Evaluation of a Sample-to-Result POCKIT Central SARS-CoV-2 PCR System
Abstract
:1. Introduction
2. Materials and Methods
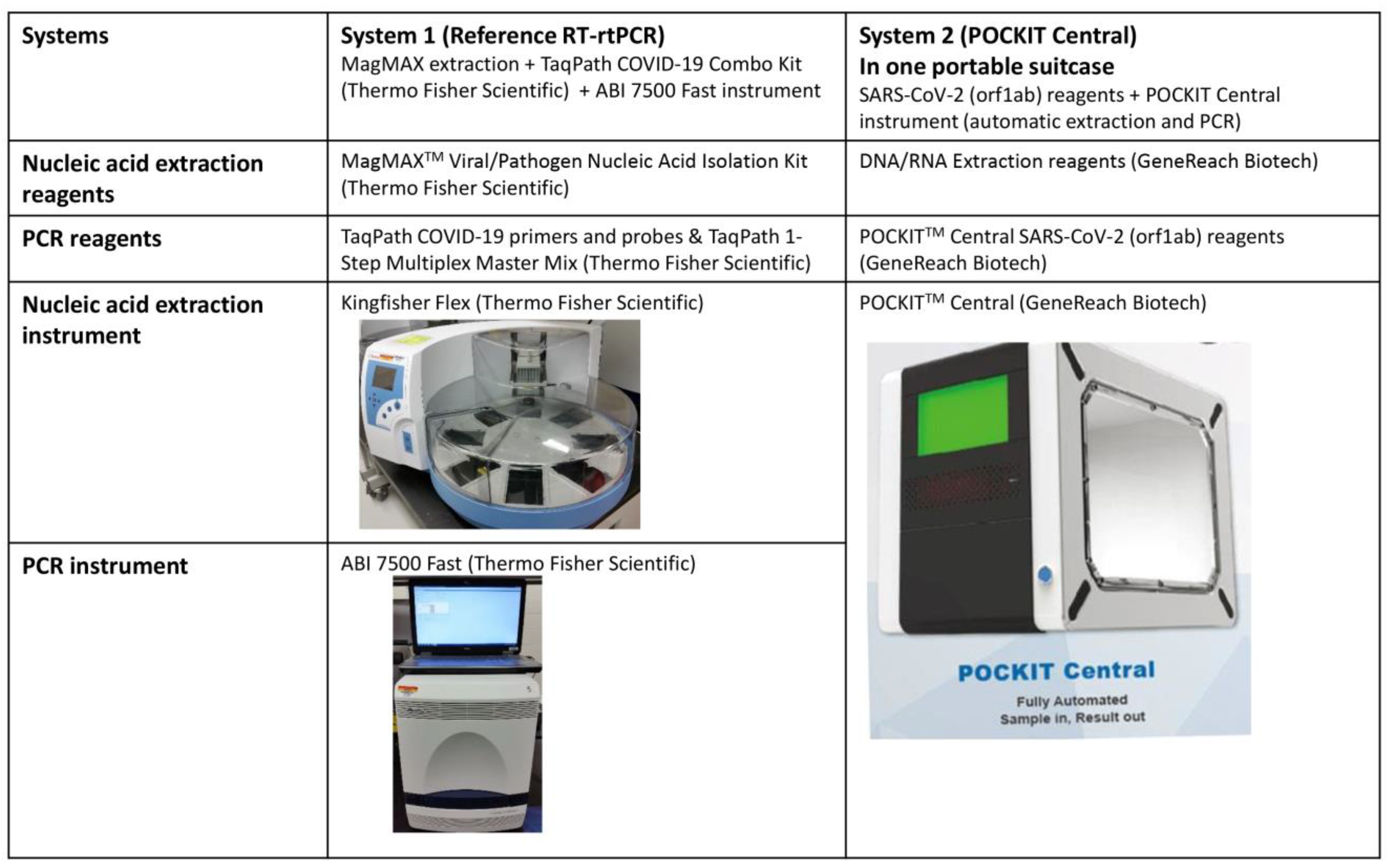
2.1. POCKIT Central SARS-CoV-2 Orf1ab RT-iiPCR System
2.2. The Reference TaqPath COVID-19 Real-Time RT-PCR System
2.3. Viral and Bacterial Pathogens
2.4. Clinical Samples
2.5. Limit of Detection of POCKIT Central SARS-CoV-2 PCR Assay and the Reference TaqPath COVID-19 PCR Assay
3. Results
3.1. Analytical Specificity of POCKIT Central SARS-CoV-2 orf1ab RT-iiPCR Assay and the Reference TaqPath COVID-19 PCR Assay
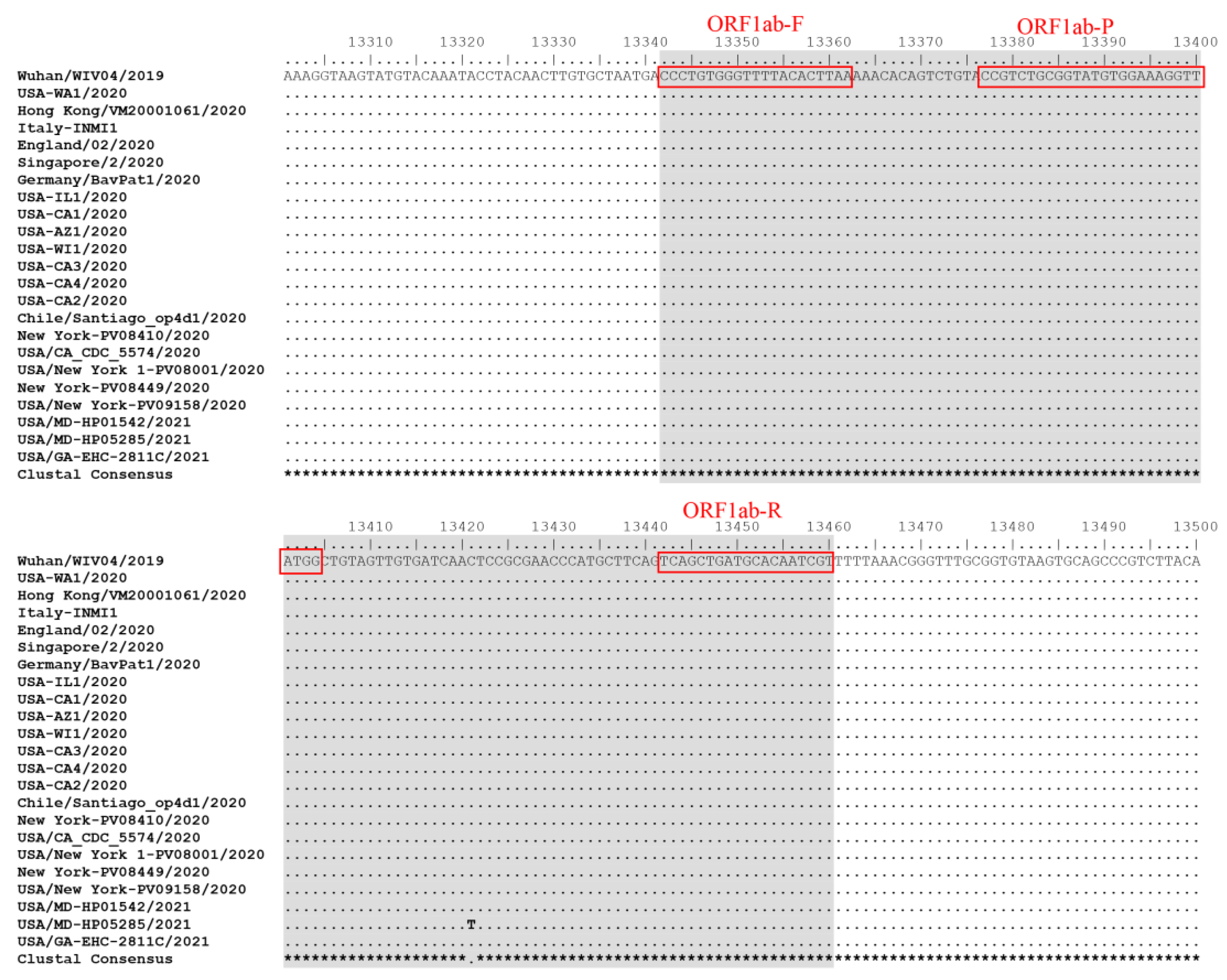
3.2. Inclusivity of POCKIT Central SARS-CoV-2 orf1ab RT-iiPCR Assay and the Reference TaqPath COVID-19 PCR Assay
3.3. Limit of Detection of POCKIT Central SARS-CoV-2 orf1ab RT-iiPCR Assay and the Reference TaqPath COVID-19 PCR Assay
3.4. Diagnostic Accuracy of POCKIT Central SARS-CoV-2 orf1ab RT-iiPCR Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pak, A.; Adegboye, O.A.; Adekunle, A.I.; Rahman, K.M.; McBryde, E.S.; Eisen, D.P. Economic Consequences of the COVID-19 Outbreak: The Need for Epidemic Preparedness. Front. Public. Health 2020, 8, 241. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Smith, D.W. COVID-19-A Novel Zoonotic Disease: A Review of the Disease, the Virus, and Public Health Measures. Asia Pac. J. Public. Health 2020, 32, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Anumudu, C.K.; Al-Sharify, Z.T.; Egele-Godswill, E.; Mbaegbu, P. COVID-19 pandemic: A review of the global lockdown and its far-reaching effects. Sci. Prog. 2021, 104, 368504211019854. [Google Scholar] [CrossRef]
- Ellis, P.; Somogyvari, F.; Virok, D.P.; Noseda, M.; McLean, G.R. Decoding Covid-19 with the SARS-CoV-2 Genome. Curr. Genet. Med. Rep. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Redondo, N.; Zaldivar-Lopez, S.; Garrido, J.J.; Montoya, M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns. Front. Immunol. 2021, 12, 708264. [Google Scholar] [CrossRef] [PubMed]
- Baric, R.S.; Fu, K.; Chen, W.; Yount, B. High recombination and mutation rates in mouse hepatitis virus suggest that coronaviruses may be potentially important emerging viruses. Adv. Exp. Med. Biol. 1995, 380, 571–576. [Google Scholar] [PubMed] [Green Version]
