Association Between Left Ventricular Global Longitudinal Strain and Hepatic Inflammation and Fibrosis in Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
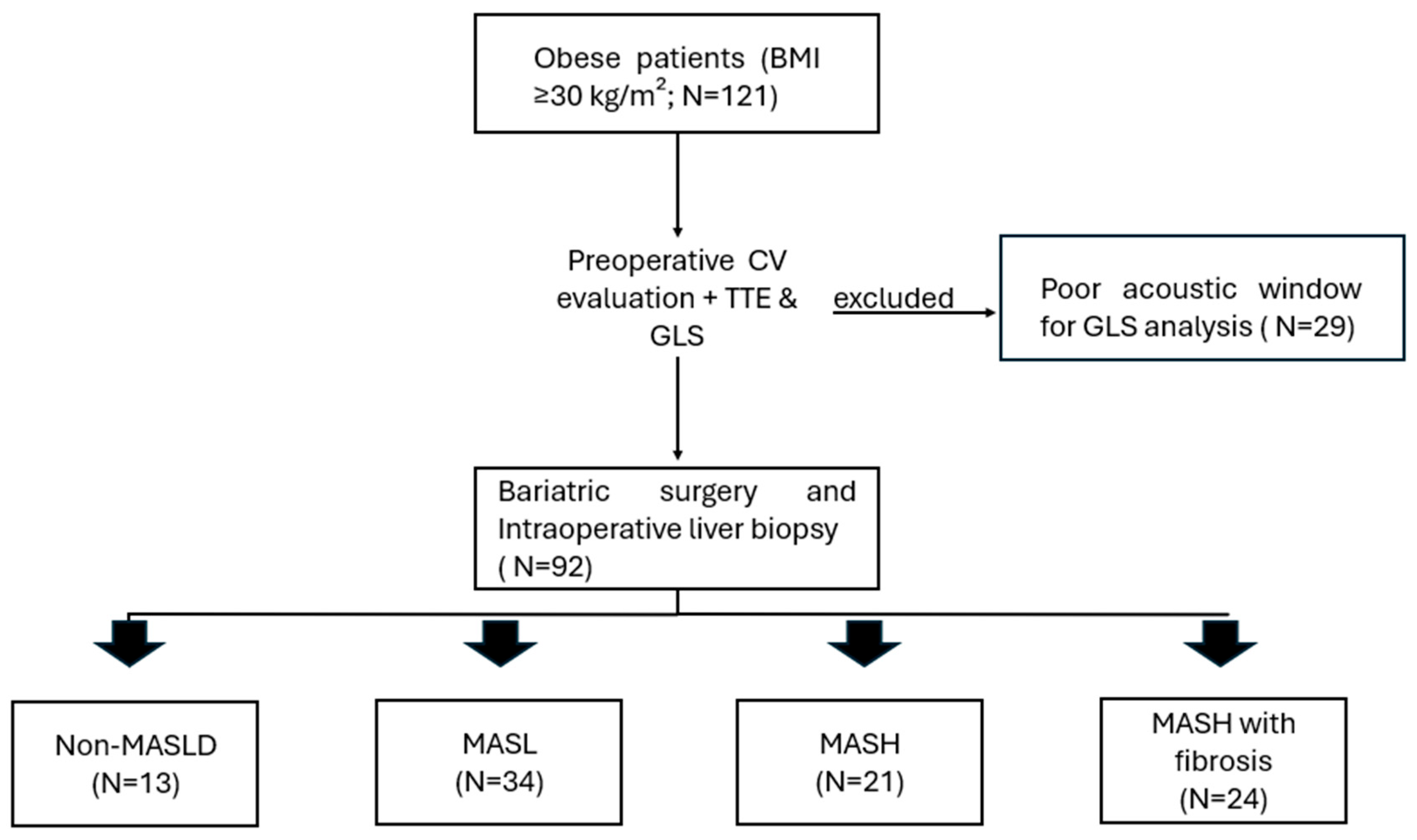
2.1. Study Design and Population
2.2. Data Collection
2.3. Cardiovascular Evaluation
2.4. Transthoracic Echocardiogram and Global Longitudinal Strain Analysis
2.5. Metabolic Dysfunction-Associated Steatotic Liver Disease Diagnosis
- -
- Group 1—Non-MASLD: Liver biopsy showing absence of steatosis.
