Relationship between Semi-Quantitative Parameters of Thallium-201 Myocardial Perfusion Imaging and Coronary Artery Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Myocardial Perfusion Imaging
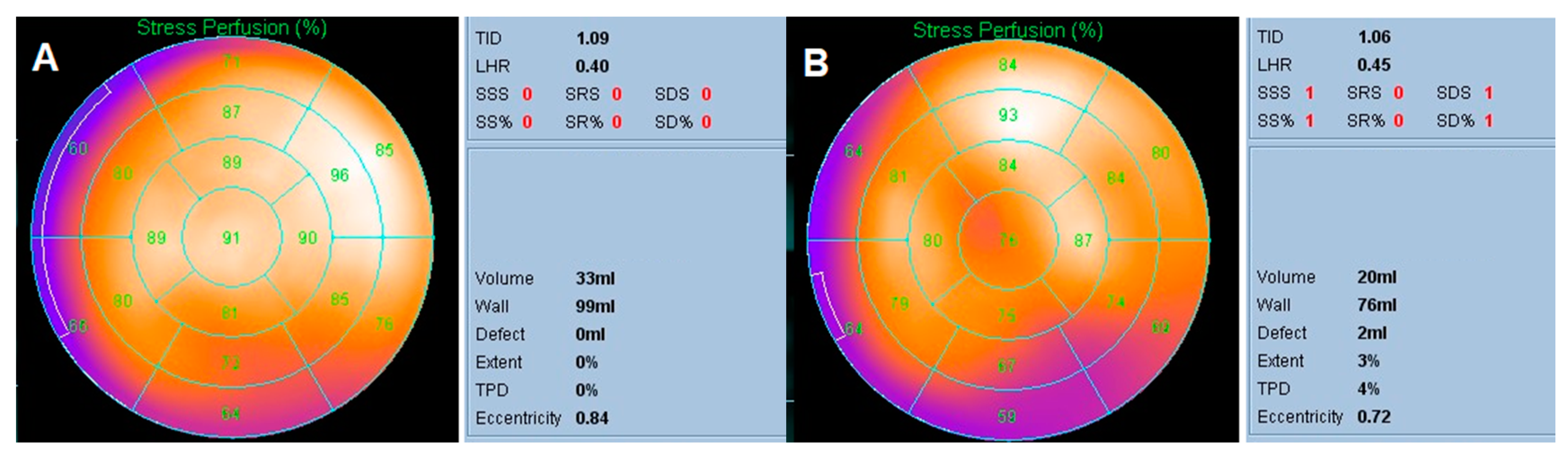
2.3. Semi-Quantitative Parameters
2.4. Cardiac Catheterization
2.5. Statistical Analysis
3. Result
3.1. Patient Characteristics
3.2. Semi-Quantitative Parameters of Myocardial Perfusion Imaging
3.3. Identifying the Most Discriminative Cutoff Values
3.4. sTPD Analysis in Different Subgroups
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, T.; Kannel, W.B. Multiple risk functions for predicting coronary heart disease: The concept, accuracy, and application. Am. Heart J. 1982, 103, 1031–1039. [Google Scholar] [CrossRef]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. Diabetes, blood lipids, and the role of obesity in coronary heart disease risk for women. The Framingham study. Ann. Intern. Med. 1977, 87, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; McGee, D.L. Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham study. Diabetes Care 1979, 2, 120–126. [Google Scholar] [CrossRef]
- Wyman, R.M.; Safian, R.D.; Portway, V.; Skillman, J.J.; McKay, R.G.; Baim, D.S. Current complications of diagnostic and therapeutic cardiac catheterization. J. Am. Coll. Cardiol. 1988, 12, 1400–1406. [Google Scholar] [CrossRef]
- Underwood, S.R.; Anagnostopoulos, C.; Cerqueira, M.; Ell, P.J.; Flint, E.J.; Harbinson, M.; Kelion, A.D.; Al-Mohammad, A.; Prvulovich, E.M.; Shaw, L.J.; et al. Myocardial perfusion scintigraphy: The evidence. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 261–291. [Google Scholar] [CrossRef] [Green Version]
- Berman, D.S.; Shaw, L.J.; Hachamovitch, R.; Friedman, J.D.; Polk, D.M.; Hayes, S.W.; Thomson, L.E.; Germano, G.; Wong, N.D.; Kang, X.; et al. Comparative use of radionuclide stress testing, coronary artery calcium scanning, and noninvasive coronary angiography for diagnostic and prognostic cardiac assessment. Semin. Nucl. Med. 2007, 37, 2–16. [Google Scholar] [CrossRef]
- Strauss, H.W.; Miller, D.D.; Wittry, M.D.; Cerqueira, M.D.; Garcia, E.V.; Iskandrian, A.S.; Schelbert, H.R.; Wackers, F.J. Procedure guideline for myocardial perfusion imaging. Society of Nuclear Medicine. J. Nucl. Med. 1998, 39, 918–923. [Google Scholar]
- Kiat, H.; Maddahi, J.; Roy, L.T.; Van Train, K.; Friedman, J.; Resser, K.; Berman, D.S. Comparison of technetium 99m methoxy isobutyl isonitrile and thallium 201 for evaluation of coronary artery disease by planar and tomographic methods. Am. Heart J. 1989, 117, 1–11. [Google Scholar] [CrossRef]
- Henzlova, M.J.; Duvall, W.L.; Einstein, A.J.; Travin, M.I.; Verberne, H.J. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J. Nucl. Cardiol. 2016, 23, 606–639. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Hayes, S.; Ali, I.; Ruddy, T.D.; Wells, R.G.; Berman, D.S.; Germano, G.; Slomka, P.J. Automatic and visual reproducibility of perfusion and function measures for myocardial perfusion SPECT. J. Nucl. Cardiol. 