- Goldstein, S.A.; Brown, J.; Pedersen, B.S.; Quinlan, A.R.; Elde, N.C. Extensive Recombination-driven Coronavirus Diversification Expands the Pool of Potential Pandemic Pathogens. Genome Biol. Evol. 2022, 14. [Google Scholar] [CrossRef]
- Hillary, V.E.; Ceasar, S.A. An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines. Heliyon 2023, 9, e13952. [Google Scholar] [CrossRef]
- Rambaut, A.; Holmes, E.C.; O’Toole, A.; Hill, V.; McCrone, J.T.; Ruis, C.; du Plessis, L.; Pybus, O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020, 5, 1403–1407. [Google Scholar] [CrossRef]
- O’Toole, A.; Scher, E.; Underwood, A.; Jackson, B.; Hill, V.; McCrone, J.T.; Colquhoun, R.; Ruis, C.; Abu-Dahab, K.; Taylor, B.; et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 2021, 7, veab064. [Google Scholar] [CrossRef]
- Ciotti, M.; Benedetti, F.; Zella, D.; Angeletti, S.; Ciccozzi, M.; Bernardini, S. SARS-CoV-2 Infection and the COVID-19 Pandemic Emergency: The Importance of Diagnostic Methods. Chemotherapy 2021, 66, 17–23. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Martini, F.; Maritati, M.; Caselli, E.; Gallenga, C.E.; Guarino, M.; De Giorgio, R.; Mazziotta, C.; Tramarin, M.L.; Badiale, G.; et al. Advanced Molecular and Immunological Diagnostic Methods to Detect SARS-CoV-2 Infection. Microorganisms 2022, 10, 1193. [Google Scholar] [CrossRef]
- La Marca, A.; Capuzzo, M.; Paglia, T.; Roli, L.; Trenti, T.; Nelson, S.M. Testing for SARS-CoV-2 (COVID-19): A systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod. Biomed. Online 2020, 41, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ge, H.; Sun, Z.; Fu, J.; Cao, L.; Feng, X.; Meng, G.; Peng, Y.; Liu, Y.; Zhao, C. A loop-mediated isothermal amplification-enabled analytical assay for the detection of SARS-CoV-2: A review. Front. Cell. Infect. Microbiol. 2022, 12, 1068015. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K. Rapid Diagnostic Testing for SARS-CoV-2. N. Engl. J. Med. 2022, 386, 264–272. [Google Scholar] [CrossRef] [PubMed]
- A, R.; Wang, H.; Wang, W.; Tan, W. Summary of the Detection Kits for SARS-CoV-2 Approved by the National Medical Products Administration of China and Their Application for Diagnosis of COVID-19. Virol. Sin. 2020, 35, 699–712. [Google Scholar] [CrossRef]
- Suzuki, H.; Akashi, Y.; Kato, D.; Takeuchi, Y.; Kiyasu, Y.; Terada, N.; Kurihara, Y.; Kuwahara, M.; Muramatsu, S.; Ueda, A.; et al. Analytical performance of the rapid qualitative antigen kit for the detection of SARS-CoV-2 during widespread circulation of the Omicron variant. J. Infect. Chemother. 2023, 29, 257–262. [Google Scholar] [CrossRef]
