Sleep-Disordered Breathing and Prognosis after Ischemic Stroke: It Is Not Apnea-Hypopnea Index That Matters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
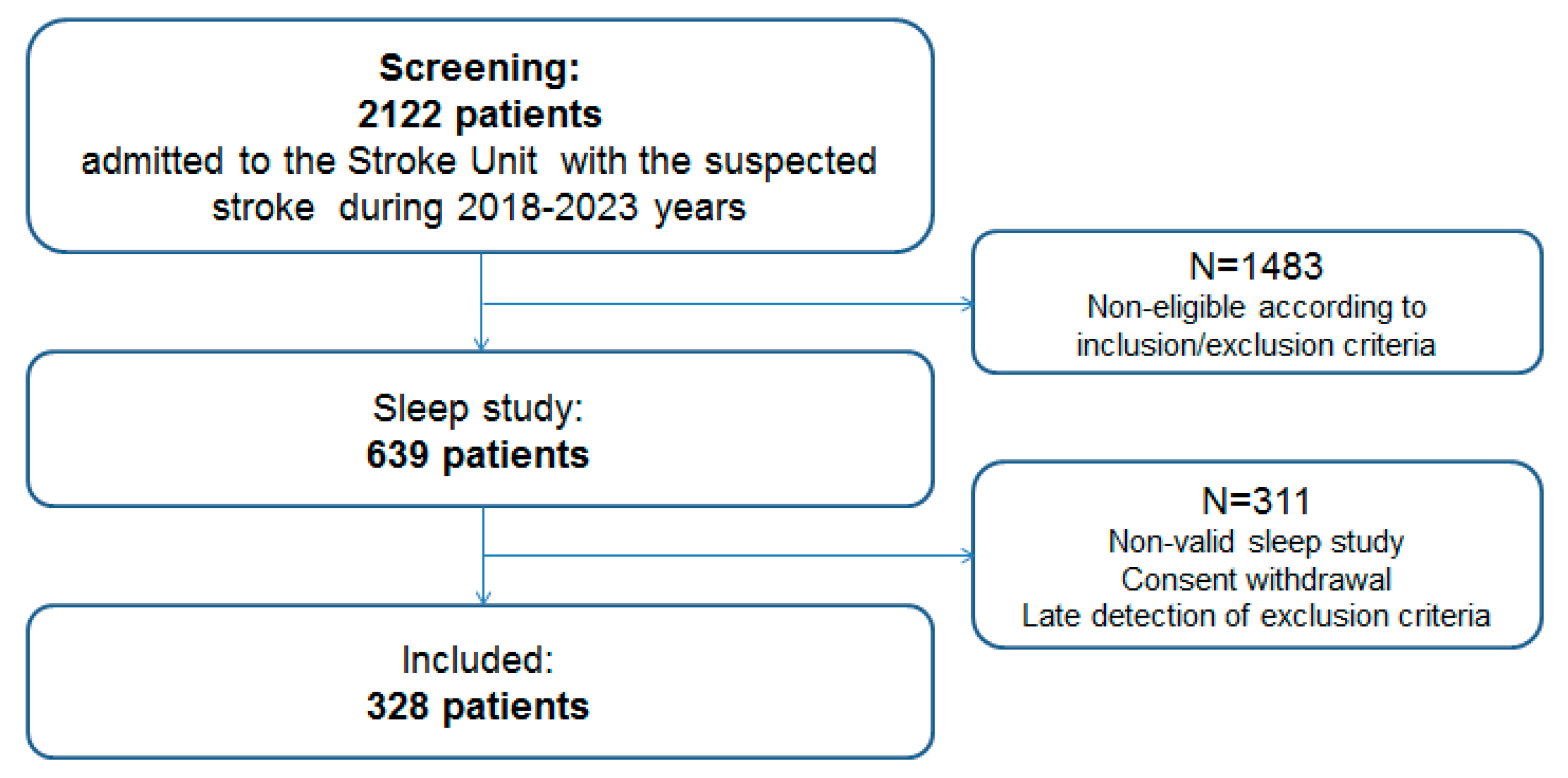
2.2. Study Cohort
2.3. Procedures
2.4. Endpoints and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Follow-Up and Outcomes
3.3. Sleep-Disordered Breathing in the Studied Cohort
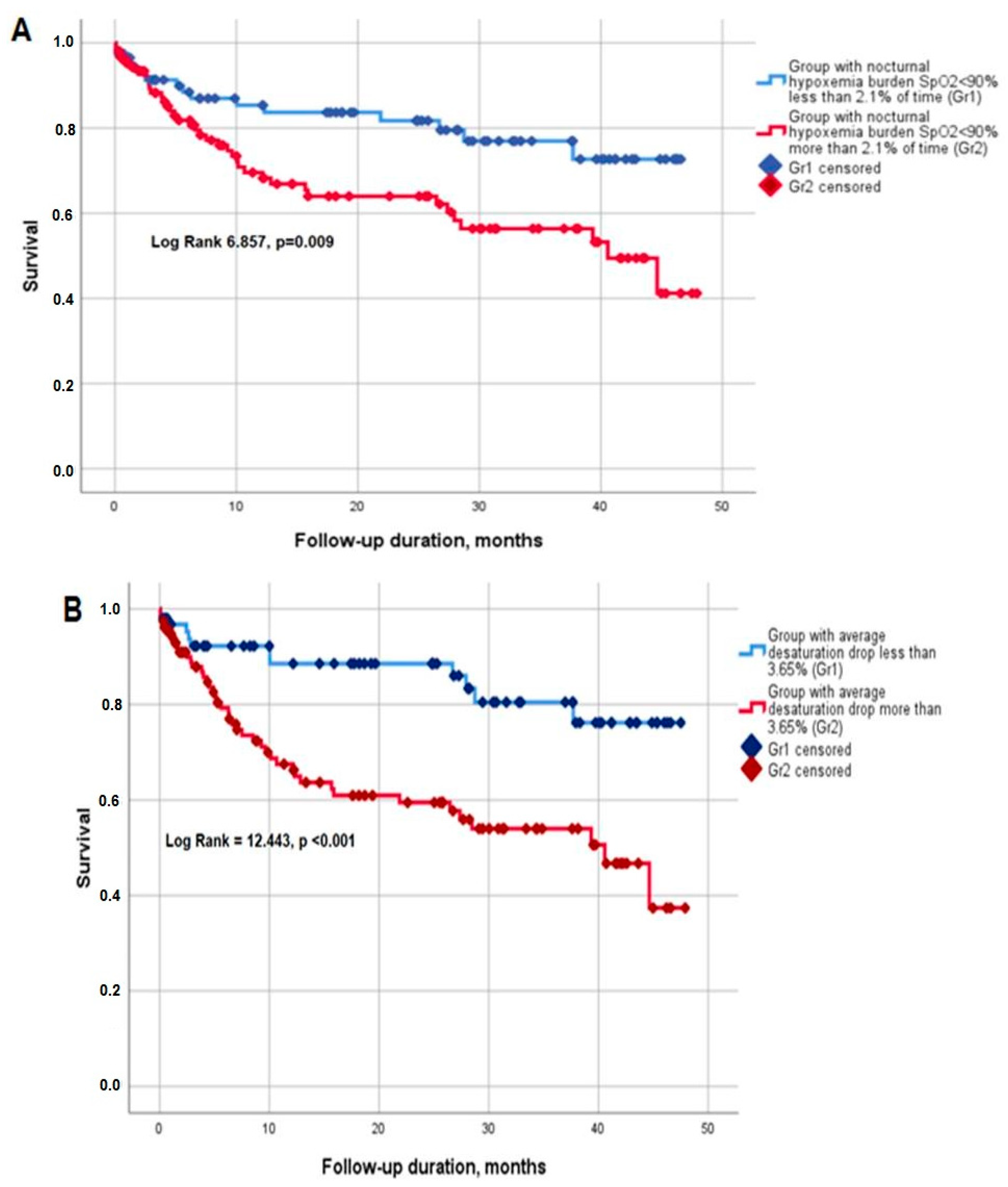
3.4. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Seiler, A.; Camilo, M.; Korostovtseva, L.; Haynes, A.G.; Brill, A.-K.; Horvath, T.; Egger, M.; Bassetti, C.L. Prevalence of sleep-disordered breathing after stroke and TIA: A meta-analysis. Neurology 2019, 92, e648–e654. [Google Scholar] [CrossRef]
- Johnson, K.G.; Johnson, D.C. Frequency of sleep apnea in stroke and TIA patient: A Meta-Analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2010, 6, 131–137. [Google Scholar] [CrossRef]
- Bassetti, C.L.A.; Randerath, W.; Vignatelli, L.; Ferini-strambi, L.; Brill, A.; Bonsignore, M.R. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur. Respir. J. 2020, 55, 1901104. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, K.H.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Ciccone, A.; Proserpio, P.; Roccatagliata, D.V.; Nichelatti, M.; Gigli, G.L.; Parati, G.; Lombardi, C.; Pizza, F.; Cirignotta, F.; Santilli, I.M.; et al. Wake-up stroke and TIA due to paradoxical embolism during long obstructive sleep apnoeas: A cross-sectional study. Thorax 2012, 68, 97–104. [Google Scholar] [CrossRef]
- Alexiev, F.; Brill, A.K.; Ott, S.R.; Duss, S.; Schmidt, M.; Bassetti, C.L. Sleep-disordered breathing and stroke: Chicken or egg? J. Thorac. Dis. 2018, 10, S4244–S4252. [Google Scholar] [CrossRef]
- Lin, H.J.; Yeh, J.H.; Hsieh, M.T.; Hsu, C.Y. Continuous positive airway pressure with good adherence can reduce risk of stroke in patients with moderate to severe obstructive sleep apnea: An updated systematic review and meta-analysis. Sleep Med. Rev. 2020, 54, 101354. [Google Scholar] [CrossRef]
- Brill, A.K.; Horvath, T.; Seiler, A.; Camilo, M.; Haynes, A.G.; Ott, S.R.; Egger, M.; Bassetti, C.L. CPAP as treatment of sleep apnea after stroke: A meta-analysis of randomized trials. Neurology 2018, 90, e1222–e1230. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Pevernagie, D. Obstructive sleep apnea: Transition from pathophysiology to an integrative disease model. J. Sleep Res. 2022, 31, e13616. [Google Scholar] [CrossRef]
- Randerath, W.; Bassetti, C.L.; Bonsignore, M.R.; Farre, R.; Ferini-Strambi, L.; Grote, L.; Al, E. Challenges and perspectives in obstructive sleep apnoea: Report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. Eur. Respir. J. 2018, 52, 1702616. [Google Scholar] [CrossRef] [PubMed]