2010, 17, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germano, G.; Kavanagh, P.B.; Berman, D.S. An automatic approach to the analysis, quantitation and review of perfusion and function from myocardial perfusion SPECT images. Int. J. Card. Imaging 1997, 13, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Hesse, B.; Tägil, K.; Cuocolo, A.; Anagnostopoulos, C.; Bardiès, M.; Bax, J.E.A.N.M.; Bengel, F.; Sokole, E.B.; Davies, G.; Dondi, M.; et al. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 855–897. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.S.; Kang, X.; Gransar, H.; Gerlach, J.; Friedman, J.D.; Hayes, S.W.; Thomson, L.E.; Hachamovitch, R.; Shaw, L.J.; Slomka, P.J.; et al. Quantitative assessment of myocardial perfusion abnormality on SPECT myocardial perfusion imaging is more reproducible than expert visual analysis. J. Nucl. Cardiol. 2009, 16, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.C.; Chen, Y.W.; Hao, C.L.; Chong, J.T.; Lee, C.I.; Tan, H.T.; Wu, M.S.; Wu, J.C. Comparison of automated 4D-MSPECT and visual analysis for evaluating myocardial perfusion in coronary artery disease. Kaohsiung J. Med. Sci. 2008, 24, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Sharir, T.; Germano, G.; Kavanagh, P.B.; Lai, S.; Cohen, I.; Lewin, H.C.; Friedman, J.D.; Zellweger, M.J.; Berman, D.S. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation 1999, 100, 1035–1042. [Google Scholar] [CrossRef] [Green Version]
- Germano, G.; Kiat, H.; Kavanagh, P.B.; Moriel, M.; Mazzanti, M.; Su, H.T.; Train, K.F.V.; Berman, D.S. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J. Nucl. Med. 1995, 36, 2138–2147. [Google Scholar]
- Leslie, W.D.; Tully, S.A.; Yogendran, M.S.; Ward, L.M.; Nour, K.A.; Metge, C.J. Prognostic value of lung sestamibi uptake in myocardial perfusion imaging of patients with known or suspected coronary artery disease. J. Am. Coll. Cardiol. 2005, 45, 1676–1682. [Google Scholar] [CrossRef] [Green Version]
- Slomka, P.J.; Nishina, H.; Berman, D.S.; Akincioglu, C.; Abidov, A.; Friedman, J.D.; Hayes, S.W.; Germano, G. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J. Nucl. Cardiol. 2005, 12, 66–77. [Google Scholar] [CrossRef]
- Abidov, A.; Bax, J.J.; Hayes, S.W.; Hachamovitch, R.; Cohen, I.; Gerlach, J.; Kang, X.; Friedman, J.D.; Germano, G.; Berman, D.S. Transient ischemic dilation ratio of the left ventricle is a significant predictor of future cardiac events in patients with otherwise normal myocardial perfusion SPECT. J. Am. Coll. Cardiol. 2003, 42, 1818–1825. [Google Scholar] [CrossRef]
- Xu, Y.; Arsanjani, R.; Clond, M.; Hyun, M.; Lemley, M.; Fish, M.; Germano, G.; Berman, D.S.; Slomka, P.J. Transient ischemic dilation for coronary artery disease in quantitative analysis of same-day sestamibi myocardial perfusion SPECT. J. Nucl. Cardiol. 2012, 19, 465–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakhki, V.R.; Sadeghi, R.; Zakavi, S.R. Assessment of transient left ventricular dilation ratio via 2-day dipyridamole Tc-99m sestamibi nongated myocardial perfusion imaging. J. Nucl. Cardiol. 2007, 14, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Romanens, M.; Grädel, C.; Saner, H.; Pfisterer, M. Comparison of 99mTc-sestamibi lung/heart ratio, transient ischaemic dilation and perfusion defect size for the identification of severe and extensive coronary artery disease. Eur. J. Nucl. Med. 2001, 28, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Krzanowski, W.J.; Hand, D.J. ROC Curves for Continuous Data, 1st ed.; Chapman and Hall/CRC: London, UK, 2009. [Google Scholar] [CrossRef]