- -
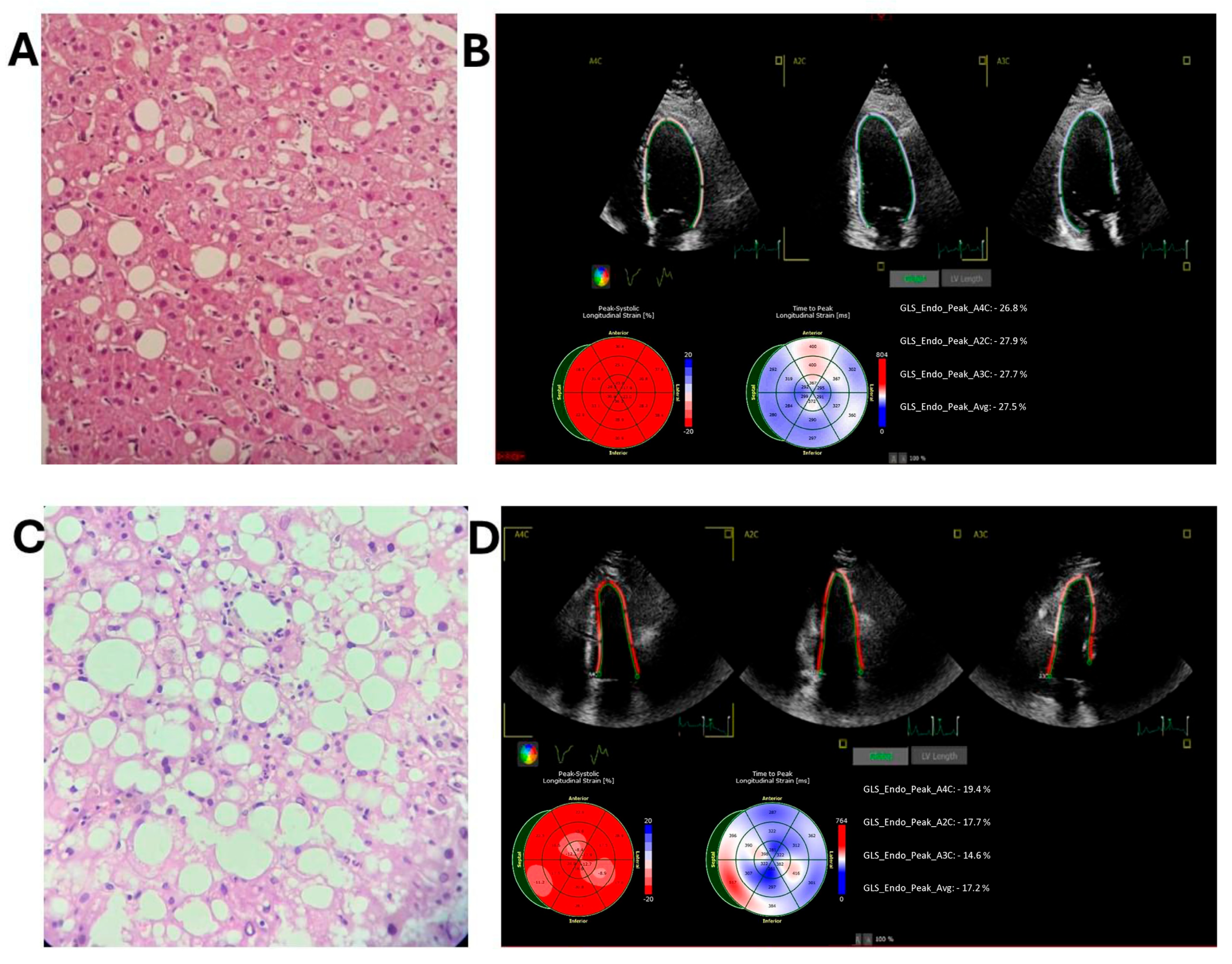
- Group 2—Metabolic dysfunction-associated steatotic liver (MASL) (Figure 2A): Liver biopsy demonstrating steatosis without signs of inflammation or fibrosis.
- -
- Group 3—Metabolic dysfunction-associated steatohepatitis (MASH) without fibrosis (Figure 2C): Liver biopsy indicating steatohepatitis without fibrosis;
- -
- Group 4—MASH with fibrosis: Liver biopsy demonstrating steatohepatitis with concurrent fibrosis.