- Jahrami, H.; Bahammam, A.S.; Algahtani, H.; Ebrahim, A.; Faris, M.; Aleid, K.; Saif, Z.; Haji, E.; Dhahi, A.; Marzooq, H.; et al. The examination of sleep quality for frontline healthcare workers during the outbreak of COVID-19|Request PDF. Sleep Breath. 2020, 2019, 503–511. [Google Scholar]
- Guo, J.; Xiao, Y. New Metrics from Polysomnography: Precision Medicine for OSA Interventions. Nat. Sci. Sleep 2023, 15, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.; Baumann, B.; Azarbarzin, A.; Allen, A.; Liu, Y.; Fels, S.; Goodfellow, S.; Singh, A.; Jen, R.; Ayas, N. Association of alternative polysomnographic features with patient outcomes in obstructive sleep apnea: A systematic review. J. Clin. Sleep Med. 2023, 19, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Audebert, H.; Turc, G.; Cordonnier, C.; Christensen, H.; Sacco, S.; Sandset, E.C.; Ntaios, G.; Charidimou, A.; Toni, D.; et al. Consensus statements and recommendations from the ESO- Karolinska Stroke Update Conference, Stockholm 11–13 November 2018. Eur. Stroke J. 2019, 4, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Schjørring, O.; Klitgaard, T.; Perner, A.; Wetterslev, J.; Lange, T.; Siegemund, M.; Bäcklund, M.; Keus, F.; Laake, J.; Morgan, M.; et al. Lower or Higher Oxygenation Targets for Acute Hypoxemic Respiratory Failure. N. Engl. J. Med. 2021, 384, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Azarbarzin, A.; Sands, S.A.; Stone, K.L.; Taranto-Montemurro, L.; Messineo, L.; Terrill, P.I.; Ancoli-Israel, S.; Ensrud, K.; Purcell, S.; White, D.P.; et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 2019, 40, 1149–1157. [Google Scholar] [CrossRef]
- Blanchard, M.; Gervès-Pinquié, C.; Feuilloy, M.; Le Vaillant, M.; Trzepizur, W.; Meslier, N.; Goupil, F.; Pigeanne, T.; Balusson, F.; Oger, E.; et al. Hypoxic burden and heart-rate variability predict stroke incidence in sleep apnoea. Eur. Respir. J. 2021, 57, 10–13. [Google Scholar] [CrossRef]
- Mehra, R.; Bena, J.; Walia, H.K. Clarifying the role of hypoxia in obstructive sleep apnea as a potential promulgator of atrial fibrillation in ischemic stroke. J. Clin. Sleep Med. 2017, 13, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Ho, C.H.; Chen, C.L.; Yu, C.C. Nocturnal desaturation is associated with atrial fibrillation in patients with ischemic stroke and obstructive sleep apnea. J. Clin. Sleep Med. 2017, 13, 729–735. [Google Scholar] [CrossRef]
- Patel, S.; Hanly, P.; Smith, E.; Chan, W.; Coutts, S. Nocturnal Hypoxemia Is Associated with White Matter Hyperintensities in Patients with a Minor Stroke or Transient Ischemic Attack. J. Clin. Sleep 2015, 11, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Roffe, C.; Sills, S.; Halim, M.; Wilde, K.; Allen, M.B.; Jones, P.W.; Crome, P. Unexpected Nocturnal Hypoxia in Patients with Acute Stroke. Stroke 2003, 34, 2641–2645. [Google Scholar] [CrossRef]
