Abstract
Background: Breast cancer (BC) is the most common cancer among women worldwide; therefore, the efforts of many scientists are aimed at finding effective biomarkers for this disease. It is known that exosomes are nanosized extracellular vesicles (EVs) that are released from various cell types, including cancer cells. Exosomes are directly involved in governing the physiological and pathological processes of an organism through the horizontal transfer of functional molecules (proteins, microRNA, etc.) from producing to receiving cells. Since the diagnosis and treatment of BC have been improved substantially with exosomes, in this study, we isolated breast carcinoma cell-derived exosomes, primary endotheliocyte-derived exosomes, and blood exosomes from BC patients (BCPs) in the first stage of disease and investigated their proteomic profiles. Methods: Exosomes were isolated from the samples by ultrafiltration and ultracentrifugation, followed by mass spectrometric and bioinformatics analyses of the data. The exosomal nature of vesicles was verified using transmission electron microscopy and flow cytometry. Results: Exosome proteins secreted by MCF-7 and BT-474 cells were found to form two clusters, one of which enhanced the malignant potential of cancer cells, while the other coincided with a cluster of HUVEC-derived exosome proteins. Despite the different ensembles of proteins in exosomes from the MCF-7 and BT-474 lines, the relevant portions of these proteins are involved in similar biological pathways. Comparison analysis revealed that more BC-associated proteins were found in the exosomal fraction of blood from BCPs than in the exosomal fraction of conditioned medium from cells mimicking the corresponding cancer subtype (89% and 81% for luminal A BC and MCF-7 cells and 86% and 80% for triple-positive BC and BT-474 cells, respectively). Conclusions: Tumor-associated proteins should be sought not in exosomes secreted by cell lines but in the composition of blood exosomes from cancer patients, while the contribution of endotheliocyte exosomes to the total pool of blood exosomes can be neglected.
1. Introduction
In recent decades, there has been a steady increase in the number of new cases of malignant neoplasms, which is primarily due to increased life expectancy, as well as changes in lifestyle and exposure to unfavorable environmental factors. Today, cancer ranks second in mortality, conceding only to cardiovascular diseases. In 2022, breast cancer (BC) accounted for 23.8% of all new cancer cases in women worldwide. In particular, there were 2.3 million new cases of BC and 666,000 deaths from BC worldwide in 2022 [1]. The incidence of BC continues to increase despite the success of mammographic screening (depending on the country, from 14.2% (Republic of Moldova) to 47.3% (Czech Republic) of patients are diagnosed at stage I) [2]. Obviously, early BC diagnosis will increase the effectiveness of anti-tumor therapy and the survival rate of tumor patients. Thus, there is an urgent need to identify more effective and non-invasive surrogate markers that can guide not only early diagnosis but also the selection of therapeutic strategies for individual patients and the accurate assessment of prognosis [3]. Furthermore, BC is characterized by considerable tissue heterogeneity, showing distinct clinical and biological features, which makes tumors respond differently to treatments and complicates their management [4]. Today, molecular profiles have been largely explored, providing a well-established classification of BCs into five well-settled subtypes: luminal A, luminal B Her2+, luminal B Her2-, basal-like, and human epidermal growth factor receptor 2 (Her2)-enriched [2,5]. These molecular subtypes of BC are established by tumor biopsy, which can cause the displacement of tumor cells, promoting metastasis and various pathological changes in breast tissue [6]. In addition, biopsy exhibits inaccuracy in determining the BC subtype and is not posed to track patient follow-up [4]. In this regard, a promising direction in molecular oncology has been the search for new tumor markers in the composition of extracellular vesicles (EVs) by liquid biopsy. Among EVs, exosomes, small vesicles 30–150 nm in diameter with a lipid bilayer membrane and tetraspanins CD9, CD63, and CD81 on their crown, are prominent [7,8]. The advantage of exosomes is the sorting of biologically active molecules (proteins, different types of RNA) at the maturation stage of these vesicles [9]. Recently, exosomes from breast cancer cell lines have been shown to be a rich source of breast cancer-related proteins and surface biomarkers and can be used for the diagnosis and prognosis of the disease [10,11]. However, when analyzing the content of exosomes circulating in the blood of cancer patients, it is necessary to take into account that in addition to tumor exosomes, exosomes from endotheliocytes and other non-tumor cells, as well as cells from the tumor microenvironment, are present in the blood.
To assess the diagnostic potential of blood exosomes and evaluate the contribution of endotheliocytes to the blood exosome proteome, we performed a differential analysis of exosomal proteomes from primary endotheliocytes, from two cell lines mimicking luminal A and luminal Her2-positive BC, and from the blood of patients with these BC subtypes.
2. Materials and Methods
2.1. Isolation and Cultivation of Human Umbilical Vein Endothelial Cells (HUVECs)
HUVECs were obtained from three donors. Each vein was washed sequentially with 50 mL phosphate-buffered saline (PBS) and 20 mL collagenase IV buffer (0.1% collagenase IV in buffer containing 1.5 mM HEPES, 14 mM NaCl, 0.4 mM KCl, 0.12 mM CaCl2, 0.04 mM MgSO4, and 0.76 mM D-glucose, pH 7.4) [12]. The vein was incubated with 0.1% collagenase IV solution at 37 °C for 15 min to release endothelial cells. The collagenase solution containing detached cells was collected and combined with an additional PBS wash of the vein. The pooled solution was centrifuged at 800× g for 10 min, and the cell pellet was resuspended in IMDM (Gibco, Aucland, New Zealand) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA) and penicillin–streptomycin (100 μg/mL) (Thermo Fisher Scientific, Waltham, MA, USA). HUVECs were cultured in a CO2 incubator (5% CO2) at 37 °C, and adherent cells were washed the next day with fresh IMDM to remove residual blood cells. HUVECs were cultured to 70–80% confluence, and cells from the first passage were used for exosome isolation. For dissociation, cells were treated with 0.1% collagenase IV.
2.2. Cancer Cell Line Cultivation
MCF-7 (ATCC® HTB-22™) and BT-474 (ATCC® HTB-20™) BC cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Gibco, USA) and penicillin–streptomycin (100 μg/mL) (Thermo Fisher Scientific, Waltham, MA, USA) in a CO2 incubator (5% CO2) at 37 °C up to 70–80% confluence. Cells were subcultured with a solution of 0.25% trypsin in PBS with 5 mM EDTA.