- Tessaro, L.; Aquino, A.; Panzenhagen, P.; Joshi, N.; Conte-Junior, C.A. A systematic review of the advancement on colorimetric nanobiosensors for SARS-CoV-2 detection. J. Pharm. Biomed. Anal. 2023, 222, 115087. [Google Scholar] [CrossRef]
- Dhar, B.C. Diagnostic assay and technology advancement for detecting SARS-CoV-2 infections causing the COVID-19 pandemic. Anal. Bioanal. Chem. 2022, 414, 2903–2934. [Google Scholar] [CrossRef]
- Zeng, R.J.; Qiu, M.H.; Wan, Q.; Huang, Z.S.; Liu, X.L.; Tang, D.P.; Knopp, D. Smartphone-Based Electrochemical Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Protein. Anal. Chem. 2022, 94, 15155–15161. [Google Scholar] [CrossRef]
- Kruger, L.J.; Klein, J.A.F.; Tobian, F.; Gaeddert, M.; Lainati, F.; Klemm, S.; Schnitzler, P.; Bartenschlager, R.; Cerikan, B.; Neufeldt, C.J.; et al. Evaluation of accuracy, exclusivity, limit-of-detection and ease-of-use of LumiraDx (TM): An antigen-detecting point-of-care device for SARS-CoV-2. Infection 2022, 50, 395–406. [Google Scholar] [CrossRef]
- Li, H.; Te, S.H.; Tavakoli, Y.; Zhang, J.J.; Gin, K.Y.H.; He, Y.L. Rapid detection methods and modelling simulations provide new insights into cyanobacteria detection and bloom management in a tropical reservoir. J. Environ. Manag. 2023, 326, 116730. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Wang, H.T.; Chang, H.F.; Tsai, C.F.; Lin, C.K.; Teng, P.H.; Su, C.; Jeng, C.C.; Lee, P.Y. Development of TaqMan probe-based insulated isothermal PCR (iiPCR) for sensitive and specific on-site pathogen detection. PLoS ONE 2012, 7, e45278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, K.H.; Lee, P.C.; Chai, H.C. Development of insulated isothermal PCR for rapid on-site malaria detection. Malar. J. 2016, 15, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, Y.Y.; Kim, Y.S.; Cheon, S.; Nam, S.; Ku, K.B.; Kim, M.; Cho, N.H.; Park, H.; Alison Lee, P.Y.; Lin, Y.C.; et al. Evaluation and Clinical Validation of Two Field-Deployable Reverse Transcription-Insulated Isothermal PCR Assays for the Detection of the Middle East Respiratory Syndrome-Coronavirus. J. Mol. Diagn. 