- Huhtakangas, J.K.; Saaresranta, T.; Bode, M.K.; Bloigu, R.; Huhtakangas, J. Stroke Volume Predicts Nocturnal Hypoxemia in the Acute Ischemic Stroke after Intravenous Thrombolysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 2481–2487. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Collen, F.M.; Wade, D.T.; Robb, G.F.; Bradshaw, C.M. The Rivermead Mobility index: A further development of the rivermead motor assessment. Disabil. Rehabil. 1991, 13, 50–54. [Google Scholar] [CrossRef]
- Wilson, J.T.; Hareendran, A.; Grant, M.; Baird, T.; Schulz, U.G.R.; Muir, K.W.; Bone, I. Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2002, 33, 2243–2246. [Google Scholar] [CrossRef]
- Adams, H.; Bendixen, B.; Kappelle, L.; Biller, J.; Love, B.; Gordon, D.; Marsh, E. Classification of subtype of acute ischemic stroke definitions for use in a multicenter clinical trial. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM scoring manual updates for 2017 (version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Pevernagie, D.A.; Gnidovec-Strazisar, B.; Grote, L.; Heinzer, R.; McNicholas, W.T.; Penzel, T.; Randerath, W.; Schiza, S.; Verbraecken, J.; Arnardottir, E.S. On the rise and fall of the apnea—hypopnea index: A historical review and critical appraisal. J. Sleep Res. 2020, 29, e13066. [Google Scholar] [CrossRef]
- Beebe, D.W.; Groesz, L.; Wells, C.; Nichols, A.; Mcgee, K. The Neuropsychological Effects of Obstructive Sleep Apnea: A Meta-Analysis of Norm-Referenced and Case-Controlled Data. Sleep 2003, 26, 298–307. [Google Scholar] [CrossRef]
- Good, D.; Henkle, J.; Gelber, D.; Welsh, J.; Verhulst, S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke 1996, 27, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Wu, H.; Han, F.; Wang, Q.; Jiang, Y.; Chen, R. Effects of severe obstructive sleep apnea on functional prognosis in the acute phase of ischemic stroke and quantitative electroencephalographic markers. Sleep Med. 2023, 101, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Birkbak, J.; Clark, A.J.; Rod, N.H. The effect of sleep disordered breathing on the outcome of stroke and transient ischemic attack: A systematic review. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2014, 10, 103–108. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Alexandrov, A.V.; Katsanos, A.H.; Barlinn, K.; Mikulik, R.; Lambadiari, V.; Bonakis, A.; Alexandrov, A.W. Noninvasive Ventilatory Correction in Patients with Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Stroke 2017, 48, 2285–2288. [Google Scholar] [CrossRef] [PubMed]