2.3. Isolation of Exosomes from Conditioned Medium
All cells were negative for mycoplasma infection, as confirmed by PCR analysis of the 16S mycoplasma ribosomal gene [13].
FBS was centrifuged at 100,000× g for 2 h at 4 °C to remove bovine exosomes. The supernatant was collected and used to prepare bovine exosome-depleted medium. Three days prior to cell harvesting, the culture medium was replaced with a depleted medium containing IMDM for HUVECs or DMEM for MCF-7 and BT-474 cells, a mixture of antibiotics, and 10% FBS devoid of bovine exosomes.
For the isolation of exosomes, after 72 h, conditioned medium was collected and subjected to two successive centrifugations at 300× g and 15,000× g for 10 min and 20 min, respectively to remove dead cells and cellular debris. To eliminate large EVs, the supernatant was filtered through 100 nm pore-size filters (Minisart high flow, 16553-K, Sartorius, Goettingen, Germany). Exosomes were isolated from the pre-cleared conditioned medium by centrifugation at 100,000× g for 90 min at 100,000× g at 4 °C. The pellets were suspended in 12 mL of PBS and again centrifuged for 90 min at 100,000× g at 4 °C. The washing stage was repeated two times. Then, the supernatant was removed, and exosomes were resuspended in 300 μL of PBS, aliquoted, frozen in liquid nitrogen, and stored at −80 °C.
2.4. Ethics Statement
The study protocol was approved by the Ethics Committee of the Institute of Chemical Biology and Fundamental Medicine. Written informed consent was obtained from every female. Human samples were obtained according to the principles expressed in the Declaration of Helsinki. Blood samples from untreated BC patients (BCPs) with from luminal A (n = 5, age range 56–61 years, median age 61) and triple-positive (n = 8, age range 44–69 years, median age 61) subtypes [2,5] (Table 1) were obtained from the Novosibirsk Regional Oncology Dispensary.

Table 1.
Clinical characteristics of untreated BCPs.
2.5. Exosome Isolation from Blood
Venous blood (9 mL) was collected by venipuncture in K3EDTA spray-coated vacutainers (Improvacuter, Guangzhou, China) and processed within one hour. To isolate total blood exosomes by ultrafiltration and differential ultracentrifugation, a previously described protocol was used [14]. The pellet containing blood exosomes was resuspended in 300 μL of PBS.
2.6. Characterization of Exosomes
The morphology and membrane integrity of the isolated exosomes was assessed by transmission electron microscopy (TEM), as described previously [15]. The initial volume of exosomes analyzed using TEM was 15 μL.
To evaluate the protein concentration of exosomes, a NanoOrange Protein Quantitation kit (NanoOrange Protein Quantitation Kit, Molecular Probes, San Jose, CA, USA) was used as described previously [16].
The presence of CD9, CD63, and CD81 tetraspanins in the exosomal crown was confirmed by flow cytometry, as described previously [16]. The median fluorescence intensity (MFI) of stained exosomes was analyzed and compared to the isotype control (BD bioscience, Heidelberg, Germany). Flow cytometry was performed using a Cytoflex instrument (Becman Coulter, BioBay, Suzhou, China) with CytExpert 2.0 Software.
2.7. Mass Spectrometry Analysis
For the identification of exosomal proteins by MALDI-TOF mass spectrometry, proteins were separated using SDS-disc electrophoresis, and then fragments of polyacrylamide gel containing the studied proteins were washed from SDS and subjected to trypsinolysis, as described previously [14]. Specifically, samples were applied to the gel in 5 replicates, with line widths of 6 mm each. After the separation of the proteins in the gel, each line was cut into pieces 5 mm thick, resulting in 20 pieces from each line. Peptides from each piece of gel were analyzed independently; a protein was considered reliably identified when it was detected in at least three out of five cases.
Peptide fragments of proteins were extracted from the gel, concentrated, and desalted on C18 ZipTips microcolumns (Milipore, Burlington, MA, USA). Next, 5 μL of a saturated solution of α-cyano-4-hydroxycinnamic acid in 70% acetonitrile was added and then spotted onto an MTP 384 ground steel target plate. After crystallization, the target was loaded into the mass spectrometer to obtain the protein molecular weight spectrum. The acquisition and registration of mass spectra were carried out on an Ultraflex III time-of-flight tandem mass spectrometer (MALDI-TOF/TOF spectrometer) (Bruker Daltonics, Bremen, Germany). To ensure the reliability and reproducibility of the results, all analyses were performed with five biological replicates for each sample.
Spectra were acquired using the following parameters: shots—150, laser frequency—66.7 Hz, laser attenuator offset—85%, laser attenuator range—21%, laser attenuator set—5_ularge, laser focus offset—0%, laser focus range—100%, and laser focus value—4%. The instrument was pre-calibrated for a mass range of 500–3800 kDa. The obtained spectra were converted into mass values using flexAnalysis software 3.4. Protein identification was performed by searching for appropriate candidates in the annotated NCBI and SwissProt databases using the Mascot program (Matrix Science Ltd., London, UK, Available online: www.matrixscience.com/search_form_select.html (accessed on 20 October 2024)) with the following search parameters: species—Homo sapiens, error tolerance—±300 ppm, maximum number of missed cleavages—2, fixed modifications—propionamide (C), variable modifications—oxidation (M), phospho (ST); the identification reliability was not lower than 95%.
The matching of at least two peptides comprising 9 or more amino acid residues was considered a reliable identification of minor proteins [17].
2.8. Bioinformatics Analysis
Data from the SwissProt database were translated into the UniProt database for further analysis using the Retrieve/ID mapping platform (Available online: https://www.uniprot.org (accessed on 11 November 2024)). Functional enrichment analysis of exosomal proteomes according to Gene Ontologies was conducted using STRING software 12.0 (Available online: https://www.string-db.org/ (accessed on 10 January 2025)). Cellular localization, molecular functions, and involvement in biological processes were determined using FunRich 3.13 software based on the Gene Ontologies (GO) component, GO function, and GO process categories. Involvement in biological pathways was assessed using the Reactome service (Available online: https://reactome.org/ (accessed on 11 January 2025)). For primary data processing and graph generation, Python 3.11 libraries such as Pandas 2.2.3, Numpy 2.2.0, Matplotlib 3.10.1, and Seaborn 0.13.2 were utilized.
