The Contribution of Sleep Texture in the Characterization of Sleep Apnea
Abstract
1. Introduction
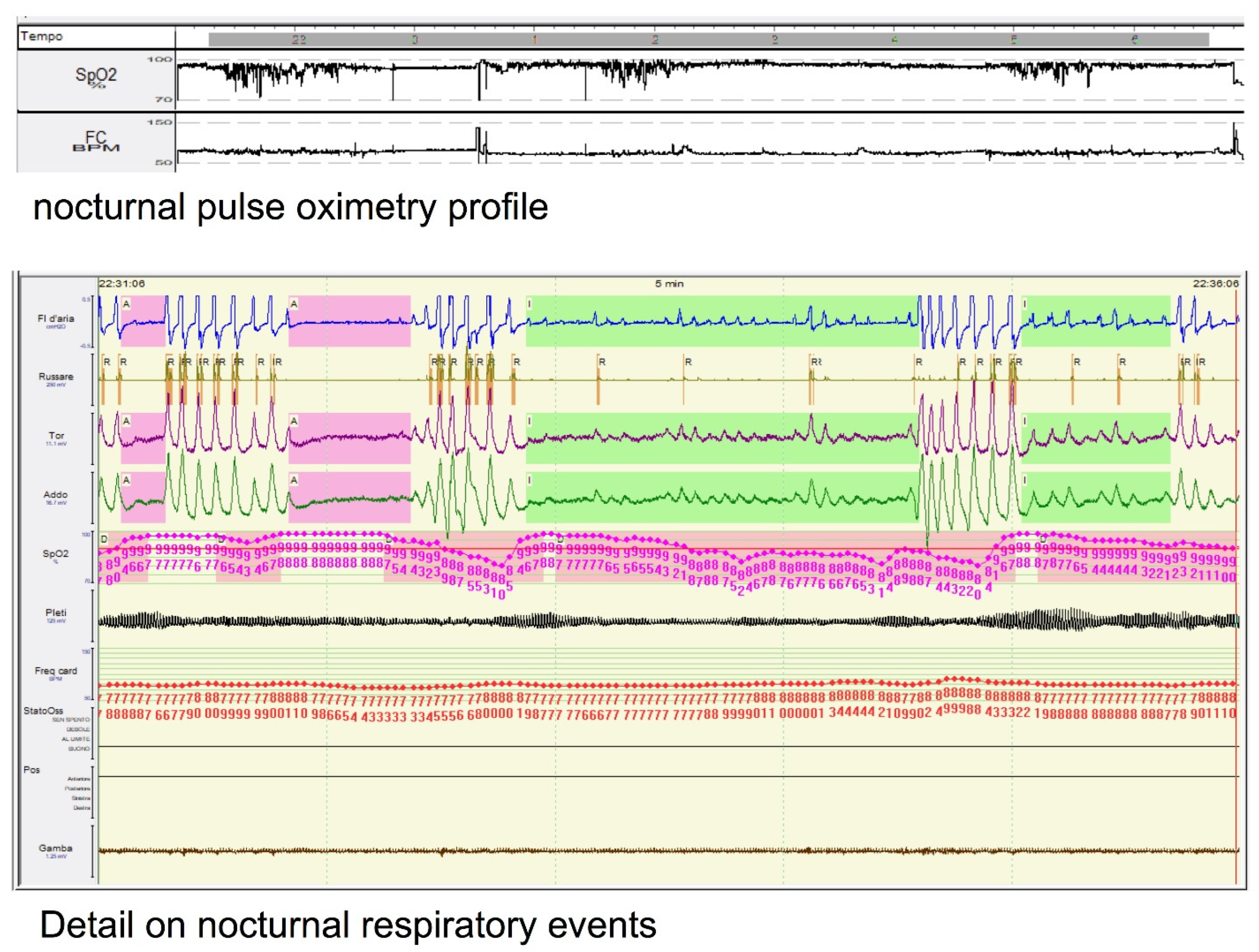
2. The Limits of AHI
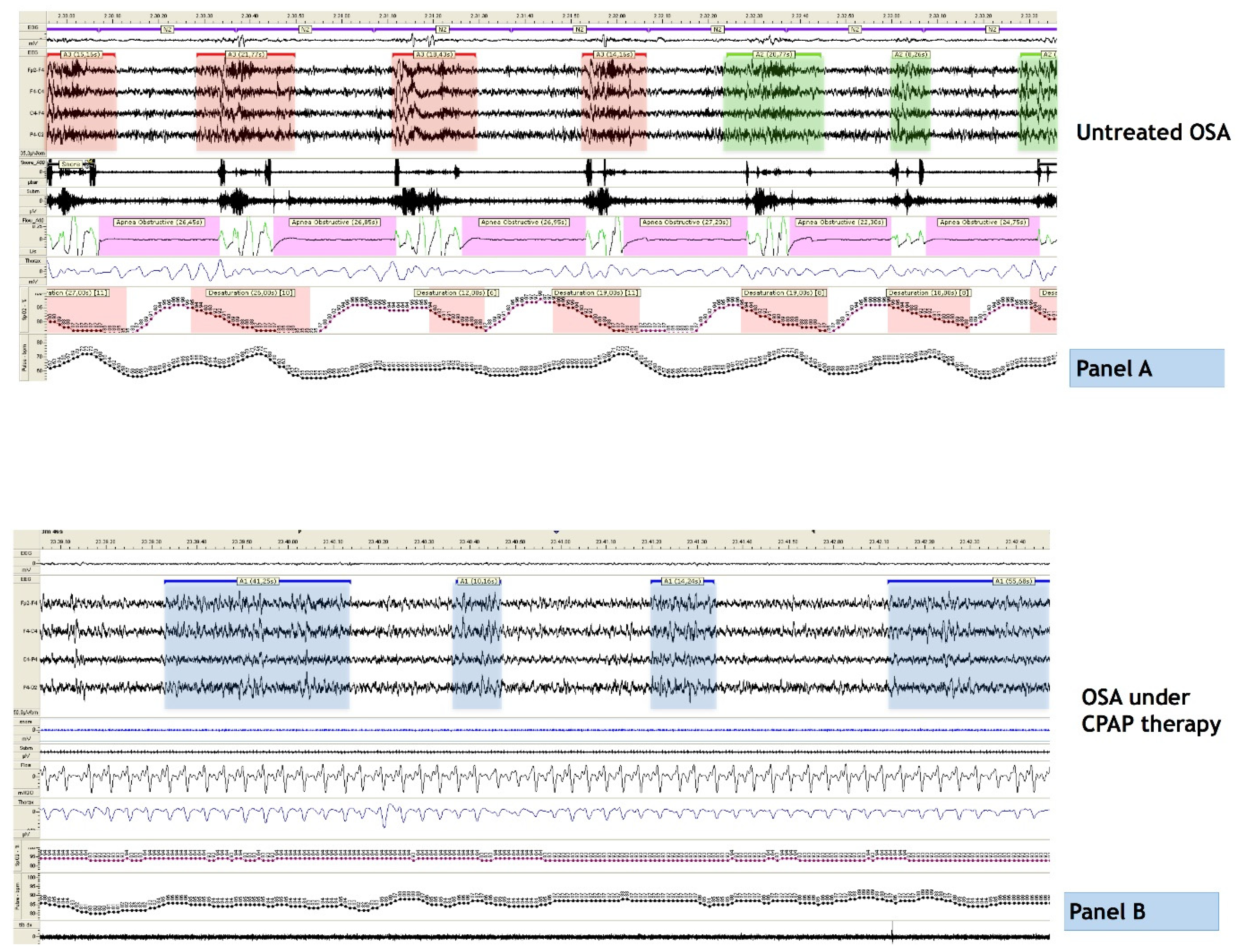
3. OSA and Sleep Architecture
4. Breathing Oscillations, Daytime Sleepiness and Treatment Outcomes
5. The Contribution of Machine Learning
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-L.; Chen, Y.-T.; Wang, K.-L.; Su, V.Y.-F.; Wu, L.-A.; Perng, D.-W.; Chang, S.-C.; Chen, Y.-M.; Chen, T.-J.; Chou, K.-T. Comorbidities and risk of mortality in patients with sleep apnea. Ann. Med. 2017, 49, 377–383. [Google Scholar] [CrossRef]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yu, W.; Wang, Z.; Huang, Z. Association between Arousals during Sleep and Subclinical Coronary Atherosclerosis in Patients with Obstructive Sleep Apnea. Brain Sci. 2022, 12, 1362. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Takamatsu, Y. Cheyne-Stokes Breathing as a Predictive Indicator of Heart Failure in Patients with Obstructive Sleep Apnea; A Retrospective Case Control Study Using Continuous Positive Airway Pressure Remote Monitoring Data. Front. Cardiovasc. Med. 2022, 9, 790331. [Google Scholar] [CrossRef]
- Silva, G.E.; Rojo-Wissar, D.M.; Quan, S.F.; Haynes, P.L. Predictive ability of the International Classification of Sleep Disorders-3 in identifying risk of obstructive sleep apnea among recently unemployed adults. Sleep Breath. 2021, 25, 1325–1334. [Google Scholar] [CrossRef]
- Wojeck, B.S.; Inzucchi, S.E.; Qin, L.; Yaggi, H.K. Polysomnographic predictors of incident diabetes and pre-diabetes: An analysis of the DREAM study. J. Clin. Sleep Med. 2023, 19, 703–710. [Google Scholar] [CrossRef]
- Arnardottir, E.S.; Islind, A.S.; Óskarsdóttir, M.; Ólafsdóttir, K.A.; August, E.; Jónasdóttir, L.; Hrubos-Strøm, H.; Saavedra, J.M.; Grote, L.; Hedner, J.; et al. The Sleep Revolution project: The concept and objectives. J. Sleep Res. 2022, 31, e13630. [Google Scholar] [CrossRef]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages in Human Subjects; National Institutes of Health Publications, Ed.; U.S. Government Printing Office: Washington, DC, USA, 1968.
