Foliar Application of Nanoselenium Enhances Drought Tolerance in Brassica oleracea var. italica Through Antioxidant Reinforcement and Pigment Stabilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Experimental Design and Treatments
2.3. Green Synthesis of SeNPs
2.4. Non-Destructive Morphophysiological Measurements
2.5. Biochemical Analyses
- Total chlorophyll content
- Total phenolic content
- Total flavonoid content
2.6. Antioxidant Capacity
- DPPH assay
- FRAP assay
- TEAC Assay
- CUPRAC assay
2.7. Hormonal Modulation
- Indole-3-Acetic Acid (IAA) Content
2.8. Markers of Drought Stress
- Proline content
- Peroxidase (POD) Activity
- Malondialdehyde (MDA) Content
2.9. Leaf Mass per Area (LMA)
2.10. Statistical Analysis
3. Results
3.1. The Morphology and Size of SeNPs Used in Treatment
3.2. The Effects of Drought Stress and SeNP Treatments on Broccoli Growth and Chlorophyll Content (SPAD)
3.3. Biochemical Parameters of Broccoli Under Drought and Nanoparticles Treatments
3.3.1. Photosynthetic Pigments
3.3.2. Bioactive Compounds and Antioxidant Capacity
3.3.3. Markers of Abiotic Factor Stress
3.3.4. Hormonal Modulation (IAA)
3.3.5. Leaf Mass per Area
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Chl | Chlorophyll |
| CUPRAC | Cupric reducing antioxidant capacity |
| DLS | Dynamic light scattering |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DS | Drought-stressed conditions |
| EDAX | Energy Dispersive X-ray Spectroscopy |
| FC | Field capacity |
| FRAP | Ferric Reducing Antioxidant Power |
| FW | Fresh weight |
| IAA | Indole-3-acetic acid |
| MDA | Malondialdehyde |
| SeNPs | Selenium nanoparticles |
| POD | Peroxidase |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| TEM | Transmission electron microscopy |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| WW | Well-watered |
References
- Verma, K.K.; Song, X.-P.; Singh, M.; Huang, H.-R.; Bhatt, R.; Xu, L.; Kumar, V.; Li, Y.-R. Influence of Nanosilicon on Drought Tolerance in Plants: An Overview. Front. Plant Sci. 2022, 13, 1014816. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Gupta, M.K.; Tripathi, S. Nanotechnology: A Promising Technology in Sustainable Agriculture. Discov. Biotechnol. 2025, 2, 12. [Google Scholar] [CrossRef]
- Samynathan, R.; Venkidasamy, B.; Ramya, K.; Muthuramalingam, P.; Shin, H.; Kumari, P.S.; Thangavel, S.; Sivanesan, I. A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance. Plants 2023, 12, 853. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Shumeyko, S.A.; Semenova, N.A.; Dorokhov, A.S.; Gudkov, S.V. Selenium Nanoparticles (Se NPs) as Agents for Agriculture Crops with Multiple Activity: A Review. Agronomy 2025, 15, 1591. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, H.; Cen, H.; Qian, W.; Wang, Y.; Ren, M.; Cheng, Y. Effects of Various Forms of Selenium Biofortification on Photosynthesis, Secondary Metabolites, Quality, and Lignin Deposition in Alfalfa (Medicago sativa L.). Field Crops Res. 2023, 292, 108801. [Google Scholar] [CrossRef]
- García Márquez, V.; Morelos Moreno, Á.; Benavides Mendoza, A.; Medrano Macías, J. Ionic Selenium and Nanoselenium as Biofortifiers and Stimulators of Plant Metabolism. Agronomy 2020, 10, 1399. [Google Scholar] [CrossRef]
- Tsivileva, O. Selenium-Containing Nanoformulations Capable of Alleviating Abiotic Stress in Plants. Int. J. Mol. Sci. 2025, 26, 1697. [Google Scholar] [CrossRef]
- Zeeshan, M.; Wang, X.; Salam, A.; Wu, H.; Li, S.; Zhu, S.; Chang, J.; Chen, X.; Zhang, Z.; Zhang, P. Selenium Nanoparticles Boost the Drought Stress Response of Soybean by Enhancing Pigment Accumulation, Oxidative Stress Management and Ultrastructural Integrity. Agronomy 2024, 14, 1372. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, C.; Huang, L.; Wu, J.; Sun, P.; Zhan, J.; Wu, J.; Liu, S.; Zhou, C.; Hu, L.; et al. Effects of Organic Selenium and Nanoselenium on Drought Stress of Pak Choi (Brassica chinensis var. pekinensis. cv. ‘Suzhouqing’) and Its Transcriptomic Analysis. Agronomy 2023, 14, 78. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez De La Lastra, J.M.; Munguira, E.B.; Juan, C.A.; Pérez-Lebeña, E. The Multifaceted Health Benefits of Broccoli—A Review of Glucosinolates, Phenolics and Antimicrobial Peptides. Molecules 2025, 30, 2262. [Google Scholar] [CrossRef]