3. Results
3.1. Characterization of Isolated Exosomes
To characterize exosomes isolated from conditioned medium and BCP blood, TEM and flow cytometry were used. TEM revealed the presence of clearly structured cup-shaped vesicles (40–95 nm) of low electron density with preserved membranes in all samples; the portion of vesicles with a size smaller than 30 nm was no more than 13% (Figure 1). We then confirmed that the exosomes isolated from all sources contained typical exosome protein markers, namely, tetraspanins CD9 and CD63. For this purpose, exosomes adsorbed onto aldehyde/sulfate latex beads using anti-CD9 antibodies were stained with FITC-labeled antibodies for the tetraspanin CD63. It was shown that the CD9 and CD63 tetraspanins were comparably represented in all vesicles (Figure 1).
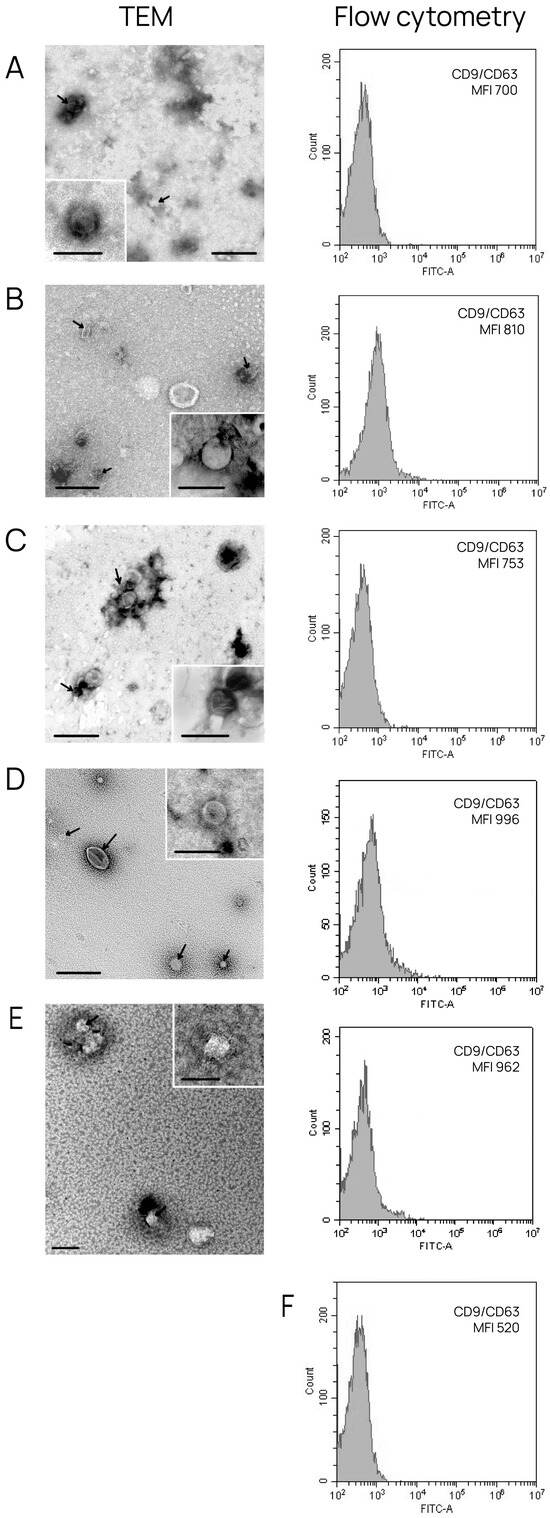
Figure 1.
Exosome characterization by TEM and flow cytometry analysis. Overview of exosome preparation and expression of CD63 on CD9-positive vesicles obtained from (A) conditioned medium from HUVECs; (B) conditioned medium from MCF-7 cells; (C) conditioned medium from BT-474 cells; (D) blood from patients with luminal A BC; and (E) blood from patients with triple-positive BC. Scale bars correspond to 100 nm. Electron microscopy, negative staining by phosphotungstate acid. For flow cytometry, mean MFI values are shown. Negative control (F) (latex beads labeled with anti-CD9 and anti-CD63 FITC antibody).
Collectively, the obtained data reveal that the sEVs isolated from conditioned medium and blood have all properties of exosomes [18].
3.2. Annotation of Identified Proteins from HUVEC-Derived Exosomes
Since in addition to tumor exosomes, exosomes from normal cells, including those of endothelial origin, circulate in the blood of cancer patients, the first stage of this work involved the identification and characterization of exosome proteins secreted by primary endothelial cells from three donors. A total of 24 proteins were identified with high confidence (p < 0.05) using MALDI-TOF mass spectrometry in the HUVEC-derived exosomes (Table S1); of these, 29% (7 proteins) were common to the three groups (Figure 2A).
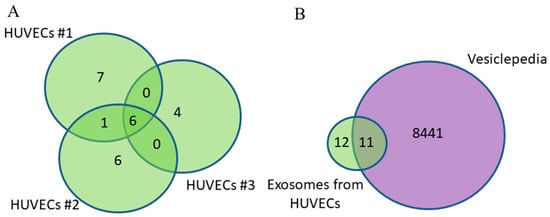
Figure 2.
Venn–Euler diagrams of proteins in exosomes from HUVECs from three donors (A) and overlap with the Vesiclepedia database (B).
The majority (70%) of identified exosomal proteins from HUVECs were previously discovered in other studies using mass spectrometry and were annotated in the Vesiclepedia database [19] (the putative CNGA1-overlapping antisense gene protein is not included in the analysis because its gene is not known). Thus, about one-third of the exosomal proteins are identified in our study for the first time as a part of EVs; previously, they were not annotated in this database (Figure 2B, Table 2).

Table 2.
HUVEC-secreted exosomal proteins not deposited in the Vesiclepedia database.
The protein–protein interaction (PPI) network was mainly concentrated on the relevance among ALB, CD9, CD63, CD81, ADAM10, and MMP9, all of which are major exosome proteins (Figure 3).
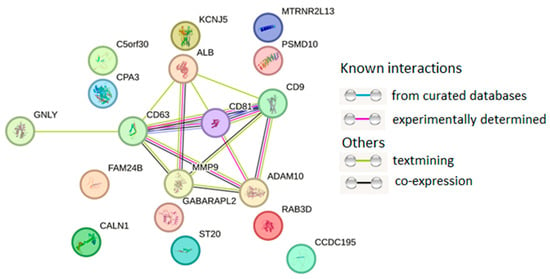
Figure 3.