2017, 19, 817–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, Y.Y.; Rajapakse, R.; Kularatne, S.A.M.; Lee, P.A.; Ku, K.B.; Nam, S.; Chou, P.H.; Tsai, Y.L.; Liu, Y.L.; Chang, H.G.; et al. A Pan-Dengue Virus Reverse Transcription-Insulated Isothermal PCR Assay Intended for Point-of-Need Diagnosis of Dengue Virus Infection by Use of the POCKIT Nucleic Acid Analyzer. J. Clin. Microbiol. 2016, 54, 1528–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carossino, M.; Li, Y.; Lee, P.A.; Tsai, C.F.; Chou, P.H.; Williams, D.; Skillman, A.; Frank Cook, R.; Brown, G.; Chang, H.G.; et al. Evaluation of a field-deployable reverse transcription-insulated isothermal PCR for rapid and sensitive on-site detection of Zika virus. BMC Infect. Dis. 2017, 17, 778. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.J.; Liu, W.L.; Lin, P.C.; Huang, B.Y.; Tsai, C.Y.; Chou, P.H.; Lee, F.C.; Ping, C.F.; Lee, P.A.; Liu, L.T.; et al. An RT-PCR panel for rapid serotyping of dengue virus serotypes 1 to 4 in human serum and mosquito on a field-deployable PCR system. PLoS ONE 2019, 14, e0214328. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.L.; Lin, C.Y.; Chen, C.P.; Lin, Y.C.; Hu, H.C.; Cheng, S.H.; Cheng, C.Y. Clinical validation of an automated reverse transcription-insulated isothermal PCR assay for the detection of severe acute respiratory syndrome coronavirus 2. J. Microbiol. Immunol. Infect. 2021, 54, 522–526. [Google Scholar] [CrossRef]
- Hogan, C.A.; Garamani, N.; Lee, A.S.; Tung, J.K.; Sahoo, M.K.; Huang, C.; Stevens, B.; Zehnder, J.; Pinsky, B.A. Comparison of the Accula SARS-CoV-2 Test with a Laboratory-Developed Assay for Detection of SARS-CoV-2 RNA in Clinical Nasopharyngeal Specimens. J. Clin. Microbiol. 2020, 58, e01072-20. [Google Scholar] [CrossRef]
- Renzoni, A.; Perez, F.; Ngo Nsoga, M.T.; Yerly, S.; Boehm, E.; Gayet-Ageron, A.; Kaiser, L.; Schibler, M. Analytical Evaluation of Visby Medical RT-PCR Portable Device for Rapid Detection of SARS-CoV-2. Diagnostics 2021, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Tricco, A.C.; Watt, J.; Tsokani, S.; Khan, P.A.; Soobiah, C.; Negm, A.; Doherty-Kirby, A.; Taylor, P.; Lunny, C.; et al. Rapid antigen-based and rapid molecular tests for the detection of SARS-CoV-2: A rapid review with network meta-analysis of diagnostic test accuracy studies. BMC Med. 2023, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Lee, C.; Pray, I.W.; Cole, D.; Bigouette, J.P.; Abedi, G.R.; Bushman, D.; Delahoy, M.J.; Currie, D.W.; Cherney, B.; et