- Wu, B.; Cai, J.; Yao, Y.; Pan, Y.; Pan, L.; Zhang, L.; Sun, Y. Relationship between sleep architecture and severity of obstructive sleep apnea. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020, 49, 455–461. (In Chinese) [Google Scholar] [CrossRef]
- Guo, D.; Peng, H.; Feng, Y.; Li, D.; Xu, T.; Li, T.; Liao, S. Effects of obstructive sleep apnea-hypopnea syndrome and age on sleep architecture. Nan Fang Yi Ke Da Xue Xue Bao 2015, 35, 922–926. (In Chinese) [Google Scholar]
- Terzano, M.G.; Parrino, L.; Spaggiari, M.C. The cyclic alternating pattern sequences in the dynamic organization of sleep. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Halász, P.; Bodizs, R.; Parrino, L.; Terzano, M. Two features of sleep slow waves: Homeostatic and reactive aspects—From long term to instant sleep homeostasis. Sleep Med. 2014, 15, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Parrino, L.; Ferri, R.; Bruni, O.; Terzano, M.G. Cyclic alternating pattern (CAP): The marker of sleep instability. Sleep Med. Rev. 2012, 16, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Parrino, L.; Grassi, A.; Milioli, G. Cyclic alternating pattern in polysomnography: What is it and what does it mean? Curr. Opin. Pulm. Med. 2014, 20, 533–541. [Google Scholar] [CrossRef]
- Terzano, M.G.; Parrino, L.; Sherieri, A.; Chervin, R.; Chokroverty, S.; Guilleminault, C.; Hirshkowitz, M.; Mahowald, M.; Moldofsky, H.; Rosa, A.; et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2001, 2, 537–553. [Google Scholar] [CrossRef]
- Hartmann, S.; Ferri, R.; Bruni, O.; Baumert, M. Causality of cortical and cardiovascular activity during cyclic alternating pattern in non-rapid eye movement sleep. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2021, 379, 20200248. [Google Scholar] [CrossRef] [PubMed]
- Mutti, C.; Azzi, N.; Halasz, P.; Szucs, A.; Parrino, L. Intra period CAP kinetics to stressful perturbation: A message from obstructive sleep apnea. Sleep Med. 2021, 80, 226–227. [Google Scholar] [CrossRef]
- Bosi, M.; Grassi, A.; Milioli, G.; Riccardi, S.; Terzano, M.G.; Cortelli, P.; Poletti, V.; Parrino, L. Can Sleep microstructure improve diagnosis of OSAS? Integrative information from CAP parameters. Arch. Ital. Biol. 2015, 153, 194–203. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Lim, D. Reply to Dr. Gold’s commentary con: Sleep fragmentation causes hypersomnolence in OSA. Sleep Med. Rev. 2021, 55, 101398. [Google Scholar] [CrossRef]