PPI network of 18 proteins from HUVEC-derived exosomes. PPI networks were plotted with STRING (Available online: http://string-db.org/ (accessed on 10 January 2025)) with the following settings: minimum interaction score—high confidence (0.400); active interaction sources—text mining, experiments, databases, co-expression.
The molecular function GO analysis revealed that HUVEC-derived exosomal proteins were commonly enriched in functions such as “metallopeptidase activity” and “transporter activity” (Table 3).

Table 3.
Analysis of the molecular functions in which HUVEC-derived exosomal proteins are involved.
Six predominant proteins (ALB, CD9, CD63, CD81, MMP9, PSMD10) were found to be significantly (p < 0.05) involved in biological pathways (Table 4). In particular, the common proteomic profiles of endothelial exosomes were enriched in terms related to biological processes such as “metabolism of RNA”, “metabolism of mRNA”, “adaptive immune system”, etc.

Table 4.
Analysis of the biological pathways in which HUVEC-derived exosomal proteins are involved.
3.3. Annotation of Protein Cargo from BC-Derived Exosomes
Using MALDI-TOF mass spectrometry, 34 and 42 proteins were identified with high reliability (p < 0.05) in the exosomes from BC cell lines BT-474 and MCF-7, respectively (Supplementary Tables S2 and S3). Of these, only 6% (five proteins) were common to both groups (Supplementary Tables S2 and S3, Figure 4A). It should be noted that the same proteins (CD9, CD24, CD63, CD81, MMP9) were common to exosomes of cancer cells and primary cells.
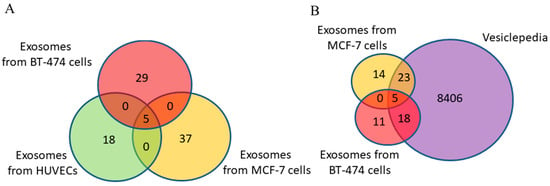
Figure 4.
Venn–Euler diagrams of proteins in exosomes from HUVECs, BT-474 cells, and MCF-7 cells (A) and overlap with the Vesiclepedia database (B).
It should be noted that due to the small size of exosomes (30–150 nm) and their high RNA content, the cargo of these vesicles contains predominantly low-molecular-weight proteins rich in lysine and arginine. As a consequence, the proportion of reliably identified proteins meeting the new criteria for proteome investigation [17] is extremely low (trypsinolysis results in a large number of peptides with fewer than nine amino acid residues).
Approximately 65% of identified exosomal proteins from cancer cells were previously discovered in other studies using mass spectrometry and were annotated in the Vesiclepedia database (Figure 4B) (the putative uncharacterized protein FLJ45840 protein is not included in the analysis because its gene is not known). The non-deposited Vesiclepedia database proteins of exosomes secreted by MCF-7 and BT-474 cells are presented in Table 5 and Table 6, respectively.

Table 5.
MCF-7 cell-secreted exosomal proteins not deposited in the Vesiclepedia database.

Table 6.
BT-474 cell-secreted exosomal proteins not deposited in the Vesiclepedia database.
For cancer-derived exosomes, the PPI network was mainly concentrated on the connections among UPF3B, SRSF6, SRSF5, RBMX, PRPF38A, KCNC4, RPS27I, VAMP8, EFNB2, CD9, CD63, CD81, ADAM10, and MMP9, among which 11 exosomal proteins formed two clusters: in addition to the cluster of major proteins identified by molecular cargo analysis of primary endotheliocytes, one cluster was identified that enhanced the malignant potential of MCF-7 and BT-474 cells (Figure 5).
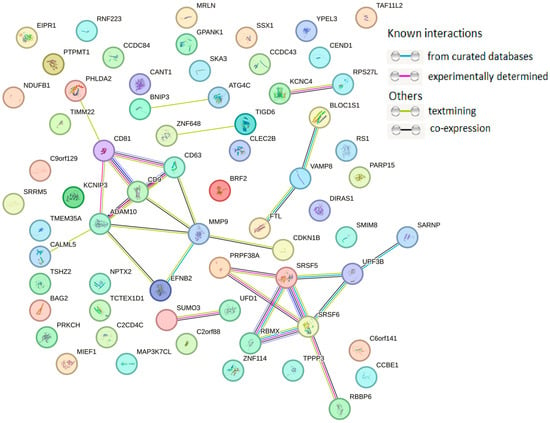
Figure 5.
PPI network of 65 proteins from cancer cell-derived exosomes. PPI networks were plotted with STRING (Available online: http://string-db.org/, accessed on 10 January 2025) with the following settings: minimum interaction score—high confidence (0.400); active interaction sources—text mining, experiments, databases, co-expression.
The molecular function GO analysis revealed that MCF-7-derived exosomal proteins were commonly enriched in functions such as “calcium ion binding”, “metallopeptidase activity”, “RNA binding”, “ubiquitin-specific protease activity”, “GTPase activity”, “DNA binding”, etc. (Table 7).

Table 7.
Analysis of the molecular functions in which MCF-7 cell-derived exosomal proteins are involved.
Analysis of the protein cargo of exosomes secreted by MCF-7 cells showed the involvement of proteins in biological pathways such as “membrane trafficking”, “Clathrin-derived vesicle budding”, “trans-Golgi network vesicle budding”, “signaling by SCF-KIT”, “Notch signaling pathway”, “signaling by EGFR”, etc. (Table 8). It should be noted that five of the eight (63%) proteins in Table 6 were identified by PPI analysis as proteins interacting with a large number of other proteins (Figure 5).

Table 8.
Analysis of the biological pathways in which MCF-7 cell-derived exosomal proteins are involved *.
The molecular functions of BT-474 cell-derived exosomal proteins were very similar to the functions of proteins secreted by MCF-7 exosomes. Specifically, bioinformatics analysis showed that the most common molecular functions of exosomal proteins were “metallopeptidase activity” and “RNA binding” (Table 9).

Table 9.
Analysis of the molecular functions in which BT-474 cell-derived exosomal proteins are involved.
Similarly, bioinformatics analysis of proteins from BT-474 cell-secreted exosomes revealed their involvement in biological pathways such as “gene expression”, “mRNA splicing”, “mRNA processing”, “transcription”, etc. (Table 10). As with exosomal proteins from MCF-7 cells, in exosomes from BT-474 cells, five of the eight proteins (63%) presented in Table 10 were also identified by PPI analysis as proteins that interact with a large number of other proteins (Figure 5).