al. Epidemiologic Characteristics Associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antigen-Based Test Results, Real-Time Reverse Transcription Polymerase Chain Reaction (rRT-PCR) Cycle Threshold Values, Subgenomic RNA, and Viral Culture Results From University Testing. Clin. Infect. Dis. 2021, 73, e1348–e1355. [Google Scholar] [PubMed]
- Barrera-Avalos, C.; Mena, J.; Luraschi, R.; Rojas, P.; Mateluna-Flores, C.; Vallejos-Vidal, E.; Imarai, M.; Sandino, A.M.; Valdes, D.; Vera, R.; et al. Sensitivity analysis of rapid antigen tests for the Omicron SARS-CoV-2 variant detection from nasopharyngeal swab samples collected in Santiago of Chile. Front. Public. Health 2022, 10, 976875. [Google Scholar] [CrossRef]
| Pathogen | ATCC or BEI Resources | POCKIT Central SARS-CoV-2 (orf 1ab) PCR Result | TaqPath COVID-19 RT-PCR Result |
|---|---|---|---|
| Pseudomonas aeruginosa | ATCC 27853 | Negative | Negative |
| Staphylococcus aureus subsp. Aureus Rosenbach | ATCC 8095 | Negative | Negative |
| Influenza A Virus (H1N1) | ATCC VR-1469 | Negative | Negative |
| Influenza B Virus | ATCC VR-1931 | Negative | Negative |
| SARS coronavirus Urbani strain RNA | BEI NR-52346 | Negative | Negative |
| MERS coronavirus EMC/2012, heat-inactivated | BEI NR-50171 | Negative | Negative |
| Human coronavirus NL63 | ATCC VR-3263SD | Negative | Negative |
| Human coronavirus 229E | ATCC VR-740 | Negative | Negative |
| Human coronavirus OC43 | ATCC VR-1558D | Negative | Negative |
| Human adenovirus 5 | ATCC VR-5 | Negative | Negative |
| Respiratory syncytial virus | ATCC VR-1540 | Negative | Negative |
| Human rhinovirus 1A | ATCC VR-1559 | Negative | Negative |
| Human parainfluenza 1 | ATCC VR-94 | Negative | Negative |
| Human parainfluenza 2 | ATCC VR-92 | Negative | Negative |
| Human parainfluenza 3 | ATCC VR-93 | Negative | Negative |
| SARS-CoV-2 Strain | Type of Material | BEI Resources | GenBank Accession Number or GISAID EPI_SET ID | WHO Label | Pango Lineage | Next strain Clade | GISAID clade | POCKIT Central SARS-CoV-2 PCR | TaqPath COVID-19 RT-PCR |
|---|---|---|---|---|---|---|---|---|---|
| USA-WA1/2020 virus | Heat-inactivated | NR-52286 | MT576653 | A | 19B | S | Positive | Positive | |
| Hong Kong/VM20001061/2020 | Genomic RNA | NR-52388 | MT644268 | A | 19B | S | Positive | Positive | |