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Cardiovascular Risk and Electrocardiogram Results
3.3. Echocardiographic Data and LV GLS Results
3.4. Discriminative Analysis and Mash Predictions:
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A2C | apical 2-chamber |
| A3C | apical 3-chamber |
| A4C | apical 4-chamber |
| ASCVD | Atherosclerotic Cardiovascular Disease Risk Score |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| AUC | the area under the ROC curve |
| AV block | atrioventricular block |
| BMI | body mass index |
| BP | blood pressure |
| CAD | coronary artery disease |
| CBC | complete blood count |
| CI | confidence interval |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| CVD | cardiovascular disease |
| CV | cardiovascular |
| EASD | European Association for the Study of Diabetes |
| EASL | European Association for the Study of the Liver |
| EASO | European Association for the Study of Obesity |
| EKG | Electrocardiograms |
| GLS | global longitudinal strain |
| HBa1c | Glycated hemoglobin |
| HDL | high-density lipoprotein |
| HF | heart failure |
| HFPEF | heart failure with preserved ejection fraction |
| HIV | human immunodeficiency virus |
| IVCD | intraventricular conduction delay |
| LAE | left atrial enlargement |
| LAFB | left anterosuperior fascicular block |
| LDL | low-density lipoprotein |
| LV | left ventricular |
| LVEDV | LV end-diastolic volume |
| LVEF | LV ejection fraction |
| LVESV | LV end-systolic volume |
| LV GLS | LV global longitudinal strain |
| LVH | left ventricular hypertrophy |
| MACE | major adverse cardiovascular events |
| MASH | metabolic dysfunction-associated steatohepatitis without fibrosis |
| MASL | metabolic dysfunction-associated steatotic liver |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| NAFLD | nonalcoholic fatty liver disease |
| Non-Masld | liver biopsy showing absence of steatosis |
| OR | odds-ratio |
| PREVENT | The Predicting Risk of Cardiovascular Disease Events calculator |
| PPLV | Posterior Wall of the LV |
| RAE | right atrial enlargement |
| RBBB | right bundle branch block |
| REDCap | Research Electronic Data Capture electronic system |
| RV | right ventricle |
| T2DM | type 2 diabetes mellitus |
| TC | total cholesterol |
| TG | triglycerides |
| TTE | transthoracic echocardiography |
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- EASL-EASD-EASO. Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhou, X.D.; Wu, S.J.; Hu, X.Q.; Tang, B.; Poucke, S.V.; Pan, X.Y.; Wu, W.J.; Gu, X.M.; Fu, S.W.; et al. Synergistic increase in cardiovascular risk in diabetes mellitus with nonalcoholic fatty liver disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 631–636. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Hagström, H.; Sundström, J.; Ludvigsson, J.F. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nationwide histology cohort. Gut 2022, 71, 1867–1875. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wai-Sun Wong, V.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quiñones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients with Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e17. [Google Scholar] [CrossRef]
- Yong, J.N.; Ng, C.H.; Lee, C.W.; Chan, Y.Y.; Tang, A.S.P.; Teng, M.; Tan, D.J.H.; Lim, W.H.; Quek, J.; Xiao, J.; et al. Non-alcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: A meta-analysis. Hepatol. Int. 2022, 16, 269–281. [Google Scholar] [CrossRef]