- Gnoni, V.; Drakatos, P.; Higgins, S.; Duncan, I.; Wasserman, D.; Kabiljo, R.; Mutti, C.; Halasz, P.; Goadsby, P.J.; Leschziner, G.D.; et al. Cyclic alternating pattern in obstructive sleep apnea: A preliminary study. J. Sleep Res. 2021, 30, e13350. [Google Scholar] [CrossRef]
- Exar, E.N.; Collop, N.A. The Upper Airway Resistance Syndrome. Chest 1999, 115, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Lopes, M.C.; Hagen, C.C.; Rosa, A. The Cyclic Alternating Pattern Demonstrates Increased Sleep Instability and Correlates with Fatigue and Sleepiness in Adults with Upper Airway Resistance Syndrome. Sleep 2007, 30, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Anaclet, C.; Ferrari, L.; Arrigoni, E.; Bass, C.E.; Saper, C.B.; Lu, J.; Fuller, P.M. The GABAergic parafacial zone is a medullary slow wave sleep–promoting center. Nat. Neurosci. 2014, 17, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Saper, C.B. Neural circuitry underlying waking up to hypercapnia. Front. Neurosci. 2019, 13, 401. [Google Scholar] [CrossRef]
- Vakulin, A.; Catcheside, P.; Baulk, S.D.; Antic, N.A.; Banks, S.; Dorrian, J.; McEvoy, D. Individual Variability and Predictors of Driving Simulator Impairment in Patients with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2014, 10, 647–655. [Google Scholar] [CrossRef]
- Parekh, A.; Mullins, A.E.; Kam, K.; Varga, A.W.; Rapoport, D.M.; Ayappa, I. Slow-wave activity surrounding stage N2 K-complexes and daytime function measured by psychomotor vigilance test in obstructive sleep apnea. Sleep 2019, 42, zsy256. [Google Scholar] [CrossRef]
- Parekh, A.; Kam, K.; Mullins, A.E.; Castillo, B.; Berkalieva, A.; Mazumdar, M.; Varga, A.W.; Eckert, D.J.; Rapoport, D.M.; Ayappa, I. Altered K-complex morphology during sustained inspiratory airflow limitation is associated with next-day lapses in vigilance in obstructive sleep apnea. Sleep 2021, 44, zsab010. [Google Scholar] [CrossRef]
- Younes, M.; Ostrowski, M.; Soiferman, M.; Younes, H.; Younes, M.; Raneri, J.; Hanly, P. Odds Ratio Product of Sleep EEG as a Continuous Measure of Sleep State. Sleep 2015, 38, 641–654. [Google Scholar] [CrossRef]