Table 10.
Analysis of the biological pathways in which BT-474 cell-derived exosomal proteins are involved *.
It was also shown that despite the different ensembles of proteins within exosomes from MCF-7 and BT-474 cell lines, these exosomal proteins are involved in many of the same biological pathways such as “post-elongation processing of intron-containing pre-mRNA”, “mRNA 3′-end processing”, “cleavage of growing transcript in the termination region”, “post-elongation processing of the transcript”, “RNA polymerase II transcription termination”, and “receptor–ligand binding initiates the second proteolytic cleavage of Notch receptor” (Table 8 and Table 10).
3.4. Comparative Proteomic Analysis of Exosomes in the Blood of BCPs with Luminal A and Triple-Positive Subtypes
Using MALDI-TOF mass spectrometry with high confidence (p < 0.05), we identified 74 and 110 proteins in exosomes from the blood of untreated BCPs with luminal A (n = 5, Table 1) and triple-positive (n = 8, Table 1) subtypes, respectively (Supplementary Tables S4 and S5). It should be noted that 8 out of 41 universal exosomal proteins were detected in half of the samples. The analysis of the identified exosomal proteins using the Vesiclepedia database revealed that among the 119 proteins that were previously found to be associated with vesicles, a total of 61 and 95 proteins were identified in the luminal A BC and triple-positive BC exosomes, respectively. Thus, 17% of proteins identified in exosomes from the blood of BCPs in this study were not previously annotated in the Vesiclepedia database (Figure 6). The non-deposited Vesiclepedia database proteins of BCP blood exosomes are presented in Table 11.
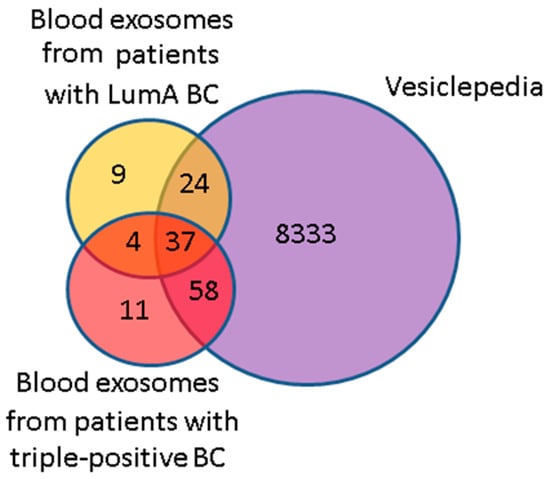
Figure 6.
Venn–Euler diagram of proteins in exosomes from the blood of BCPs with luminal A and triple-positive subtypes and those in the Vesiclepedia database.

Table 11.
BCP blood exosomal proteins not deposited in the Vesiclepedia database.
The molecular functions of BCP blood exosome proteins were similar to those of proteins within exosomes secreted by MCF-7 and BT-474 cells. In particular, bioinformatics analysis showed that the universal molecular functions of exosomal proteins were “calcium ion binding”, “DNA binding”, “metallopeptidase activity”, and “RNA binding” (Table 7, Table 9, and Table 12).

Table 12.
Analysis of the molecular functions in which BCP blood exosomal proteins are involved.
At the same time, the bioinformatic analysis also revealed terms unique to BCP blood exosome proteins such as “protease inhibitor activity”, “protein binding”, etc.
It should be noted that barrier-to-autointegration factor (BANF1) and zinc finger protein 638 (ZNF638) were found earlier as DNA-binding proteins in exosomes [20]. In general, these exosomal DNA-binding proteins have many molecular functions and are involved in important biological processes [21,22,23,24]. Thus, exosomes carry biologically active DNA-binding proteins, mainly in the form of internal contents from donor cells to recipient cells, causing changes in the behavior of recipient cells.
To determine whether the exosomal proteins we identified were found in breast neoplasms, the list of proteins was analyzed using FunRich 3.13 software to search a publicly available database for proteins in BC, namely, the Human Protein Atlas (HPA). It was shown that 66 BC-associated proteins from blood exosomes of BCPs with a luminal A subtype and 34 from MCF-7-derived exosomes were annotated in the HPA database; all 5 major exosomal proteins were also found in the HPA database (Figure 7A).
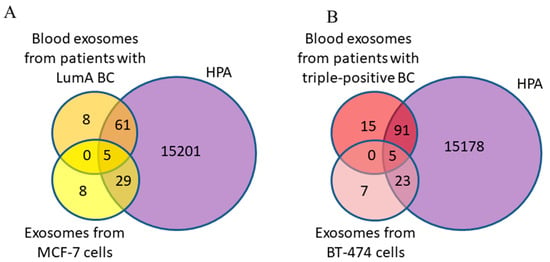
Figure 7.
Venn–Euler diagram of exosomal proteins from cancer cells and blood samples from BCPs with luminal A (A) and triple-positive (B) subtypes compared with the HPA database, composed using QuickGO 2.0 and FunRich 3.13 software.
Similar results were obtained for protein cargo of exosomes from the blood of triple-positive BCPs and from BT-474 conditioned medium (Figure 7B). Thus, more BC-associated proteins were found in the composition of exosomes from the blood of BCPs than in the exosomes from the conditioned medium of cells mimicking the corresponding cancer subtype (89% and 81% for luminal A BC and MCF-7 cells and 86% and 80% for triple-positive BC and BT-474 cells, respectively).
4. Discussion
Tumor exosomes able to transport biologically active molecules (RNA, proteins, and metabolites) in recipient cells have been recognized as fundamental mediators of cell-to-cell communication in cancer, including BC. Since the molecular cargo of exosomes reflects the composition of the parent cell, in the field of molecular diagnostics, great potential is associated with the identification of tumor-specific signatures in the composition of exosomes for the development of a method for the diagnosis of malignant neoplasms by liquid biopsy [25,26]. One of the undoubted advantages of exosomal proteome research is the possibility of removing ballast proteins from blood plasma and increasing the concentration of tumor-specific proteins, including membrane proteins. It should be noted that the membrane of vesicles protects their contents from the action of proteases and nucleases, and vesicle preparations are stable and can be stored for a long time in laboratory conditions [27].