- Lim, W.H.; Chew, N.W.; Quek, J.; Ng, C.H.; Tan, D.J.H.; Xiao, J.; Nah, B.; Lee, G.H.; Huang, D.Q.; Tan, E.X.X.; et al. Echocardiographic assessment of cardiovascular function and clinical outcomes in liver transplant recipients. Clin. Transplant. 2022, 36, e14793. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Millán-Rodríguez, C.; Castelló, C.P.; Caballero-Valderrama, M.R.; Esquivias, G.B. Clinical Management of Non-alcoholic Steatohepatitis and the Role of the Cardiologist. Eur. Cardiol. 2023, 18, e64. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Oikonomidou, A.C.; Doundoulakis, I.; Antza, C.; Kalopitas, G.; Dardavessis, T.; Chourdakis, M. Evaluation of subclinical cardiac damage in biopsy-proven nonalcoholic fatty liver disease: A systematic review and meta-analysis. Ann. Gastroenterol. 2021, 34, 424. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Wilcox, J.E.; Ning, H.; Lewis, C.E.; Carr, J.J.; Rinella, M.E.; Shah, S.J.; Lima, J.A.C.; Lloyd-Jones, D.M. Longitudinal Association of Non-Alcoholic Fatty Liver Disease With Changes in Myocardial Structure and Function: The CARDIA Study. J. Am. Heart Assoc. 2020, 9, e014279. [Google Scholar] [CrossRef]
- Karabay, C.Y.; Kocabay, G.; Kalayci, A.; Colak, Y.; Oduncu, V.; Akgun, T.; Kalkan, S.; Guler, A.; Kirma, C. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: A speckle-tracking echocardiography study. Eur. J. Gastroenterol. Hepatol. 2014, 26, 325–331. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Cerini, F.; Nicolosi, G.L.; Lombardo, M.; Rumi, M.G.; Viganò, M. Left ventricular strain predicts subclinical atherosclerosis in nonadvanced nonalcoholic fatty liver disease patients. Eur. J. Gastroenterol. Hepatol. 2022, 34, 707–716. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, H.; Sun, L.; Wang, Y.; Li, Y.; Chang, W.; Li, G.; Huang, D. Assessment of left ventricular function in type 2 diabetes mellitus patients with non-alcoholic fatty liver disease using three-dimensional speckle-tracking echocardiography. Anatol. J. Cardiol. 2020, 23, 41–48. [Google Scholar] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [PubMed]
- Zeng, J.; Fan, J.G.; Francque, S.M. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United Eur. Gastroenterol. J. 2024, 12, 177–186. [Google Scholar] [CrossRef]
- Semlitsch, T.; Stigler, F.L.; Jeitler, K.; Horvath, K.; Siebenhofer, A. Management of overweight and obesity in primary care-A systematic overview of international evidence-based guidelines. Obes. Rev. 2019, 20, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.; Mancini, M.C.; de Melo, M.E.; Lamounier, R.N.; Moreira, R.O.; Carra, M.K.; Kyle, T.K.; Cercato, C.; Boguszewski, C.L. Proposal of an obesity classification based on weight history: An official document by the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society for the Study of Obesity and Metabolic Syndrome (ABESO). Arch. Endocrinol. Metab. 2022, 66, 139–151. [Google Scholar] [CrossRef]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.M.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Mion Júnior, D.; et al. Brazilian Guidelines of Hypertension—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef]
- Bertoluci, M.C.; Salles, J.E.N.; Silva-Nunes, J.; Pedrosa, H.C.; Moreira, R.O.; da Silva Duarte, R.M.C.; da Costa Carvalho, D.M.; Trujilho, F.R.; Dos Santos Raposo, J.F.C.; Parente, E.B.; et al. Portuguese-Brazilian evidence-based guideline on the management of hyperglycemia in type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2020, 12, 45. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inf. 2019, 95, 103208. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Grams, M.E.; Surapaneni, A.; Blaha, M.J.; Carson, A.P.; Chang, A.R.; Ciemins, E.; et al. Development and validation of the American Heart Association’s PREVENT equations. Circulation 2024, 149, 430–449. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. Circulation 2014, 129 (Suppl. S2), S49–S73. [Google Scholar] [CrossRef] [PubMed]
- Samesima, N.; God, E.G.; Kruse, J.C.L.; Leal, M.G.; Pinho, C.; França, F.F.A.C.; Pimenta, J.; Cardoso, A.F.; Paixão, A.; Fonseca, A.; et al. Brazilian Society of Cardiology Guidelines on the Analysis and Issuance of Electrocardiographic Reports—2022. Arq. Bras. Cardiol. 2022, 119, 638–680. [Google Scholar]