- Younes, M.; Azarbarzin, A.; Reid, M.; Mazzotti, D.R.; Redline, S. Characteristics and reproducibility of novel sleep EEG biomarkers and their variation with sleep apnea and insomnia in a large community-based cohort. Sleep 2021, 44, zsab145. [Google Scholar] [CrossRef]
- Younes, M.; Hanly, P.J. Immediate postarousal sleep dynamics: An important determinant of sleep stability in obstructive sleep apnea. J. Appl. Physiol. 2016, 120, 801–808. [Google Scholar] [CrossRef]
- Bosi, M.; Milioli, G.; Riccardi, S.; Melpignano, A.; Vaudano, A.E.; Cortelli, P.; Poletti, V.; Parrino, L. Arousal responses to respiratory events during sleep: The role of pulse wave amplitude. J. Sleep Res. 2018, 27, 259–267. [Google Scholar] [CrossRef]
- Parrino, L.; Rausa, F.; Azzi, N.; Pollara, I.; Mutti, C. Cyclic alternating patterns and arousals: What is relevant in obstructive sleep apnea? In Memoriam Mario Giovanni Terzano. Curr. Opin. Pulm. Med. 2021, 27, 496–504. [Google Scholar] [CrossRef]
- Terzano, M.G.; Parrino, L.; Boselli, M.; Spaggiari, M.C.; Di Giovanni, G. Polysomnographic Analysis of Arousal Responses in Obstructive Sleep Apnea Syndrome by Means of the Cyclic Alternating Pattern. J. Clin. Neurophysiol. 1996, 13, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Zitting, K.-M.; Lockyer, B.B.J.; Azarbarzin, A.; Sands, S.A.; Wang, W.; Wellman, A.; Quan, S.F. Association of cortical arousals with sleep-disordered breathing events. J. Clin. Sleep Med. 2023, 19, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Bilecenoglu, N.T.; Aksu, M.; Yoldas, T.K. Cyclic Alternating Pattern in Obstructive Sleep Apnea Patients with versus without Excessive Sleepiness. Sleep Disord. 2018, 2018, 8713409. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Q.; Zou, X.; Zhong, Z.; Ouyang, Q.; Wang, M.; Luo, Y.; Yao, D. Effects of CPAP Treatment on Electroencephalographic Activity in Patients with Obstructive Sleep Apnea Syndrome during Deep Sleep with Consideration of Cyclic Alternating Pattern. Nat. Sci. Sleep 2022, 14, 2075–2089. [Google Scholar] [CrossRef]
- Parrino, L.; Thomas, R.J.; Smerieri, A.; Spaggiari, M.C.; Del Felice, A.; Terzano, M.G. Reorganization of sleep patterns in severe OSAS under prolonged CPAP treatment. Clin. Neurophysiol. 2005, 116, 2228–2239. [Google Scholar] [CrossRef]