| Italy-INMI1 | Genomic RNA | NR-52498 | MT077125 | B | 19A | V | Positive | Positive | |
| England/02/2020 | Genomic RNA | NR-52499 | EPI_ISL_407073 | A | 19B | S | Positive | Positive | |
| Singapore/2/2020 | Genomic RNA | NR-52501 | EPI_ISL_407987 | B | 19A | L | Positive | Positive | |
| Germany/BavPat1/2020 | Genomic RNA | NR-52502 | MT270101 | B.1 | 20A | G | Positive | Positive | |
| USA-IL1/2020 | Genomic RNA | NR-52503 | MN988713 | B | 19A | Other | Positive | Positive | |
| USA-CA1/2020 | Genomic RNA | NR-52504 | MN994467 | A | 19B | S | Positive | Positive | |
| USA-AZ1/2020 | Genomic RNA | NR-52505 | MN997409 | A | 19B | S | Positive | Positive | |
| USA-WI1/2020 | Genomic RNA | NR-52506 | MT039887 | B | 19A | L | Positive | Positive | |
| USA-CA3/2020 | Genomic RNA | NR-52507 | MT027062 | B | 19A | L | Positive | Positive | |
| USA-CA4/2020 | Genomic RNA | NR-52508 | MT027063 | B | 19A | L | Positive | Positive | |
| USA-CA2/2020 | Genomic RNA | NR-52509 | MN994468 | B | 19A | Other | Positive | Positive | |
| Chile/Santiago_op4d1/2020 | Genomic RNA | NR-52510 | EPI_ISL_415661 | A.2 | 19B | S | Positive | Positive | |
| New Yor-PV08410/2020 | Genomic RNA | NR-53518 | MT370900 | B.1 | 20C | GH | Positive | Positive | |
| USA/New York 1-PV08001/2020 | Genomic RNA | NR-52389 | MT370904 | B.4 | 19A | Other | Positive | Positive | |
| New York-PV08449/2020 | Genomic RNA | NR-53519 | MT370902 | B.1.319 | 20C | GH | Positive | Positive | |
| USA/New York-PV09158/2020 | Genomic RNA | NR-53520 | MT371034 | B.1 | 20C | GH | Positive | Positive | |
| USA/CA_CDC_5574/2020 virus | Heat-inactivated | NR-55245 | EPI_ISL_751801 | Alpha variant | B.1.1.7 | 20I | GRY | Positive | Positive |
| USA/MD-HP01542/2021 virus | Heat-inactivated | NR-55350 | EPI_ISL_890360 | Beta variant | B.1.351 | 20H | GH | Positive | Positive |
| USA/MD-HP05285/2021 virus | Heat-inactivated | NR-56128 | EPI_ISL_2103264 | Delta variant | B.1.617.2 | 21I | GK | Positive | Positive |
| USA/GA-EHC-2811C/2021 virus | Heat-inactivated | NR-56495 | OL744074 | Omicron variant | B.1.1.529 / BA.1 | 21K | GRA | Positive | Positive |
| Isolate Concentration (TCID50/mL) | Isolate Concentration (Genomic Copies/mL) | POCKIT Central SARS-CoV-2 (orf 1ab) PCR | TaqPath COVID-19 RT-PCR | ||
|---|---|---|---|---|---|