- D’Elia, N.; Caselli, S.; Kosmala, W.; Lancellotti, P.; Morris, D.; Muraru, D.; Takeuchi, M.; van den Bosch, A.; van Grootel, R.W.J.; Villarraga, H.; et al. Normal Global Longitudinal Strain: An Individual Patient Meta-Analysis. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 167–169. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathologic patterns and biopsy evaluation in clinical research. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2012. [Google Scholar]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; Tan, D.J.H.; Tang, A.S.P.; Tay, P.; Xiao, J.; et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 20–30. [Google Scholar] [CrossRef]
- Wong, R.J. Epidemiology of metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD). Metab. Target Organ Damage 2024, 4, 35. [Google Scholar] [CrossRef]
- Thiele, M.; Pose, E.; Juanola, A.; Mellinger, J.; Ginès, P. Population screening for cirrhosis. Hepatol. Commun. 2024, 8, e0512. [Google Scholar] [CrossRef] [PubMed]
- Maung, S.T.; Tanpowpong, N.; Satja, M.; Treeprasertsuk, S.; Chaiteerakij, R. MRI for hepatocellular carcinoma and the role of abbreviated MRI for surveillance of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2024, 39, 1969–1981. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef]
- Xu, J.; Dai, L.; Zhang, Y.; Wang, A.; Li, H.; Wang, Y.; Meng, X.; Wu, S.; Wang, Y. Severity of Nonalcoholic Fatty Liver Disease and Risk of Future Ischemic Stroke Events. Stroke 2021, 52, 103–110. [Google Scholar] [CrossRef]
- Laakso, M.; Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 2014, 10, 293–302. [Google Scholar] [CrossRef]
- Targher, G.; Valbusa, F.; Bonapace, S.; Bertolini, L.; Zenari, L.; Pichiri, I.; Mantovani, A.; Zoppini, G.; Bonora, E.; Barbieri, E.; et al. Association of nonalcoholic fatty liver disease with QTc interval in patients with type 2 diabetes. Nutrition, Metabolism and Cardiovascular Diseases 2014, 24, 663–669. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Long QT Syndrome: An Emerging Role for Inflammation and Immunity. Front. Cardiovasc. Med. 2015, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Rigolon, R.; Pichiri, I.; Bonapace, S.; Morani, G.; Zoppini, G.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease is associated with an increased risk of heart block in hospitalized patients with type 2 diabetes mellitus. PLoS ONE 2017, 12, e0185459. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Krieg, S.; Krieg, A.; Vaghiri, S.; Mohr, R.; Konrad, M.; Luedde, M.; Luedde, T.; Kostev, K.; Loosen, S.H. Non-Alcoholic Fatty Liver Disease (NAFLD) and risk of new-onset heart failure: A retrospective analysis of 173,966 patients. Clin. Res. Cardiol. 2023, 112, 1446–1453. [Google Scholar] [CrossRef]
- Goliopoulou, A.; Theofilis, P.; Oikonomou, E.; Anastasiou, A.; Pantelidis, P.; Gounaridi, M.I.; Zakynthinos, G.E.; Katsarou, O.; Kassi, E.; Lambadiari, V.; et al. Non-Alcoholic Fatty Liver Disease and Echocardiographic Parameters of Left Ventricular Diastolic Function: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 14292. [Google Scholar] [CrossRef]
- Almeida, A.L.C.; Melo, M.D.T.; Bihan, D.C.S.L.; Vieira, M.L.C.; Pena, J.L.B.; Del Castillo, J.M.; Abensur, H.; Hortegal, R.A.; Otto, M.E.B.; Piveta, R.B.; et al. Position Statement on the Use of Myocardial Strain in Cardiology Routines by the Brazilian Society of Cardiology’s Department of Cardiovascular Imaging—2023. Arq. Bras. Cardiol. 2023, 120, e20230646. [Google Scholar] [CrossRef]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef]
- Abou, R.; van der Bijl, P.; Bax, J.J.; Delgado, V. Global longitudinal strain: Clinical use and prognostic implications in contemporary practice. Heart 2020, 106, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Gherbesi, E.; Gianstefani, S.; Angeli, F.; Ryabenko, K.; Bergamaschi, L.; Armillotta, M.; Guerra, E.; Tuttolomondo, D.; Gaibazzi, N.; Squeri, A.; et al. Myocardial strain of the left ventricle by speckle tracking echocardiography: From physics to clinical practice. Echocardiography 2024, 41, e15753. [Google Scholar] [CrossRef]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.R.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 63, 447–456. [Google Scholar] [CrossRef]