- Parrino, L.; Smerieri, A.; Boselli, M.; Spaggiari, M.C.; Terzano, M.G. Sleep reactivity during acute nasal CPAP in obstructive sleep apnea syndrome. Neurology 2000, 54, 1633–1640. [Google Scholar] [CrossRef]
- Gnoni, V.; Mesquita, M.; O’Regan, D.; Delogu, A.; Chakalov, I.; Antal, A.; Young, A.H.; Bucks, R.S.; Jackson, M.L.; Rosenzweig, I. Distinct cognitive changes in male patients with obstructive sleep apnoea without co-morbidities. Front. Sleep 2023, 2, 1097946. [Google Scholar] [CrossRef]
- Gu, Y.; Gagnon, J.; Kaminska, M. Sleep electroencephalography biomarkers of cognition in obstructive sleep apnea. J. Sleep Res. 2023; ahead of print. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Wang, D.; Wang, Q.; Han, F.; Jyothi, K.; Chen, R. Correlation of sleep microstructure with daytime sleepiness and cognitive function in young and middle-aged adults with obstructive sleep apnea syndrome. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 3525–3532. [Google Scholar] [CrossRef]
- Karimzadeh, F.; Nami, M.; Boostani, R. Sleep microstructure dynamics and neurocognitive performance in obstructive sleep apnea syndrome patients. J. Integr. Neurosci. 2017, 16, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Miano, S.; Castelnovo, A.; Bruni, O.; Manconi, M. Sleep microstructure in attention deficit hyperactivity disorder according to the underlying sleep phenotypes. J. Sleep Res. 2021, 31, e13426, Erratum in J. Sleep Res. 2023, 32, e13780. [Google Scholar] [CrossRef] [PubMed]
- Miano, S.; Bruni, O.; Elia, M.; Scifo, L.; Smerieri, A.; Trovato, A.; Verrillo, E.; Terzano, M.G.; Ferri, R. Sleep phenotypes of intellectual disability: A polysomnographic evaluation in subjects with Down syndrome and Fragile-X syndrome. Clin. Neurophysiol. 2008, 119, 1242–1247. [Google Scholar] [CrossRef]
- Bailly, S.; Grote, L.; Hedner, J.; Schiza, S.; McNicholas, W.T.; Basoglu, O.K.; Lombardi, C.; Dogas, Z.; Roisman, G.; Pataka, A.; et al. Clusters of sleep apnoea phenotypes: A large pan-European study from the European Sleep Apnoea Database (ESADA). Respirology 2021, 26, 378–387. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Gentry, M.J.; Concato, J.; Yaggi, H.K. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med. Rev. 2017, 35, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Takaya, H.; Qian, J.; Petocz, P.; Ng, A.T.; Cistulli, P.A. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 861–868. [Google Scholar] [CrossRef]