- Siomos, A.S.; Koularmanis, K.; Tsouvaltzis, P. The Impacts of the Emerging Climate Change on Broccoli (Brassica oleracea L. var. italica Plenck.) Crop. Horticulturae 2022, 8, 1032. [Google Scholar] [CrossRef]
- Chevilly, S.; Dolz-Edo, L.; López-Nicolás, J.M.; Morcillo, L.; Vilagrosa, A.; Yenush, L.; Mulet, J.M. Physiological and Molecular Characterization of the Differential Response of Broccoli (Brassica oleracea var. Italica) Cultivars Reveals Limiting Factors for Broccoli Tolerance to Drought Stress. J. Agric. Food Chem. 2021, 69, 10394–10404. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Khan, M.A.; Kang, S.-M.; Hamayun, M.; Lee, I.-J. Enhancement of Drought-Stress Tolerance of Brassica oleracea var. italica L. by Newly Isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500–1509. [Google Scholar] [CrossRef]
- Mohan, V.R.; MacDonald, M.T.; Abbey, L. Impact of Water Deficit Stress on Brassica Crops: Growth and Yield, Physiological and Biochemical Responses. Plants 2025, 14, 1942. [Google Scholar] [CrossRef]
- Šola, I.; Gmižić, D.; Miškec, K.; Ludwig-Müller, J. Impact of Water Stress on Metabolic Intermediates and Regulators in Broccoli Sprouts, and Cellular Defense Potential of Their Extracts. Int. J. Mol. Sci. 2025, 26, 632. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and Silica Nanostructure-Based Recovery of Strawberry Plants Subjected to Drought Stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Peijnenburg, W. Mitigation of the Effect of Drought on Growth and Yield of Pomegranates by Foliar Spraying of Different Sizes of Selenium Nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Priyanka, A.S.; Haripriya, S.; Kalarani, M.K.; Umapathi, M. Nanoselenium Improves Drought Tolerance of Sorghum via Reduced Transpiration Rate and Osmolytic Accumulation. S. Afr. J. Bot. 2024, 172, 93–108. [Google Scholar] [CrossRef]
- Vicas, S.I.; Cavalu, S.; Laslo, V.; Tocai, M.; Costea, T.O.; Moldovan, L. Growth, Photosynthetic Pigments, Phenolic, Glucosinolates Content and Antioxidant Capacity of Broccoli Sprouts in Response to Nanoselenium Particles Supply. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 821–828. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. Nano-Enabled Agriculture: How Do Nanoparticles Cross Barriers in Plants? Plant Commun. 2022, 3, 100346. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Rui, Y. Nanomaterials in Broccoli Production: Current Applications and Future Prospects. Agronomy 2025, 15, 1193. [Google Scholar] [CrossRef]
- Daler, S.; Korkmaz, N.; Kılıç, T.; Hatterman-Valenti, H.; Karadağ, A.; Kaya, O. Modulatory Effects of Selenium Nanoparticles against Drought Stress in Some Grapevine Rootstock/Scion Combinations. Chem. Biol. Technol. Agric. 2024, 11, 108. [Google Scholar] [CrossRef]
- Kang, L.; Jia, Y.; Wu, Y.; Liu, H.; Zhao, D.; Ju, Y.; Pan, C.; Mao, J. Selenium Nanoparticle and Melatonin Treatments Improve Melon Seedling Growth by Regulating Carbohydrate and Polyamine. Int. J. Mol. Sci. 2024, 25, 7830. [Google Scholar] [CrossRef] [PubMed]
- Khai, H.D.; Mai, N.T.N.; Tung, H.T.; Luan, V.Q.; Cuong, D.M.; Ngan, H.T.M.; Chau, N.H.; Buu, N.Q.; Vinh, N.Q.; Dung, D.M.; et al. Selenium Nanoparticles as in Vitro Rooting Agent, Regulates Stomata Closure and Antioxidant Activity of Gerbera to Tolerate Acclimatization Stress. Plant Cell Tissue Organ Cult. 2022, 150, 113–128. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V. Modulatory Effects of Selenium Nanoparticle against Drought Stress in Canola Plants. BMC Plant Biol. 2025, 25, 532. [Google Scholar] [CrossRef]