- Xu, L.; Wang, N.; Chen, X.; Liang, Y.; Zhou, H.; Yan, J. Quantitative evaluation of myocardial layer-specific strain using two-dimensional speckle tracking echocardiography among young adults with essential hypertension in China. Medicine 2018, 97, e12448. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, T.; Biering-Sørensen, S.R.; Olsen, F.J.; Sengeløv, M.; Jørgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.S. Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbidity and mortality in a low-risk general population: The Copenhagen City Heart Study. Circ. Cardiovasc. Imaging 2017, 10, e005521. [Google Scholar] [CrossRef] [PubMed]
- Imbalzano, E.; Zito, C.; Carerj, S.; Oreto, G.; Mandraffino, G.; Cusmà-Piccione, M.; Di Bella, G.; Saitta, C.; Saitta, A. Left ventricular function in hypertension: New insight by speckle tracking echocardiography. Echocardiography 2011, 28, 649–657. [Google Scholar] [CrossRef]
- Tzortzis, S.; Ikonomidis, I.; Triantafyllidi, H.; Trivilou, P.; Pavlidis, G.; Katsanos, S.; Katogiannis, K.; Birba, D.; Thymis, J.; Makavos, G.; et al. Optimal Blood Pressure Control Improves Left Ventricular Torsional Deformation and Vascular Function in Newly Diagnosed Hypertensives: A 3-Year Follow-up Study. J. Cardiovasc. Transl. Res. 2020, 13, 814–825. [Google Scholar] [CrossRef]
- Silva, T.R.W.; Silva, R.L.; Martins, A.F.; Marques, J.L.B. Role of Strain in the Early Diagnosis of Diabetic Cardiomyopathy. Arq. Bras. Cardiol. Imagem Cardiovasc. 2022, 35, eabc293. [Google Scholar]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Angeli, F.; Paolisso, P.; Belmonte, M.; Raschi, E.; Di Dalmazi, G.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; et al. Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 2025, 270, 108861. [Google Scholar] [CrossRef]

| Variable | Total (n = 92) | Non-MASLD (n = 13) | MASL (n = 34) | MASH (n = 21) | MASH with Fibrosis (n = 24) | p |
|---|---|---|---|---|---|---|
| Clinical Data | ||||||
| Age, years | 38 (31–46) | 36 (33–43) | 38.5 (31–46) | 44 (36–55) | 35 (30–43) | 0.194 |
| Caucasian | 76 (82.6) | 9 (69.2) | 28 (82.4) | 16 (76.2) | 23 (95.8) | 0.190 |
| Male sex | 14 (15.2) | 0 | 4 (11.8) | 5 (23.8) | 5 (20.8) | 0.101 |
| Active smoking | 2 (2.2) | 0 | 0 | 2 (9.5) | 0 | 0.268 |
| Previous smoking | 13 (14.1) | 3 (23.1) | 5 (14.7) | 3 (14.3) | 2 (8.3) | 0.268 |
| Hypertension | 36 (39.1) | 6 (46.2) | 15 (44.1) | 5 (23.8) | 10 (41.7) | 0.430 |
| Untreated hypertension | 6 (6.5) | 0 | 3 (8.8) | 3 (14.3) | 0 | 0.077 |
| Dyslipidemia | 75 (81.5) | 8 (61.5) | 26 (76.4) | 21 (100) | 20 (83.3) | 0.576 |
| Untreated dyslipidemia | 66 (71.7) | 8 (61.5) | 23 (67.6) | 17 (81) | 18 (75) | 0.576 |
| Diabetes mellitus | 11 (12) | 1 (7.7) | 2 (5.9) | 1 (4.8) | 7 (29.2) | 0.044 |
| Antihypertensive use | 30 (32.6) | 0 a | 12 (35.3) | 8 (38.1) | 10 (41.7) a | 0.009 |
| ACEi/ARBs use | 25 (27.2) | 0 | 10 (29.4) | 6 (28.6) | 9 (37.5) | 0.023 |
| CCBs use | 5 (5.4) | 0 | 0 a | 0 | 5 (20.8) a | 0.003 |
| BMI, kg/m2 | 39 (37–44) | 42 (37.5–45) | 38 (37–45.25) | 41 (37.5–43.5) | 39 (37–43.75) | 0.710 |
| Systolic BP, mmHg | 120 (110–130) | 110 (110–129) | 120 (111.5–130) | 125 (120–130) | 120 (112.5–137.5) | 0.262 |
| Diastolic BP, mmHg | 75 (70–80) | 70 (60–77.5) a,b | 80 (70–71.5) b,c,d | 70 (60–70) c,d | 80 (70–80) a,c | 0.012 |
| CKD-EPI, mL/min 1.73 m2 | 110.5 (99.2–118) | 111 (101–114) | 111.5 (98.7–118.2) | 99 (88–113.5) a | 114.5 (107.2–121.2) a | 0.035 |
| Glucose, mg/dL | 93 (84–104.7) | 85 (79–96) | 91.5 (82.7–104.2) | 94 (87.5–109) | 94 (84.7–109.7) | 0.225 |
| HbA1c, % | 5.5 (5.2–5.9) | 5.3 (5.15–5.8) | 5.5 (5.3–5.8) | 5.3 (5.0–5.9) | 5.6 (5.3–6.0) | 0.651 |