- Haba-Rubio, J.; Vujica, J.; Franc, Y.; Michel, P.; Heinzer, R. Effect of CPAP treatment of sleep apnea on clinical prognosis after ischemic stroke: An observational study. J. Clin. Sleep Med. 2019, 15, 839–847. [Google Scholar] [CrossRef]
- Liu, X.; Lam, D.C.L.; Chan, K.P.F.; Chan, H.Y.; Ip, M.S.M.; Lau, K.K. Prevalence and Determinants of Sleep Apnea in Patients with Stroke: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2021, 30, 106129. [Google Scholar] [CrossRef]
- Takala, M.; Puustinen, J.; Rauhala, E.; Holm, A. Pre-screening of sleep-disordered breathing after stroke: A systematic review. Brain Behav. 2018, 8, e01146. [Google Scholar] [CrossRef]
- Schütz, S.G.; Lisabeth, L.D.; Shafie-Khorassani, F.; Case, E.; Sanchez, B.N.; Chervin, R.D.; Brown, D.L. Clinical phenotypes of obstructive sleep apnea after ischemic stroke: A cluster analysis. Sleep Med. 2019, 60, 178–181. [Google Scholar] [CrossRef]
- Poli, M.; Philip, P.; Taillard, J.; Debruxelles, S.; Renou, P.; Orgogozo, J.M.; Rouanet, F.; Sibon, I. Atrial fibrillation is a major cause of stroke in apneic patients: A prospective study. Sleep Med. 2017, 30, 251–254. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskina, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Freiberg, J.; Tybjærg-Hansen, A.; Jensen, J.; Nordestgaard, B.G. Nonfasting triglycerides, cholesterol, and ischemic stroke in the general population. J. Am. Med. Acad. 2008, 300, 2142–2152. [Google Scholar] [CrossRef]
- Jain, M.; Jain, A.; Yerragondu, N.; Brown, R.D.; Rabinstein, A.; Jahromi, B.S.; Vaidyanathan, L.; Blyth, B.; Stead, L.G. The Triglyceride Paradox in Stroke Survivors: A Prospective Study. Neurosci. J. 2013, 2013, 870608. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.J.; Sattar, N.; Walters, M.R.; Lees, K.R. Low triglyceride, not low cholesterol concentration, independently predicts poor outcome following acute stroke. Cerebrovasc. Dis. 2003, 16, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, T.; Slowik, A.; Gryz, E.A.; Szczudlik, A. Lower serum triglyceride level is associated with increased stroke severity. Stroke 2004, 35, e151–e152. [Google Scholar] [CrossRef] [PubMed]
- Pikija, S.; Trkulja, V.; Juvan, L.; Ivanec, M.; Dukši, D. Higher on-admission serum triglycerides predict less severe disability and lower all-cause mortality after acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, e15–e24. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Park, M.S.; Kim, J.T.; Chang, J.; Nam, T.S.; Choi, S.M.; Lee, S.H.; Kim, B.C.; Kim, M.K.; Cho, K.H. Serum triglyceride level is an important predictor of early prognosis in patients with acute ischemic stroke. J. Neurol. Sci. 2012, 319, 111–116. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Total (n = 328) | Patients with Poor Survival (n = 65) | Patients Free of Events (n = 263) | p-Level |