- Appleton, S.L.; Vakulin, A.; Martin, S.A.; Lang, C.J.; Wittert, G.A.; Taylor, A.W.; McEvoy, R.D.; Antic, N.A.; Catcheside, P.G.; Adams, R.J. Hypertension Is Associated with Undiagnosed OSA During Rapid Eye Movement Sleep. Chest 2016, 150, 495–505. [Google Scholar] [CrossRef]
- Aurora, R.N.; Crainiceanu, C.; Gottlieb, D.J.; Kim, J.S.; Punjabi, N.M. Obstructive Sleep Apnea during REM Sleep and Cardiovascular Disease. Am. J. Respir. Crit. Care Med. 2018, 197, 653–660. [Google Scholar] [CrossRef]
- Joosten, S.A.; Landry, S.A.; Wong, A.-M.; Mann, D.L.; Terrill, P.I.; Sands, S.A.; Turton, A.; Beatty, C.; Thomson, L.; Hamilton, G.S.; et al. Assessing the Physiologic Endotypes Responsible for REM- and NREM-Based OSA. Chest 2021, 159, 1998–2007. [Google Scholar] [CrossRef]
- Thomas, R.J.; Terzano, M.G.; Parrino, L.; Weiss, J.W. Obstructive Sleep-Disordered Breathing with a Dominant Cyclic Alternating Pattern—A Recognizable Polysomnographic Variant with Practical Clinical Implications. Sleep 2004, 27, 229–234. [Google Scholar] [CrossRef]
- Smales, E.T.; Edwards, B.A.; Deyoung, P.N.; McSharry, D.G.; Wellman, A.; Velasquez, A.; Owens, R.; Orr, J.E.; Malhotra, A. Trazodone Effects on Obstructive Sleep Apnea and Non-REM Arousal Threshold. Ann. Am. Thorac. Soc. 2015, 12, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Puranik, S.; Morales, A.W. Heart Rate Estimation of PPG Signals with Simultaneous Accelerometry Using Adaptive Neural Network Filtering. IEEE Trans. Consum. Electron. 2019, 66, 69–76. [Google Scholar] [CrossRef]
- Khor, Y.H.; Khung, S.-W.; Ruehland, W.R.; Jiao, Y.; Lew, J.; Munsif, M.; Ng, Y.; Ridgers, A.; Schulte, M.; Seow, D.; et al. Portable evaluation of obstructive sleep apnea in adults: A systematic review. Sleep Med. Rev. 2023, 68, 101743. [Google Scholar] [CrossRef] [PubMed]
- Murarka, S.; Wadichar, A.; Bhurane, A.; Sharma, M.; Acharya, U.R. Automated classification of cyclic alternating pattern sleep phases in healthy and sleep-disordered subjects using convolutional neural network. Comput. Biol. Med. 2022, 146, 105594. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Patel, V.; Tiwari, J.; Acharya, U.R. Automated Characterization of Cyclic Alternating Pattern Using Wavelet-Based Features and Ensemble Learning Techniques with EEG Signals. Diagnostics 2021, 11, 1380. [Google Scholar] [CrossRef]