The search for tumor markers in exosomes is complicated by the high individual variability of the exosome proteome, even in the group of healthy donors. In a comparison of the proteomic profiles of blood plasma exosomes from fifteen clinically healthy donors, only 9 out of 109 identified exosomal proteins were present in all samples [28]. Our profiling of the proteome of HUVEC-derived exosomes partially confirms these data: only 29% of proteins within primary endotheliocytes were common to the three human umbilical vein donors.
To avoid the problem of low reproducibility between different samples, as well as to solve the problem of the amount of protein needed for mass spectrometry, most researchers work with cell culture exosomes, which is reflected in the Vesiclepedia database. As a result, the authors provide data on thousands of proteins in the composition of exosomes obtained from the conditioned media of various cell lines. In particular, Altelaar’s group successfully identified exosome proteomes from cell lines mimicking triple-negative (BT-549, Hs578T, LM2, MDA-MB-231), HER-2-positive (HCC1419, HCC1954, JIMT1, SKBR-3), and luminal A (MCF-7) BC subtypes [29], identifying 4648 proteins. However, the authors did not take into account the fact that if the size of exosomes is less than 150 nm, the vesicle cannot contain more than 100 proteins. Thus, there is no point in obtaining excessively large quantities of exosomes and describing proteins that can be detected in blood exosomes with very low probability.
Modern BC diagnostic methods (mammography, breast ultrasound, and MRI) can detect neoplasms larger than 0.5 cm; however, these tests are often ambiguous and have documented drawbacks [3]. For simplicity of calculation, we assume that only the surface cells of the tumor secrete exosomes to the external space. Then, considering that the surface area of a 5 mm diameter sphere-shaped tumor is 78.5 mm2, and taking into account the average diameter of a breast carcinoma cell of 20 µm, a quarter of a million cells will be located on the outer surface of the tumor. Taking into account the above calculations, in the current work, for the analysis of exosome proteins by mass spectrometry, the proteins in exosomes secreted by 500,000 MCF-7 or BT-474 cells were applied to a gel and separated. The obtained data on the proteomic profiles of exosomes from the conditioned media of breast carcinoma lines correspond to the number of exosomes secreted by the tumor at stage T1. A comparative analysis of proteins of secreted by primary endotheliocyte exosomes and breast carcinoma cell exosomes showed that it is possible to neglect the contribution of the endotheliocyte exosome proteome when searching for tumor markers in the composition of blood exosomes. Moreover, since the five proteins CD9, CD24, CD63, CD81, and MMP9 are universal and present in exosomes secreted by both primary endotheliocytes and breast carcinoma cells, they cannot be part of the diagnostic panels being developed.
To confirm that EVs are ideal diagnostic tools, Hoshino’s group analyzed the proteomes of EVs from different sources. It was shown that EV proteins from human plasma overlapped best with human serum-derived EVs (r2 = 0.92), followed by human bone marrow (r2 = 0.65) and lymphatic fluid EVs (r2 = 0.64); these proteins correlated least with human cell line- (r2 = 0.15) and tissue explant-derived EVs (r2 = 0.24), suggesting that the complexity of plasma and lymph EV proteomes may drive the divergence of tissue EV proteomes [30]. Our study also revealed an extremely weak correlation between exosome proteins secreted by MCF-7 cells and blood exosomes from patients with the luminal A subtype of BC, as well as between exosome proteins secreted by BT-474 cells and blood exosomes from patients with the triple-positive subtype of BC. However, when comparing protein profiles of blood exosomes from patients and tissue proteins, a significant coincidence of proteins was found. It should be noted that breast tumors are characterized by significant variability in their cellular composition, as well as histological, expression, and genotypic heterogeneity. In particular, the intratumoral morphologic heterogeneity of invasive breast carcinoma of a nonspecific type, the most common histologic form of BC (incidence rate up to 80%), has been described [31]. As a consequence, exosomes secreted by tumor cells have a more diverse composition than exosomes originating from MCF-7 or BT-474 cells. In addition, in the body, besides cancer cells, exosomes are secreted by other cells, in particular cells from the tumor microenvironment [32]. Thus, the exosomal content in the blood of BCPs at the T1 stage reflects the cancer-associated changes occurring not only in the developing primary tumor but also the tumor microenvironment. Despite previous studies searching for protein biomarkers within exosomes secreted by breast carcinoma cell lines [11,33,34], there is no consensus on exosomal tumor markers due to limited EV proteomic datasets from human samples and appropriate controls for data analysis and interpretation.
It should be noted that only three (GTSE1, VAV3, and SOCS3) proteins identified in our study were deposited simultaneously in Vesiclepedia and were shown to be strongly associated with BC according to the HPA database. Specifically, for the G2 and S phase-expressed-1 (GTSE1) protein, its expression is known to be significantly upregulated in BC tissues and cell lines, with high levels of this protein correlating with tumor prevalence and poor prognosis [35]. It has been shown that GTSE1 promotes tumor cell proliferation by activating the AKT signaling pathway and enhances metastasis through the regulation of the epithelial–mesenchymal transition [36].
Another protein, VAV3, has been identified as a critical player in the modulation of immune responses and cancer cell behavior. VAV3 has been shown to be involved in the activation of Rho/Rac signaling pathways that regulate cytoskeleton dynamics, cell migration, and invasion. VAV3 is known to be upregulated in various types of cancer, including breast cancer [37]. VAV3 has been found to be associated with poor prognosis and aggressive breast cancer subtypes [38].
SOCS3 is a critical negative regulator of cytokine signaling pathways, primarily modulating the Janus kinase/signal transducer and activator of transcription (JAK/STAT) axis. It achieves this through direct binding to phosphorylated tyrosine residues on activated receptors, thereby inhibiting STAT activation [39]. The dysregulation of SOCS3 expression is associated with aberrant JAK/STAT signaling, contributing to the development and progression of several cancers [40]. SOCS3 functions as a tumor suppressor in many contexts, where its downregulation correlates with enhanced tumor growth, angiogenesis, and immune evasion [41]. SOCS3 also exerts broader regulatory effects on other oncogenic pathways, including PI3K/AKT and MAPK signaling, by interacting with receptor tyrosine kinases and downstream effectors. These interactions modulate cell proliferation, survival, and migration [39].