| Total cholesterol, mg/dL | 190 (165.2–211.5) | 164 (158.5–194.5) | 191 (165.7–210.5) | 196 (163–217.5) | 190 (177–216.7) | 0.274 |
| HDL, mg/dL | 45.5 (38–53) | 49 (36.5–60.5) | 48.5 (38.7–57) | 42 (39.5–49) | 44.5 (36.2–51.7) | 0.375 |
| LDL, mg/dL | 114 (90.5–130) | 102 (88.5–108.6) | 116 (96.7–132) | 118 (90.2–134.5) | 113.5 (79.2–128) | 0.340 |
| Triglycerides, mg/dL | 125 (97.2–177) | 99 (78.5–152.5) | 122.5 (87.5–166) | 134 (107.5–200) | 140 (106.5–210) | 0.183 |
| AST, mg/dL | 23 (19–28) | 21 (20–26) | 23 (19–28) | 24 (16.5–28) | 23.5 (21–30.5) | 0.314 |
| ALT, mg/dL | 27 (21–36) | 22 (17–31) a | 24 (20.7–31) b | 27 (18.5–44.5) c | 33 (27–46.7) a,b,c | 0.011 |
| Hematocrit, % | 40.65 (38.8–42.4) | 39.8 (38.4–41.8) b | 39.7 (38.5–41.5) a | 43.1 (40.1–46.1) a,b | 40.95 (38.8–42.7) | 0.022 |
| Hemoglobin, mg/dL | 13.75 (13.1–14.2) | 13.9 (12.9–14.1) | 13.5 (12.9–13.8) a | 14 (13.6–15.6) a | 13.75 (13.1–14.3) | 0.017 |
| Normal abdominal ultrasound | 21 (22.8) | 9 (69.2) a,b,c | 10 (29.4)b | 2 (9.5) c | 0 a | <0.001 |
| Risk Score | Total (n = 92) | Non-MASLD (n = 13) | MASL (n = 34) | MASH (n = 21) | MASH with Fibrosis (n = 24) | p |
|---|---|---|---|---|---|---|
| ASCVD, % | 1.1 (0.5–2.1) | 0.4 (0.3–1.1) a,b,c | 0.85 (0.5–1.5) | 1.7 (0.9–2.8) b,c | 1.4 (0.7–2.6) a | 0.002 |
| Low (<5%) | 86 (93) | 11 (84.6) | 33 (97.1) | 19 (90.5) | 23 (95.8) | 0.491 |
| Intermediate (7.5–19.9%) | 3 (3.3) | 1 (7.7) | 1 (2.9) | 1 (4.8) | 0 | |
| High Risk (≥20%) | 0 | 0 | 0 | 0 | 0 | |
| Framingham, % | 2.9 (1.7–5.0) | 1.5 (1.1–2.3) a,b | 2.9 (1.8–4.5) | 4.5 (2–7.5) b | 3.4 (1.9–5.6) a | 0.011 |
| Low (<10%) | 85 (92.4) | 12 (92.3) | 32 (94.1) | 20 (95.2) | 21 (87.5) | 0.333 |
| Intermediate (10–20%) | 6 (6.5) | 0 | 2 (5.9) | 1 (4.8) | 3 (12.5) | |
| High Risk (>20%) | 1 (1.1) | 1 (7.7) | 0 | 0 | 0 | |
| PREVENT, % | 0.8 (0.4–1.5) | 0.3 (0.3–0.8) a,b | 0.8 (0.3–1) b | 1.3 (0.6–2.2) c | 1.15 (0.4–1.8) a,c | 0.021 |
| Low (<5%) | 89 (96.7) | 12 (92.3) | 32 (94.1) | 21 (100) | 24 (100) | 0.516 |
| Intermediate (7.5–19.9%) | 1 (1) | 0 | 1 (2.9) | 0 | 0 | |
| High Risk (≥20%) | 0 | 0 | 0 | 0 | 0 |
| Variable | Total (n = 92) | Non-MASLD (n = 13) | MASLD (n = 34) | MASH (n = 21) | MASH with Fibrosis (n = 24) | p |
|---|---|---|---|---|---|---|
| Sinus Rhythm | 92 (100) | - | - | - | - | - |
| Heart Rate (HR) | 75 (68–85) | 79 (68.5–87.5) | 73.5 (60–82.5) | 75 (68.5–83.5) | 76.5 (70.25–85.75) | 0.410 |
| Intraventricular Blocks | 44 (47.8) | 3 (23.1) | 21 (61.8) | 8 (38.1) | 12 (50.0) | 0.083 |
| Left Anterosuperior Fascicular Block (LAFB) | 2 (2.2) | 0 | 1 (2.9) | 0 | 1 (4.2) | 0.586 |
| Right Bundle-Branch Block (RBBB) | 1 (1.1) | 0 | 0 | 0 | 1 (4.2) | 0.437 |
| First-Degree AV Block | 1 (1.1) | 0 | 1 (2.9) | 0 | 0 | 0.570 |
| Intraventricular Conduction Delay (IVCD) | 41 (44.6) | 3 (23.1) | 20 (58.8) | 8 (38.1) | 10 (41.7) | 0.129 |
| Supraventricular Ectopic Beats | 1 (1.1) | 0 | 0 | 1 (4.8) | 0 | 0.393 |
| Ventricular Ectopic Beats | 2 (2.2) | 1 (7.7) | 0 | 1 (4.8) | 0 | 0.243 |
| LAE | 26 (28.3) | 2 (15.4) | 13 (38.2) | 5 (23.8) | 6 (25.0) | 0.375 |
| RAE | 1 (1.1) | 0 | 0 | 1 (4.8) | 0 | 0.393 |
| LVH | 1 (1.1) | 0 | 1 (2.9) | 0 | 0 | 0.570 |
| QT Interval (corrected)—Bazzett, ms | 423 (403.5–446.75) | 438 (414.5–464) | 422.5 (400.75–443) | 426 (407.5–446) | 412 (402.75–452.25) | 0.295 |
| QT Interval (corrected)—Fredericia, ms | 404 (389–427.75) | 423 (396.5–449) | 403 (388.25–429) | 409 (391.5–423) | 399.5 (382–428.5) | 0.260 |
| QT Interval (corrected)—Hodges, ms | 405 (390.25–425) | 419 (395.5–444.5) | 406 (389.75–428) | 407 (392–420) | 397 (384–424.75) | 0.271 |
| Prolonged QT Interval | 5 (5.4) | 0 | 3 (8.8) | 1 (4.8) | 1 (4.2) | 0.532 |
| Variable | Total (n = 92) | Non-MASLD (n = 13) | MASL (n = 34) | MASH (n = 21) | MASH with Fibrosis (n = 24) | p |
|---|---|---|---|---|---|---|
| Indexed Aortic Diameter, mm/m2 | 14 (13–16) | 14 (12.5–15.5) | 14 (13–16.2) | 14 (13–16) | 14 (13–15) | 0.314 |