|---|---|---|---|---|
| Sex (m), n (%) | 181 (55.2%) | 40 (61.5%) | 141 (53.6%) | χ2 = 1.043, p = 0.31 |
| Age, years | 65.8 ± 11.2 | 69.5 ± 9.5 | 64.7 ± 11.5 | p = 0.005 |
| HTN, n (%) | 262 (79.8%) | 64 (98.5%) | 198 (75.3%) | χ2 = 5.700, p = 0.017 |
| HTN crisis, n (%) | 35 (10.7%) | 5 (7.7%) | 30 (11.4%) | χ2 = 1.667, p = 0.20 |
| AF, n (%) | 82 (25%) | 33 (50.8%) | 49 (18.6%) | χ2 = 19.880, p<0.001 |
| Pacemaker, n (%) | 4 (1.2%) | 1 (1.5%) | 3 (1.1%) | χ2 = 0.144, p = 0.70 |
| CAD, n (%) | 138 (42.1%) | 42 (64.6%) | 96 (36.5%) | χ2 = 8.861, p = 0.003 |
| Previous MI, n (%) | 57 (17.4%) | 19 (29.2%) | 38 (14.5%) | χ2 = 4.455, p = 0.035 |
| CAD-related heart surgery, n (%) | 28 (8.5%) | 8 (12.3%) | 20 (7.6%) | χ2 = 0.576, p = 0.45 |
| Valvular disease, n (%) | 33 (10.1%) | 8 (12.3%) | 25 (9.5%) | χ2 = 0.041, p = 0.84 |
| Valvular surgery, n (%) | 4 (1.2%) | 0 | 4 (1.9%) | χ2 = 1.202, p = 0.27 |
| Pulmonary thromboembolism, n (%) | 4 (1.2%) | 3 (4.6%) | 1 (0.4%) | χ2 = 6.257, p = 0.012 |
| Pulmonary hypertension, n (%) | 17 (5.2%) | 3 (4.6%) | 14 (5.3%) | χ2 < 0.001, p = 0.99 |
| Other CVD (myxoma, foramen ovale) | 8 (2.4%) | 0 | 8 (3%) | χ2 = 2.127, p = 0.15 |
| Diabetes mellitus, n (%) | 70 (21.3%) | 18 (27.7%) | 52 (19.8%) | χ2 = 0.432, p = 0.51 |
| Dyslipidemia, n (%) | 157 (47.9%) | 35 (53.8%) | 122 (46.4%) | χ2 = 0.065, p = 0.80 |
| Obesity, n (%) | 70 (21.3%) | 22 (33.8%) | 48 (18.3%) | χ2 = 3.889, p = 0.049 |
| CKD, n (%) | 24 (7.3%) | 1 (1.5%) | 23 (8.7%) | χ2 = 3.378, p = 0.066 |
| COPD, n (%) | 23 (7.0%) | 5 (7.7%) | 18 (6.8%) | χ2 = 0.018, p = 0.89 |
| Bronchial asthma, n (%) | 5 (1.5%) | 0 | 5 (1.9%) | χ2 = 1.267, p = 0.26 |
| Previous stroke/TIA, n (%) | 93 (28.4%) | 21 (32.3%) | 72 (27.3%) | χ2 = 0.006, p = 0.94 |
| PAD, n (%) | 21 (6.4%) | 7 (10.8%) | 14 (5.3%) | χ2 = 3.404, p = 0.065 |
| Thyroid disease, n (%) | 22 (6.7%) | 3 (4.6%) | 19 (7.2%) | χ2 = 0.269, p = 0.60 |
| Cancer, n (%) | 16 (4.9%) | 2 (3.1%) | 14 (5.3%) | χ2 = 0.386, p = 0.53 |
| Varicose vein disease, n (%) | 31 (9.5%) | 6 (9.2%) | 25 (9.5%) | χ2 = 0.34, p = 0.85 |
| Parameter | Total (n = 328) | Patients with Poor Survival (n = 65) | Patients Free of Events (n = 263) | p-Level |
|---|---|---|---|---|
| TOAST classification | p = 0.023 | |||
| Atherothrombotic | 61 (18.6%) | 13 (20%) | 48 (18.3%) | |
| Cardioembolic | 103 (31.4%) | 30 (46.2%) | 73 (27.8%) | |
| Small-vessel disease | 33 (10%) | 2 (3.1%) | 31 (11.8%) | |
| Other determined etiology | 5 (1.5%) | 1 (1.5%) | 4 (1.5%) | |
| Undetermined etiology | 128 (37.8%) | 19 (28.8%) | 109 (41.4%) | |
| Affected cerebral artery | p = 0.55 | |||
| Vertebrobasilar | 52 (15.9%) | 9 (13.8%) | 43 (16.3%) | |
| Anterior cerebral artery | 3 (0.9%) | 0 | 3 (1.1%) | |