Taken together, our results support the idea that tumor-associated proteins should be sought not in exosomes secreted by cell lines but in the composition of blood exosomes from cancer patients, while the contribution of endotheliocyte exosomes to the total pool of blood exosomes can be neglected.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics15081028/s1, Table S1: Proteins identified in exosomes secreted by HUVECs; Table S2: Proteins identified in exosomes secreted by BT-474 cells; Table S3: Proteins identified in exosomes secreted by MCF-7 cells; Table S4: Exosomal proteins identified in the blood of luminal A BCPs; Table S5: Exosomal proteins identified in the blood of triple positive BCPs.
Author Contributions
Conceptualization, Y.T. and S.T.; methodology, A.S., L.Y., O.T., A.G. and N.Y.; formal analysis, A.S.; investigation, A.S., L.Y., K.P., O.T., A.G. and N.Y.; resources, Y.T.; data curation, S.T.; writing—original draft preparation, A.S., L.Y. and S.T.; writing—review and editing, Y.T. and S.T.; project administration, L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Russian Science Foundation, N23-25-00462, https://rscf.ru/project/23-25-00462/, accessed on 25 January 2023.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Local Ethics Committee of the Institute of Chemical Biology and Fundamental Medicine (the protocol N3 from 2 September 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Gao, H. The Role of the Tumor Microenvironment in Triple-Positive Breast Cancer Progression and Therapeutic Resistance. Cancers 2023, 15, 5493. [Google Scholar] [CrossRef] [PubMed]
- Shefer, A.; Yalovaya, A.; Tamkovich, S. Exosomes in breast cancer: Involvement in tumor dissemination and prospects for liquid biopsy. Int. J. Mol. Sci. 2022, 23, 8845. [Google Scholar] [CrossRef] [PubMed]
- Bandini, E.; Rossi, T.; Scarpi, E.; Gallerani, G.; Vannini, I.; Salvi, S.; Azzali, I.; Melloni, M.; Salucci, S.; Battistelli, M.; et al. Early Detection and Investigation of Extracellular Vesicles Biomarkers in Breast Cancer. Front. Mol. Biosci. 2021, 8, 732900. [Google Scholar] [CrossRef]
- Johnson, K.S.; Conant, E.F.; Soo, M.S. Molecular Subtypes of Breast Cancer: A Review for Breast Radiologists. J. Breast Imaging 2021, 3, 12–24. [Google Scholar] [CrossRef]
- Bilous, M. Breast core needle biopsy: Issues and controversies. Mod. Pathol. 2010, 23 (Suppl. S2), S36–S45. [Google Scholar] [CrossRef]
- Lee, Y.; Ni, J.; Wasinger, V.C.; Graham, P.; Li, Y. Comparison Study of Small Extracellular Vesicle Isolation Methods for Profiling Protein Biomarkers in Breast Cancer Liquid Biopsies. Int. J. Mol. Sci. 2023, 24, 15462. [Google Scholar] [CrossRef]
- Yunusova, N.V.; Tugutova, E.A.; Tamkovich, S.N.; Kondakova, I.V. The role of exosomal tetraspanins and proteases in tumor progression. Biochem. Suppl. Ser. B Biomed. Chem. 2018, 12, 191–202. [Google Scholar]
- Neagu, A.-N.; Whitham, D.; Bruno, P.; Morrissiey, H.; Darie, C.A.; Darie, C.C. Omics-Based Investigations of Breast Cancer. Molecules 2023, 28, 4768. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef]
- Gangoda, L.; Liem, M.; Ang, C.S.; Keerthikumar, S.; Adda, C.G.; Parker, B.S.; Mathivanan, S. Proteomic Profiling of Exosomes Secreted by Breast Cancer Cells with Varying Metastatic Potential. Proteomics 2017, 17, 1600370. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.O.; Laktionov, P.P.; Cherepanova, A.V.; Chernonosova, V.S.; Shevelev, G.Y.; Zaporozhchenko, I.A.; Karaskov, A.M.; Laktionov, P.P. General Study and Gene Expression Profiling of Endotheliocytes Cultivated on Electrospun Materials. Materials 2019, 12, 4082. [Google Scholar] [CrossRef] [PubMed]
- Cherepanova, A.V.; Bushuev, A.V.; Kharkova, M.V.; Vlassov, V.V.; Laktionov, P.P. DNA inhibits dsRNA-induced secretion of pro-inflammatory cytokines by gingival fibroblasts. Immunobiology 2013, 218, 272–280. [Google Scholar] [CrossRef]
- Tutanov, O.; Orlova, E.; Proskura, K.; Grigor’eva, A.; Yunusova, N.; Tsentalovich, Y.; Alexandrova, A.; Tamkovich, S. Proteomic Analysis of Blood Exosomes from Healthy Females and Breast Cancer Patients Reveals an Association between Different Exosomal Bioactivity on Non-tumorigenic Epithelial Cell and Breast Cancer Cell Migration In Vitro. Biomolecules 2020, 10, 495. [Google Scholar] [CrossRef]
- Tamkovich, S.; Tutanov, O.; Efimenko, A.; Grigor’eva, A.; Ryabchikova, E.; Kirushina, N.; Vlassov, V.; Tkachuk, V.; Laktionov, P. Blood Circulating Exosomes Contain Distinguishable Fractions of Free and Cell-Surface-Associated Vesicles. Curr. Mol. Med. 2019, 19, 273–285. [Google Scholar] [CrossRef]
- Tamkovich, S.N.; Yunusova, N.V.; Tugutova, E.; Somov, A.K.; Proskura, K.V.; Kolomiets, L.A.; Stakheeva, M.N.; Grigor’eva, A.E.; Laktionov, P.P.; Kondakova, I.V. Protease cargo in circulating exosomes of breast cancer and ovarian cancer patients. Asian Pac. J. Cancer Prev. 2019, 20, 255–262. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Lane, L.; Overall, C.M.; Bandeira, N.; Baker, M.S.; Pineau, C.; Moritz, R.L.; Corrales, F.; Orchard, S.; Van Eyk, J.E.; et al. Human Proteome Project Mass Spectrometry Data Interpretation Guidelines 3.0. J. Proteome Res. 2019, 18, 4108–4116. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles. 2024, 13, e12404. [Google Scholar] [CrossRef]
- Vesiclepedia Database. Available online: www.microvesicles.org (accessed on 30 November 2024).