- Nayek, S.; Choudhury, I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Memete, A.R.; Sărac, I.; Teusdea, A.C.; Budău, R.; Bei, M.; Vicas, S.I. Bioactive Compounds and Antioxidant Capacity of Several Blackberry (Rubus spp.) Fruits Cultivars Grown in Romania. Horticulturae 2023, 9, 556. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Miere (Groza), F.; Teusdea, A.C.; Laslo, V.; Fritea, L.; Moldovan, L.; Costea, T.; Uivarosan, D.; Vicas, S.I.; Pallag, A. Natural Polymeric Beads for Encapsulation of Stellaria Media Extract with Antioxidant Properties. Mater. Plast. 2019, 56, 671–679. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (TEAC) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Sarker, A.; Al-Rashid, J. Analytical Protocol for Determination of Indole 3 Acetic Acid (IAA) Production by Plant Growth Promoting Bacteria (PGPB); Technical Report; Bangladesh Agricultural University: Mymensingh, Bangladesh, 2013. [Google Scholar]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for Determination of Proline in Plants. In Plant Stress Tolerance; Sunkar, R., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 317–331. ISBN 978-1-60761-701-3. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yoo, Y.J. Peroxidase Production from Carrot Hairy Root Cell Culture. Enzym. Microb. Technol. 1996, 18, 531–535. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The Role of Selenium and Nano Selenium on Physiological Responses in Plant: A Review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Mahmud, J.A.; Nahar, K.; Fujita, M. Selenium in Plants: Boon or Bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium Application in Two Methods Promotes Drought Tolerance in Solanum Lycopersicum Plant by Inducing the Antioxidant Defense System. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Jiao, S.; Zeng, F.; Huang, Y.; Zhang, L.; Mao, J.; Chen, B. Physiological, Biochemical and Molecular Responses Associated with Drought Tolerance in Grafted Grapevine. BMC Plant Biol. 2023, 23, 110. [Google Scholar] [CrossRef]
- Luo, Q.; Xie, H.; Chen, Z.; Ma, Y.; Yang, H.; Yang, B.; Ma, Y. Morphology, Photosynthetic Physiology and Biochemistry of Nine Herbaceous Plants under Water Stress. Front. Plant Sci. 2023, 14, 1147208. [Google Scholar] [CrossRef]
- Miettinen, I.; Zhang, C.; Alonso, L.; Fernández-Marín, B.; García-Plazaola, J.I.; Grebe, S.; Porcar-Castell, A.; Atherton, J. Hyperspectral Imaging Reveals Differential Carotenoid and Chlorophyll Temporal Dynamics and Spatial Patterns in Scots Pine Under Water Stress. Plant Cell Environ. 2025, 48, 1535–1554. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Khan, N. Health Effects of Dark Chocolate. Emerg. Chall. Agric. Food Sci. 2023, 8, 76–87. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Siddika, A.; Bardhan, K.; Hosen, M.S.; Prasad, P.V.V. Selenium and Its Nanoparticles Modulate the Metabolism of Reactive Oxygen Species and Morpho-Physiology of Wheat (Triticum aestivum L.) to Combat Oxidative Stress under Water Deficit Conditions. BMC Plant Biol. 2024, 24, 578. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xin, L.; Gao, F.; Liu, H.; Wang, X. Effects of Foliar Selenium Application on Oxidative Damage and Photosynthetic Properties of Greenhouse Tomato under Drought Stress. Plants 2024, 13, 302. [Google Scholar] [CrossRef]
- Su, Y.; Fu, F.; Ou, X.; Gong, L.; Liu, H.; Sun, Y. Response of Selenium Pools to Drought Stress by Regulating Physio-biochemical Attributes and Anatomical Changes in Gentiana Macrophylla. Ecotoxicol. Environ. Saf. 2024, 280, 116591. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Hauenstein, M.; Kräutler, B. Chlorophyll Breakdown—Regulation, Biochemistry and Phyllobilins as Its Products. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 90, pp. 213–271. ISBN 978-0-08-102752-3. [Google Scholar]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Abd El-Lateef, H.M.; Yasir, M.; Tanveer Shah, S.; Ullah, I.; Elsayed Mohamed Mohamed, M.; Ali, I.; Ali, F.; et al. Effect of Azospirillum and Azotobacter Species on the Performance of Cherry Tomato under Different Salinity Levels. Gesunde Pflanz. 2022, 74, 487–499. [Google Scholar] [CrossRef]