| Median cerebral artery | 247 (75.3%) | 49 (75.4%) | 198 (75.3%) | |
| Several arteries | 28 (8.5%) | 7 (10.8%) | 21 (8.0%) | |
| Medical interventions | ||||
| Thrombolytic therapy, n (%) | 43 (13.1%) | 7 (10.8%) | 36 (13.7%) | χ2 = 0.39, p = 0.53 |
| Emergency stroke-related vascular surgery | 66 (20.1%) | 14 (21.5%) | 52 (19.8%) | χ2 = 0.101, p = 0.75 |
| Parameter | Total (n = 328) | Patients with Poor Survival (n = 65) | Patients Free of Events (n = 263) | p-Level |
|---|---|---|---|---|
| Glucose, mmol/L | 6.29 (2.79; 33.33) | 6.37 (2.79; 16.03) | 6.22 (3.68; 33.33) | 0.99 |
| Total cholesterol, mmol/L | 4.79 (1.97; 9.27) | 4.65 (1.97; 7.64) | 4.80 (2.20; 9.27) | 0.63 |
| Triglycerides, mmol/L | 1.39 (0.53; 13.44) | 1.27 (0.53; 3.28) | 1.40 (0.59; 13.44) | 0.011 |
| HDL Cholesterol, mmol/L | 1.08 (0.25; 3.43) | 1.09 (0.25; 3.24) | 1.08 (0.52; 3.43) | 0.94 |
| LDL Cholesterol, mmol/L | 2.89 (0.52; 7.62) | 2.81 (1.19; 5.86) | 2.9 (0.52; 7.62) | 0.99 |
| Fibrinogen, mg/L | 3.6 (0.87; 12.7) | 3.9 (0.99; 12.70) | 3.6 (0.87; 6.60) | 0.012 |
| C-reactive protein, mg/mL | 3.57 (0.02; 138.32) | 5.69 (0.02; 138.32) | 3.2 (0.18; 130.62) | 0.011 |
| Hemoglobin, g/L | 143.2 (69.9; 197.5) | 142.5 (81.7; 171.2) | 143.3 (69.9; 197.5) | 0.52 |
| Creatinine, mcmol/L | 80.7 (38; 222.4) | 81.0 (44.0; 129.0) | 80.3 (38.0; 222.4) | 0.61 |
| eGFR, ml/min/1.73 m2 | 85.6 (51.2; 140.0) | 83.1 (64.5; 104.6) | 86.2 (51.2; 140.0) | 0.046 |
| Red blood cell count, *1012/L | 4.76 (2.70; 6.21) | 4.7 (3.5; 6.0) | 4.8 (2.7; 6.2) | 0.79 |
| White blood cell count, *109/L | 8.1 (2.4; 20.8) | 8.8 (3.4; 18.3) | 7.9 (2.4; 20.8) | 0.13 |
| Hematocrit, % | 42.7 (30; 109) | 42.5 (30; 52.5) | 42.8 (30; 109) | 0.82 |
| Platelets, *109/L | 218 (67; 506) | 228 (67; 386) | 213 (48; 506) | 0.14 |
| Parameter | Total (n = 328) | Patients with Poor Survival (n = 65) | Patients Free of Events (n = 263) | p-Level |
|---|---|---|---|---|
| NIHSS baseline, score | 4 (1; 25) | 6 (1; 25) | 4 (1; 25) | 0.025 |
| NIHSS baseline ≥ 5 scores, n (%) | 135 (41.2%) | 38 (58.5%) | 97 (36.9%) | χ2 = 4.904, p = 0.030 |
| NIHSS discharge, score | 3 (0; 23) | 3(0; 23) | 3 (0; 19) | 0.083 |
| Barthel index baseline, score | 60 (0; 100) | 50 (0; 100) | 60 (0; 100) | 0.132 |
| Barthel index discharge, score | 90 (0; 100) | 80 (0; 100) | 90 (0; 100) | 0.018 |
| mRS baseline, score | 3 (0; 5) | 4 (0; 5) | 3 (1; 5) | 0.124 |
| mRS discharge, score | 3 (0; 6) | 3 (0; 6) | 2 (0; 5) | 0.005 |
| Rivermead index baseline, score | 3.5 (0; 15) | 3 (0; 15) | 4 (0; 15) | 0.105 |
| Rivermead index on the 2nd day (at neurology department), score | 5 (0; 15) | 3.5 (0; 15) | 5 (0; 15) | 0.045 |
| Rivermead index discharge, score | 12 (0; 15) | 8 (0; 15) | 12 (0; 15) | 0.019 |