| % (No. of Pos for Target) | % (No. of Pos for Internal Control) | % (No. of Pos for Target) | % (No. of Pos for Internal Control) | ||
| 16 | 3.75 × 104 | 100% (5/5) | 100% (5/5) | 100% (5/5) | 100% (5/5) |
| 12 | 2.81 × 104 | 100% (5/5) | 100% (5/5) | 100% (5/5) | 100% (5/5) |
| 8 | 1.87 × 104 | 100% (5/5) | 100% (5/5) | 100% (5/5) | 100% (5/5) |
| 4 | 9.37 × 103 | 100% (5/5) | 100% (5/5) | 100% (5/5) | 100% (5/5) |
| 1.6 | 3.75 × 103 | 100% (5/5) | 100% (5/5) | 100% (5/5) | 100% (5/5) |
| 0.8 | 1.87 × 103 | 100% (20/20) | 100% (20/20) | 100% (20/20) | 100% (20/20) |
| 0.4 | 9.37 × 102 | 90% (18/20) | 100% (20/20) | 100% (20/20) | 100% (20/20) |
| 0.16 | 3.75 × 102 | 60% (12/20) | 100% (20/20) | 100% (20/20) | 100% (20/20) |
| 0.08 | 1.87 × 102 | 15% (3/20) | 100% (20/20) | 90% (18/20) | 100% (20/20) |
| 0.04 | 9.37 × 101 | 65% (13/20) | 100% (20/20) | ||
| 0.01 | 2.34 × 101 | 10% (2/20) | 100% (20/20) | ||
| TaqPath COVID-19 RT-PCR | Total | |||
|---|---|---|---|---|
| Positive | Negative | |||
| POCKIT Central SARS-CoV-2 (orf 1ab) PCR | Positive | 121 | 0 | 121 |
| Negative | 11 | 51 | 62 | |
| Total | 132 | 51 | 183 | |
| Sensitivity 91.7%; specificity 100%; agreement 94.0% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.-H.; Tai, C.-H.; Ping, C.-F.; Chou, P.-H.; Tsai, Y.-L.; Chung, S.; Bradner, L.; Pentella, M.; Gauger, P.; Zhang, J. Evaluation of a Sample-to-Result POCKIT Central SARS-CoV-2 PCR System. Diagnostics 2023, 13, 2219. https://doi.org/10.3390/diagnostics13132219
Zhu J-H, Tai C-H, Ping C-F, Chou P-H, Tsai Y-L, Chung S, Bradner L, Pentella M, Gauger P, Zhang J. Evaluation of a Sample-to-Result POCKIT Central SARS-CoV-2 PCR System. Diagnostics. 2023; 13(13):2219. https://doi.org/10.3390/diagnostics13132219
Chicago/Turabian StyleZhu, Jin-Hui, Chia-Hsing Tai, Chia-Fong Ping, Pin-Hsing Chou, Yun-Long Tsai, Simon Chung, Laura Bradner, Michael Pentella, Phillip Gauger, and Jianqiang Zhang. 2023. "Evaluation of a Sample-to-Result POCKIT Central SARS-CoV-2 PCR System" Diagnostics 13, no. 13: 2219. https://doi.org/10.3390/diagnostics13132219
APA StyleZhu, J.-H., Tai, C.-H., Ping, C.-F., Chou, P.-H., Tsai, Y.-L., Chung, S., Bradner, L., Pentella, M., Gauger, P., & Zhang, J. (2023). Evaluation of a Sample-to-Result POCKIT Central SARS-CoV-2 PCR System. Diagnostics, 13(13), 2219. https://doi.org/10.3390/diagnostics13132219