- Brennan, H.L.; Kirby, S.D. The role of artificial intelligence in the treatment of obstructive sleep apnea. J. Otolaryngol.-Head Neck Surg. 2023, 52, 7. [Google Scholar] [CrossRef]
- Parrino, L.; Halasz, P.; Szucs, A.; Thomas, R.J.; Azzi, N.; Rausa, F.; Pizzarotti, S.; Zilioli, A.; Misirocchi, F.; Mutti, C. Sleep medicine: Practice, challenges and new frontiers. Front. Neurol. 2022, 13, 966659. [Google Scholar] [CrossRef]
- Penzel, T.; Kantelhardt, J.W.; Bartsch, R.P.; Riedl, M.; Kraemer, J.F.; Wessel, N.; Garcia, C.; Glos, M.; Fietze, I.; Schöbel, C. Modulations of Heart Rate, ECG, and Cardio-Respiratory Coupling Observed in Polysomnography. Front. Physiol. 2016, 7, 460. [Google Scholar] [CrossRef]
- Parrino, L. Now that automatic processing makes CAP scoring fast and reliable is the sleep field ready for a paradigm shift? Sleep 2023, 46, zsac255. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutti, C.; Pollara, I.; Abramo, A.; Soglia, M.; Rapina, C.; Mastrillo, C.; Alessandrini, F.; Rosenzweig, I.; Rausa, F.; Pizzarotti, S.; et al. The Contribution of Sleep Texture in the Characterization of Sleep Apnea. Diagnostics 2023, 13, 2217. https://doi.org/10.3390/diagnostics13132217
Mutti C, Pollara I, Abramo A, Soglia M, Rapina C, Mastrillo C, Alessandrini F, Rosenzweig I, Rausa F, Pizzarotti S, et al. The Contribution of Sleep Texture in the Characterization of Sleep Apnea. Diagnostics. 2023; 13(13):2217. https://doi.org/10.3390/diagnostics13132217
Chicago/Turabian StyleMutti, Carlotta, Irene Pollara, Anna Abramo, Margherita Soglia, Clara Rapina, Carmela Mastrillo, Francesca Alessandrini, Ivana Rosenzweig, Francesco Rausa, Silvia Pizzarotti, and et al. 2023. "The Contribution of Sleep Texture in the Characterization of Sleep Apnea" Diagnostics 13, no. 13: 2217. https://doi.org/10.3390/diagnostics13132217
APA StyleMutti, C., Pollara, I., Abramo, A., Soglia, M., Rapina, C., Mastrillo, C., Alessandrini, F., Rosenzweig, I., Rausa, F., Pizzarotti, S., Salvatelli, M. l., Balella, G., & Parrino, L. (2023). The Contribution of Sleep Texture in the Characterization of Sleep Apnea. Diagnostics, 13(13), 2217. https://doi.org/10.3390/diagnostics13132217