| Parameter | Total (n = 328) | Patients with Poor Survival (n = 65) | Patients Free of Events (n = 263) | p-Level |
|---|---|---|---|---|
| AHI, episodes/h | 15.4 (0.0; 87.8) | 17.2 (0.6; 87.8) | 14.6 (0.0; 87.8) | 0.42 |
| AHI supine, episodes/h | 19.4 (0.0; 86.9) | 14.0 (0.0; 86.2) | 20.1 (0.0; 86.9) | 0.39 |
| HI, episodes/h | 5.9 (0.0; 51.2) | 5.0 (0.0; 38.0) | 6.1 (0.0; 51.2) | 0.39 |
| HI supine, episodes/h | 7.0 (0.0; 47.8) | 4.7 (0.0; 30.7) | 7.8 (0.0; 47.8) | 0.008 |
| ODI, episodes/h | 12.0 (0.0; 80.9) | 13.7 (0.0; 80.4) | 12.0 (0.0; 80.9) | 0.57 |
| AI, episodes/h | 5.6 (0.0; 82.9) | 7.1 (0.0; 52.8) | 4.7 (0.0; 82.9) | 0.12 |
| OAH, episodes/h | 3.3 (0.0; 56.7) | 4.8 (0.0; 50.6) | 2.9 (0.0; 56.7) | 0.038 |
| CAH, episodes/h | 0.4 (0.0; 68.3) | 0.2 (0.0; 26.9) | 0.4 (0.0; 68.3) | 0.17 |
| MAH, episodes/h | 0.1 (0.0; 31.9) | 0.1 (0.0; 19.6) | 0.1 (0.0; 31.9) | 0.44 |
| Respiratory rate at night, breaths/min | 16.1 (10.6; 29.0) | 17.0 (11.3; 27.8) | 15.9 (9.1; 29.0) | 0.018 |
| Average SpO2, % | 92.9 (78.7; 97.8) | 92.7 (78.7; 97.7) | 92.9 (83.0; 97.8) | 0.45 |
| Minimal SpO2, % | 81.0 (51.0; 95.0) | 80.0 (52.0; 93.0) | 81.0 (51.0; 95.0) | 0.12 |
| Average desaturation drop, % | 3.9 (1.3; 9.4) | 4.1 (1.3; 7.1) | 3.8 (2.5; 9.4) | 0.023 |
| SpO2 < 90%, percent of total analyzed time | 3.7 (0.0; 87.9) | 6.2 (0.0; 48.3) | 2.9 (0.0; 87.9) | 0.018 |
| SpO2 < 85%, percent of total analyzed time | 0.1 (0.0; 64.0) | 0.35 (0.0; 23.2) | 0.1 (0.0; 64.0) | 0.033 |
| Time in the supine position, percent of total analyzed time | 65.8 (0.0; 100.0) | 84.8 (0.0; 100.0) | 64.2 (0.0; 100.0) | 0.026 |
| Time in the non-supine position, percent of total analyzed time | 30.1 (0.0; 100.0) | 11.6 (0.0; 100.0) | 34.6 (0.0; 100.0) | 0.029 |
| SDB severity | p = 0.074 | |||
| No SDB, n (%) | 108 (32.9%) | 15 (23.1%) | 93 (35.4%) | |
| Mild, n (%) | 71 (21.6%) | 16 (24.6%) | 55 (20.9%) | |
| Moderate, n (%) | 60 (18.3%) | 12 (18.5%) | 48 (18.3%) | |
| Severe, n (%) | 89 (27.1%) | 22 (33.8%) | 67 (25.5%) | |
| Tachypnoe at night ≥ 15.6/min, n (%) | 160 (48.8%) | 40 (61.5%) | 120 (45.6%) | χ2 = 3.837, p = 0.050 |
| Hypoxemia (SpO2 < 90%) ≥2.1% of total analyzed time, n (%) | 165 (50.3%) | 42 (64.6%) | 123 (46.8%) | χ2 = 5.002, p = 0.025 |
| Average desaturation drop ≥ 3.65%, n (%) | 163 (49.7%) | 45 (69.2%) | 118 (44.8%) | χ2 = 9.764, p = 0.002 |
| Model 1 (Incl. Hypoxemia Time < 2.1% versus ≥2.1% Nocturnal Time) | Model 2 (Incl. Average Nocturnal Desaturation Drop < 3.65% versus ≥3.65%) | |||
|---|---|---|---|---|
| Parameter | HR (95% CI) | p-Level | HR (95% CI) | p-Level |
| Age | 1.091 (1.040; 1.140) | <0.001 | 1.062 (1.020; 1.107) | 0.004 |
| Sex (male) | excl | 0.985 | excl | 0.368 |
| TOAST | 1.339 (0.974; 1.839) | 0.072 | 1.455 (1.065; 1.989) | 0.019 |