- Germano, G.; Kavanagh, P.B.; Slomka, P.J.; Van Kriekinge, S.D.; Pollard, G.; Berman, D.S. Quantitation in gated perfusion SPECT imaging: The Cedars-Sinai approach. J. Nucl. Cardiol. 2007, 14, 433–454. [Google Scholar] [CrossRef]
- Ficaro, E.P.; Lee, B.C.; Kritzman, J.N.; Corbett, J.R. Corridor4DM: The Michigan method for quantitative nuclear cardiology. J. Nucl. Cardiol. 2007, 14, 455–465. [Google Scholar] [CrossRef]
- Liu, Y.H. Quantification of nuclear cardiac images: The Yale approach. J. Nucl. Cardiol. 2007, 14, 483–491. [Google Scholar] [CrossRef]
- Garcia, E.V.; Faber, T.L.; Cooke, C.D.; Folks, R.D.; Chen, J.; Santana, C. The increasing role of quantification in clinical nuclear cardiology: The Emory approach. J. Nucl. Cardiol. 2007, 14, 420–432. [Google Scholar] [CrossRef]
- Slomka, P.; Xu, Y.; Berman, D.; Germano, G. Quantitative analysis of perfusion studies: Strengths and pitfalls. J. Nucl. Cardiol. 2012, 19, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Petretta, M.; Acampa, W.; Daniele, S.; Petretta, M.P.; Nappi, C.; Assante, R.; Zampella, E.; Costanzo, P.; Perrone-Filardi, P.; Cuocolo, A. Transient ischemic dilation in SPECT myocardial perfusion imaging for prediction of severe coronary artery disease in diabetic patients. J. Nucl. Cardiol. 2013, 20, 45–52. [Google Scholar] [CrossRef]
- Peace, R.A.; Adams, P.C.; Lloyd, J.J. Effect of sex, age, and weight on ejection fraction and end-systolic volume reference limits in gated myocardial perfusion SPECT. J. Nucl. Cardiol. 2008, 15, 86–93. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Nakazato, R.; Hayes, S.; Hachamovitch, R.; Cheng, V.Y.; Gransar, H.; Miranda-Peats, R.; Hyun, M.; Shaw, L.J.; Friedman, J.; et al. Prognostic value of automated vs visual analysis for adenosine stress myocardial perfusion SPECT in patients without prior coronary artery disease: A case-control study. J. Nucl. Cardiol. 2011, 18, 1003–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holly, T.A.; Abbott, B.G.; Al-Mallah, M.; Calnon, D.A.; Cohen, M.C.; DiFilippo, F.P.; Ficaro, E.P.; Freeman, M.R.; Hendel, R.C.; Jain, D.; et al. Single photon-emission computed tomography. J. Nucl. Cardiol. 2010, 17, 941–973. [Google Scholar] [CrossRef]
- Gould, K.L.; Lipscomb, K.; Calvert, C. Compensatory changes of the distal coronary vascular bed during progressive coronary constriction. Circulation 1975, 51, 1085–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Value (%) |
|---|---|
| Age | |
| Mean ± SD | 62.5 ± 12.1 |
| Range | 34–89 |
| Gender | |
| Male | 100 (76.9%) |
| Female | 30 (23.1%) |
| Type 2 diabetes | 60 (49.0%) |
| Hypertension | 73 (56.2%) |
| Dyslipidemia | 62 (47.7%) |
| End-stage renal disease | 14 (10.8%) |
| Smoking history | 35 (26.9%) |
| Prior myocardial infarction | 13 (10.0%) |
| Coronary artery disease | 83 (63.9%) |
| AUC | p | 95% CI | Cutoff Value | Sensitivity | Specificity | 95% CI | |
|---|---|---|---|---|---|---|---|
| TID | 0.630 | 0.0140 | 0.531–0.728 | 1.0 | 0.506 | 0.723 | 0.574–0.844 |