| Indexed Left Atrial Volume, mL/m2 | 28 (25–32) | 31 (28–33) | 28 (25–32) | 27.5 (23.2–31.7) | 28 (24–30.7) | 0.271 |
| Right Ventricle Diameter, mm | 26 (24–27) | 26 (23.5–27) | 25 (23.7–27) | 26 (24–28) | 25 (23.2–28) | 0.682 |
| Left Ventricle Diastolic Diameter, mm | 50 (47–52) | 50 (47.5–51.5) | 52 (47–52) | 49 (46–51.5) | 50.5 (48–52.7) | 0.574 |
| Left Ventricle Systolic Diameter, mm | 31 (29–33) | 31 (28–33) | 32 (28.7–33.2) | 30 (29–33) | 31 (30–34.5) | 0.614 |
| Septal Thickness, mm | 9 (8–9) | 8 (7.5–9) a,b | 8 (8–9) | 9 (8–10) a | 9 (8–10) b | 0.040 |
| PPLV Wall, mm | 8 (8–9) | 8 (7.5–8) a,b | 8 (8–9) | 8 (8–9.5) a | 9 (8–9) b | 0.017 |
| Relative Thickness of LV Wall | 0.33 (0.31–0.36) | 0.31 (0.3–0.34) | 0.33 (0.3–0.36) | 0.34 (0.32–0.37) | 0.35 (0.32–0.37) | 0.044 |
| Indexed LV Diastolic Volume, mL/m2 | 55 (47.2–61) | 55 (48–59) | 57 (47.5–64.2) | 53 (47.5–57.5) | 55 (47–60.7) | 0.443 |
| Indexed LV Systolic Volume, mL/m2 | 18 (14.2–20.7) | 17 (14–20.5) | 19 (16–21.5) | 17 (14–18.5) | 17.5 (15–20.5) | 0.459 |
| Indexed LV Mass, g/m2 | 67 (59.2–76) | 62 (57–68) | 70 (59.7–76.2) | 66 (57–78) | 70 (60.2–75.7) | 0.241 |
| Ejection Fraction (%) | 66 (64–69.6) | 66 (65–67.5) | 66 (64–70.2) | 69 (64–71) | 66 (64.2–69) | 0.662 |
| Diastolic Dysfunction, n (%) | 4 (4.3) | 0 | 1 (2.9) | 1 (4.8) | 2 (8.3) | 0.557 |
| Systolic Dysfunction, n (%) | 1 (1.1) | 0 | 0 | 0 | 1 (4.3) | 0.426 |
| Global Longitudinal Strain (GLS) | Total (n = 92) | Non-MASLD (n = 13) | MASL (n = 34) | MASH (n = 21) | MASH with Fibrosis (n = 24) | p |
|---|---|---|---|---|---|---|
| GLS, % | 23.4 (22.2–25.1) | 24.2 (22.8–25.2) a | 24.2 (22.7–25.4) b | 23.1 (22.2–25.5) | 22.4 (20.9–23.2) a,b | 0.011 |
| GLS ≤ 18% | 2 (2.2%) | 0 | 1 (2.9%) | 1 (4.8%) | 0 | 0.531 |
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% (CI) | 95% (CI) | |||||||
| OR | Lower | Upper | p | OR | Lower | Upper | p | |
| LV GLS, % | 0.757 | 0.614 | 0.932 | 0.009 | 0.784 | 0.637 | 0.965 | 0.022 |
| HDL, mg/dL | 0.962 | 0.930 | 0.996 | 0.028 | 0.969 | 0.934 | 1.006 | 0.099 |
| LV mass, g/m2 | 1.015 | 1.000 | 1.030 | 0.044 | 1.011 | 0.995 | 1.028 | 0.164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hüning, A.R.; Egypto Rosa, V.E.; Piardi, D.S.; Assis, D.V.R.; Ferreira, T.V.; Griseli, L.; Moreira, F.C.; Carli, L.A.D.; Hartmann, C.R.; Coral, G.P.; et al. Association Between Left Ventricular Global Longitudinal Strain and Hepatic Inflammation and Fibrosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Diagnostics 2025, 15, 3007. https://doi.org/10.3390/diagnostics15233007
Hüning AR, Egypto Rosa VE, Piardi DS, Assis DVR, Ferreira TV, Griseli L, Moreira FC, Carli LAD, Hartmann CR, Coral GP, et al. Association Between Left Ventricular Global Longitudinal Strain and Hepatic Inflammation and Fibrosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Diagnostics. 2025; 15(23):3007. https://doi.org/10.3390/diagnostics15233007
Chicago/Turabian StyleHüning, Alberto Rodolpho, Vitor Emer Egypto Rosa, Diogo Silva Piardi, Daniara Viegas Rebelo Assis, Tainá Vanes Ferreira, Leonardo Griseli, Fabio Cañellas Moreira, Luiz Alberto De Carli, Carolina Rigatti Hartmann, Gabriela Perdomo Coral, and et al. 2025. "Association Between Left Ventricular Global Longitudinal Strain and Hepatic Inflammation and Fibrosis in Metabolic Dysfunction-Associated Steatotic Liver Disease" Diagnostics 15, no. 23: 3007. https://doi.org/10.3390/diagnostics15233007
APA StyleHüning, A. R., Egypto Rosa, V. E., Piardi, D. S., Assis, D. V. R., Ferreira, T. V., Griseli, L., Moreira, F. C., Carli, L. A. D., Hartmann, C. R., Coral, G. P., Sampaio, R. O., Vieira, M. L. C., Tarasoutchi, F., Leães, P. E., & Mattos, A. A. D. (2025). Association Between Left Ventricular Global Longitudinal Strain and Hepatic Inflammation and Fibrosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Diagnostics, 15(23), 3007. https://doi.org/10.3390/diagnostics15233007