| Atrial fibrillation (yes) | 5.075 (1.961; 13.137) | 0.001 | 4.231 (1.666; 10.741) | 0.002 |
| Thrombolytic therapy (yes) | excl | 0.447 | Excl | 0.330 |
| Emergency vascular intervention (yes) | excl | 0.708 | Excl | 0.724 |
| Previous stroke/TIA | 2.100 (0.897; 4.916) | 0.087 | Excl | 0.114 |
| Obesity (yes) | excl | 0.249 | Excl | 0.279 |
| Diabetes mellitus (yes) | 2.303 (1.037; 5.118) | 0.041 | excl | 0.305 |
| Coronary artery disease (yes) | 2.261 (0.950; 5.381) | 0.065 | excl | 0.137 |
| Hypertensive crisis at stroke onset (yes) | excl | 0.332 | excl | 0.463 |
| NIHSS at discharge | 1.158 (1.071; 1.252) | <0.001 | 1.106 (1.026; 1.191) | 0.008 |
| eGFR | excl | 0.569 | excl | 0.221 |
| Triglycerides | 0.456 (0.218; 0.953) | 0.037 | 0.515 (0.256; 1.036) | 0.063 |
| C-reactive protein | 1.019 (1.005; 1.032) | 0.007 | 1.024 (1.010; 1.038) | 0.001 |
| Platelet count | 1.009 (1.004; 1.015) | 0.001 | 1.009 (1.004; 1.015) | 0.001 |
| Respiratory rate ≥ 15.6/min | excl | 0.268 | excl | 0.921 |
| SpO2 < 90% during ≥2.1% of total analyzed time | 3.693 (1.517; 8.992) | 0.004 | - | - |
| Average nocturnal desaturation drop ≥ 3.65% | - | - | 4.257 (1.612; 11.240) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korostovtseva, L.; Bochkarev, M.; Amelina, V.; Nikishkina, U.; Osipenko, S.; Vasilieva, A.; Zheleznyakov, V.; Zabroda, E.; Gordeev, A.; Golovkova-Kucheryavaia, M.; et al. Sleep-Disordered Breathing and Prognosis after Ischemic Stroke: It Is Not Apnea-Hypopnea Index That Matters. Diagnostics 2023, 13, 2246. https://doi.org/10.3390/diagnostics13132246
Korostovtseva L, Bochkarev M, Amelina V, Nikishkina U, Osipenko S, Vasilieva A, Zheleznyakov V, Zabroda E, Gordeev A, Golovkova-Kucheryavaia M, et al. Sleep-Disordered Breathing and Prognosis after Ischemic Stroke: It Is Not Apnea-Hypopnea Index That Matters. Diagnostics. 2023; 13(13):2246. https://doi.org/10.3390/diagnostics13132246
Chicago/Turabian StyleKorostovtseva, Lyudmila, Mikhail Bochkarev, Valeria Amelina, Uliana Nikishkina, Sofia Osipenko, Anastasia Vasilieva, Vladislav Zheleznyakov, Ekaterina Zabroda, Alexey Gordeev, Maria Golovkova-Kucheryavaia, and et al. 2023. "Sleep-Disordered Breathing and Prognosis after Ischemic Stroke: It Is Not Apnea-Hypopnea Index That Matters" Diagnostics 13, no. 13: 2246. https://doi.org/10.3390/diagnostics13132246
APA StyleKorostovtseva, L., Bochkarev, M., Amelina, V., Nikishkina, U., Osipenko, S., Vasilieva, A., Zheleznyakov, V., Zabroda, E., Gordeev, A., Golovkova-Kucheryavaia, M., Yanishevskiy, S., Sviryaev, Y., & Konradi, A. (2023). Sleep-Disordered Breathing and Prognosis after Ischemic Stroke: It Is Not Apnea-Hypopnea Index That Matters. Diagnostics, 13(13), 2246. https://doi.org/10.3390/diagnostics13132246