- Tutanov, O.; Shtam, T.; Grigor’eva, A.; Tupikin, A.; Tsentalovich, Y.; Tamkovich, S. Blood Plasma Exosomes Contain Circulating DNA in Their Crown. Diagnostics 2022, 12, 854. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, C.; Shi, J.; Wen, K.; Wang, X. AIM2 Inhibits the Proliferation, Invasion and Migration, and Promotes the Apoptosis of Osteosarcoma Cells by Inactivating the PI3K/AKT/mTOR Signaling Pathway. Mol. Med. Rep. 2022, 25, 53. [Google Scholar] [CrossRef]
- Corona, R.I.; Seo, J.H.; Lin, X.; Hazelett, D.J.; Reddy, J.; Fonseca, M.A.S.; Abassi, F.; Lin, Y.G.; Mhawech-Fauceglia, P.Y.; Shah, S.P.; et al. Non-Coding Somatic Mutations Converge on the PAX8 Pathway in Ovarian Cancer. Nat. Commun. 2020, 11, 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, A.; Li, C.; Sun, J.; Yi, G.; Cheng, H.; Liu, X.; Wang, Z.; Zhou, Y.; Yao, G.; et al. Methylation-Induced Silencing of ALDH2 Facilitates Lung Adenocarcinoma Bone Metastasis by Activating the MAPK Pathway. Front. Oncol. 2020, 10, 1141. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Ma, X.; Meruvu, S.; Hugendubler, L.; Mueller, E. The Adipogenic Transcriptional Cofactor ZNF638 Interacts with Splicing Regulators and Influences Alternative Splicing. J. Lipid Res. 2014, 55, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.; Mohamed, B.M.; Lewis, F.; O’Toole, S.; O’Leary, J.J. Biomarkers in high grade serous ovarian cancer. Biochim. Biophys. Acta Rev. Cancer. 2024, 1879, 189224. [Google Scholar] [CrossRef]
- Bao, H.; Min, L.; Bu, F.; Wang, S.; Meng, J. Recent advances of liquid biopsy: Interdisciplinary strategies toward clinical decision-making. Interdiscip. Med. 2023, 1, e20230021. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomedicine 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Bastos-Amador, P.; Royo, F.; Gonzalez, E.; Conde-Vancells, J.; Palomo-Diez, L.; Borras, F.E.; Falcon-Perez, J.M. Proteomic analysis of microvesicles from plasma of healthy donors reveals high individual variability. J. Proteomics 2012, 75, 3574–3584. [Google Scholar] [CrossRef]
- Rontogianni, S.; Synadaki, E.; Li, B.; Liefaard, M.C.; Lips, E.H.; Wesseling, J.; Wu, W.; Altelaar, M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun. Biol. 2019, 2, 325. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef]
- Gerashchenko, T.S.; Zavyalova, M.V.; Denisov, E.V.; Krakhmal, N.V.; Pautova, D.N.; Litviakov, N.V.; Vtorushin, S.V.; Cherdyntseva, N.V.; Perelmuter, V.M. Intratumoral Morphological Heterogeneity of Breast Cancer as an Indicator of the Metastatic Potential and Tumor Chemosensitivity. Acta Naturae 2017, 9, 56–67. [Google Scholar] [CrossRef]
- Eguchi, T.; Sheta, M.; Fujii, M.; Calderwood, S.K. Cancer extracellular vesicles, tumoroid models, and tumor microenvironment. Semin. Cancer Biol. 2022, 86, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, L.; Barone, I.; Caruso, A.; Giordano, F.; Brindisi, M.; Morello, G.; Accattatis, F.M.; Panza, S.; Cappello, A.R.; Bonofiglio, D.; et al. Proteomic Profiling of Extracellular Vesicles Released by Leptin-Treated Breast Cancer Cells: A Potential Role in Cancer Metabolism. Int. J. Mol. Sci. 2022, 23, 12941. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Cheng, J.; Zhu, J.; Ji, S.; Gu, K.; Zhao, Y.; Yu, S.; Meng, Y. Using Weighted Gene Co-Expression Network Analysis to Identify Increased MND1 Expression as a Predictor of Poor Breast Cancer Survival. Int. J. Gen. Med. 2022, 15, 4959–4974. [Google Scholar] [CrossRef]
- Lin, F.; Xie, Y.J.; Zhang, X.K.; Huang, T.J.; Xu, H.F.; Mei, Y.; Liang, H.; Hu, H.; Lin, S.T.; Luo, F.F.; et al. GTSE1 is involved in breast cancer progression in p53 mutation-dependent manner. J. Exp. Clin. Cancer Res. 2019, 38, 152. [Google Scholar] [CrossRef]
- Ojala, V.K.; Knittle, A.M.; Kirjalainen, P.; Merilahti, J.A.M.; Kortesoja, M.; Tvorogov, D.; Vaparanta, K.; Lin, S.; Kast, J.; Pulliainen, A.T.; et al. The guanine nucleotide exchange factor VAV3 participates in ERBB4-mediated cancer cell migration. J. Biol. Chem. 2020, 295, 11559–11571. [Google Scholar] [CrossRef]
- Chang, Y.T.; Hong, Z.J.; Tsai, H.H.; Feng, A.C.; Huang, T.Y.; Yu, J.C.; Hsu, K.F.; Huang, C.C.; Lin, W.Z.; Chu, C.M.; et al. Hub metastatic gene signature and risk score of breast cancer patients with small tumor sizes using WGCNA. Breast Cancer 2024, 31, 1114–1129. [Google Scholar] [CrossRef]
- Thacker, G.; Henry, S.; Nandi, A.; Debnath, R.; Singh, S.; Nayak, A.; Susnik, B.; Boone, M.M.; Zhang, Q.; Kesmodel, S.B.; et al. Immature natural killer cells promote progression of triple-negative breast cancer. Sci. Transl. Med. 2023, 15, eabl4414. [Google Scholar] [CrossRef]
- Chu, J.; Hu, X.C.; Li, C.C.; Li, T.Y.; Fan, H.W.; Jiang, G.Q. KLF14 alleviated breast cancer invasion and M2 macrophages polarization through modulating SOCS3/RhoA/Rock/STAT3 signaling. Cell Signal 2022, 92, 110242. [Google Scholar] [CrossRef]
- Li, X.; Peng, B.; Li, J.; Tian, M.; He, L. Unleashing Breast Cancer Progression: miR-455-5p’s Targeting of SOCS3 Drives Proliferation, Migration, and Invasion. Protein Pept. Lett. 2023, 30, 992–1000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).