| LHR | 0.609 | 0.0400 | 0.509–0.708 | 0.4 | 0.277 | 0.915 | 0.796–0.976 |
| SSS | 0.780 | <0.0001 | 0.703–0.856 | 8.5 | 0.482 | 1.000 | 0.925–1.000 |
| SRS | 0.786 | <0.0001 | 0.708–0.864 | 0.5 | 0.711 | 0.787 | 0.643–0.893 |
| SDS | 0.650 | 0.0050 | 0.554–0.745 | 1.5 | 0.699 | 0.532 | 0.381–0.679 |
| sTPD | 0.813 | <0.0001 | 0.741–0.884 | 3.5 | 0.735 | 0.745 | 0.597–0.861 |
| rTPD | 0.705 | <0.0001 | 0.617–0.793 | 3.5 | 0.651 | 0.681 | 0.529–0.809 |
| iTPD | 0.675 | 0.0010 | 0.584–0.766 | 0.5 | 0.578 | 0.745 | 0.597–0.861 |
| sTPD | ||
|---|---|---|
| Median (Interquartile Range) | p Value | |
| Gender | 0.0014 * | |
| Male | 5.0 (15.5) | |
| Female | 2.0 (4.0) | |
| Type 2 diabetes | 0.0010 * | |
| Yes | 6.5 (18.5) | |
| No | 2.0 (8.0) | |
| Hypertension | 0.1522 | |
| Yes | 4.0 (9.0) | |
| No | 5.0 (16.0) | |
| Dyslipidemia | 0.0078 * | |
| Yes | 8.0 (19.0) | |
| No | 3.0 (6.0) | |
| End-stage renal disease | 0.0302 * | |
| Yes | 8.5 (31.0) | |
| No | 4.0 (9.5) | |
| History of smoking | 0.8351 | |
| Yes | 4.0 (15.0) | |
| No | 4.0 (9.0) | |
| Prior myocardial infarction | 0.1854 | |
| Yes | 10.0 (20.3) | |
| No | 4.0 (10.3) | |
| AUC | p Value | 95% CI | Optimal Cutoff Value | Sensitivity | Specificity | 95% CI | |
|---|---|---|---|---|---|---|---|
| All | 0.813 | <0.0001 | 0.741–0.884 | 3.5 | 0.735 | 0.745 | 0.597–0.861 |
| Male | 0.811 | <0.0001 | 0.727–0.895 | 8.5 | 0.549 | 0.931 | 0.772–0.992 |
| Female | 0.766 | 0.0150 | 0.588–0.944 | 3.0 | 0.667 | 0.778 | 0.524–0.936 |
| Type 2 diabetes | |||||||
| Positive | 0.828 | <0.0001 | 0.715–0.942 | 8.5 | 0.531 | 1.000 | 0.715–1.000 |
| Negative | 0.777 | <0.0001 | 0.667–0.886 | 2.5 | 0.706 | 0.722 | 0.548–0.858 |
| Dyslipidemia | |||||||
| Positive | 0.857 | <0.0001 | 0.764–0.950 | 8.5 | 0.644 | 0.941 | 0.713–0.999 |
| Negative | 0.767 | <0.0001 | 0.656–0.878 | 3.5 | 0.658 | 0.767 | 0.577–0.901 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Yang, M.-H.; Liu, C.-T.; Chu, H.-L.; Lin, C.-Y.; Yen, W.-J.; Chung, C.-Y.; Ho, S.-Y.; Tyan, Y.-C. Relationship between Semi-Quantitative Parameters of Thallium-201 Myocardial Perfusion Imaging and Coronary Artery Disease. Diagnostics 2020, 10, 772. https://doi.org/10.3390/diagnostics10100772
Chang C-C, Yang M-H, Liu C-T, Chu H-L, Lin C-Y, Yen W-J, Chung C-Y, Ho S-Y, Tyan Y-C. Relationship between Semi-Quantitative Parameters of Thallium-201 Myocardial Perfusion Imaging and Coronary Artery Disease. Diagnostics. 2020; 10(10):772. https://doi.org/10.3390/diagnostics10100772
Chicago/Turabian StyleChang, Chin-Chuan, Ming-Hui Yang, Chih-Ting Liu, Hsiu-Lan Chu, Chia-Yang Lin, Wei-Jheng Yen, Chao-Yu Chung, Sheng-Yow Ho, and Yu-Chang Tyan. 2020. "Relationship between Semi-Quantitative Parameters of Thallium-201 Myocardial Perfusion Imaging and Coronary Artery Disease" Diagnostics 10, no. 10: 772. https://doi.org/10.3390/diagnostics10100772
APA StyleChang, C.-C., Yang, M.-H., Liu, C.-T., Chu, H.-L., Lin, C.-Y., Yen, W.-J., Chung, C.-Y., Ho, S.-Y., & Tyan, Y.-C. (2020). Relationship between Semi-Quantitative Parameters of Thallium-201 Myocardial Perfusion Imaging and Coronary Artery Disease. Diagnostics, 10(10), 772. https://doi.org/10.3390/diagnostics10100772