPV-Associated Head and Neck Squamous Cell Carcinoma. Diagnostics 2019, 9, 89. https://doi.org/10.3390/diagnostics9030089
Burbelo PD, Chaturvedi A, Notkins AL, Gunti S. Luciferase-Based Detection of Antibodies for the Diagnosis of HPV-Associated Head and Neck Squamous Cell Carcinoma. Diagnostics. 2019; 9(3):89. https://doi.org/10.3390/diagnostics9030089
Chicago/Turabian StyleBurbelo, Peter D., Adrija Chaturvedi, Abner L. Notkins, and Sreenivasulu Gunti. 2019. "Luciferase-Based Detection of Antibodies for the Diagnosis of HPV-Associated Head and Neck Squamous Cell Carcinoma" Diagnostics 9, no. 3: 89. https://doi.org/10.3390/diagnostics9030089
APA StyleBurbelo, P. D., Chaturvedi, A., Notkins, A. L., & Gunti, S. (2019). Luciferase-Based Detection of Antibodies for the Diagnosis of HPV-Associated Head and Neck Squamous Cell Carcinoma. Diagnostics, 9(3), 89. https://doi.org/10.3390/diagnostics9030089