- Yu, H.; Miao, P.; Li, D.; Wu, Y.; Zhou, C.; Pan, C. Improving Red Pitaya Fruit Quality by Nano-Selenium Biofortification to Enhance Phenylpropanoid and Betalain Biosynthesis. Ecotoxicol. Environ. Saf. 2023, 267, 115653. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Plant Hormesis: The Energy Aspect of Low and High-Dose Stresses. Plant Stress 2024, 14, 100628. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, S.; Li, K.; Guo, Y. Selenium Nanoparticles Regulate Antioxidant Enzymes and Flavonoid Compounds in Fagopyrum Dibotrys. Plants 2024, 13, 3098. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, P.; Nie, M.; Zhan, J.; Huang, L.; Wu, J.; Zhang, J.; He, X.; Li, N.; Hu, L.; et al. Integration of Metabolomics and Transcriptomics Analyses Reveals the Effects of Nano-Selenium on Pak Choi. Sci. Rep. 2025, 15, 11215. [Google Scholar] [CrossRef]
- Asghari, J.; Mahdavikia, H.; Rezaei-Chiyaneh, E.; Banaei-Asl, F.; Amani Machiani, M.; Harrison, M.T. Selenium Nanoparticles Improve Physiological and Phytochemical Properties of Basil (Ocimum basilicum L.) under Drought Stress Conditions. Land 2023, 12, 164. [Google Scholar] [CrossRef]
- Ma, J.; Wu, X.; Xie, H.; Geng, G.; Qiao, F. Molecular Regulation of Phenylpropanoid and Flavonoid Biosynthesis Pathways Based on Transcriptomic and Metabolomic Analyses in Oat Seedlings Under Sodium Selenite Treatment. Biology 2025, 14, 1131. [Google Scholar] [CrossRef]
- Ikram, S.; Li, Y.; Lin, C.; Yi, D.; Heng, W.; Li, Q.; Tao, L.; Hongjun, Y.; Weijie, J. Selenium in Plants: A Nexus of Growth, Antioxidants, and Phytohormones. J. Plant Physiol. 2024, 296, 154237. [Google Scholar] [CrossRef] [PubMed]
- Azizi, I.; Esmaielpour, B.; Fatemi, H. Effect of Foliar Application of Selenium on Morphological and Physiological Indices of Savory (Satureja hortensis) under Cadmium Stress. Food Sci. Nutr. 2020, 8, 6539–6549. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cui, H.; Li, H.; Qiang, X.; Han, Q.; Liu, H. Foliar Application of Selenium Enhances Drought Tolerance in Tomatoes by Modulating the Antioxidative System and Restoring Photosynthesis. Agronomy 2024, 14, 1184. [Google Scholar] [CrossRef]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H. Selenium Promotes the Growth and Photosynthesis of Tomato Seedlings Under Salt Stress by Enhancing Chloroplast Antioxidant Defense System. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases Have More Functions than a Swiss Army Knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Sadeghzadeh, N.; Ebrahimi, N.; Sadeghzadeh, B.; Mohammadi, S.A. Influence of Selenium in Drought-Stressed Wheat Plants under Greenhouse and Field Conditions. Acta Agric. Slov. 2015, 105, 175–191. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A Compelling Platform for Sophisticated Plant Science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-Proline Biosynthesis, Signal Transduction, Transport, Accumulation and Its Vital Role in Plants during Variable Environmental Conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2024, 14, 2. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in Action: Signalling, Transport and the Control of Plant Growth and Development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Shani, E.; Salehin, M.; Zhang, Y.; Sanchez, S.E.; Doherty, C.; Wang, R.; Mangado, C.C.; Song, L.; Tal, I.; Pisanty, O.; et al. Plant Stress Tolerance Requires Auxin-Sensitive Aux/IAA Transcriptional Repressors. Curr. Biol. 2017, 27, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Blumwald, E. Hormone Balance and Abiotic Stress Tolerance in Crop Plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass per Area (LMA): A Meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of Leaf Stomatal Density to Water Status and Its Relationship with Photosynthesis in a Grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
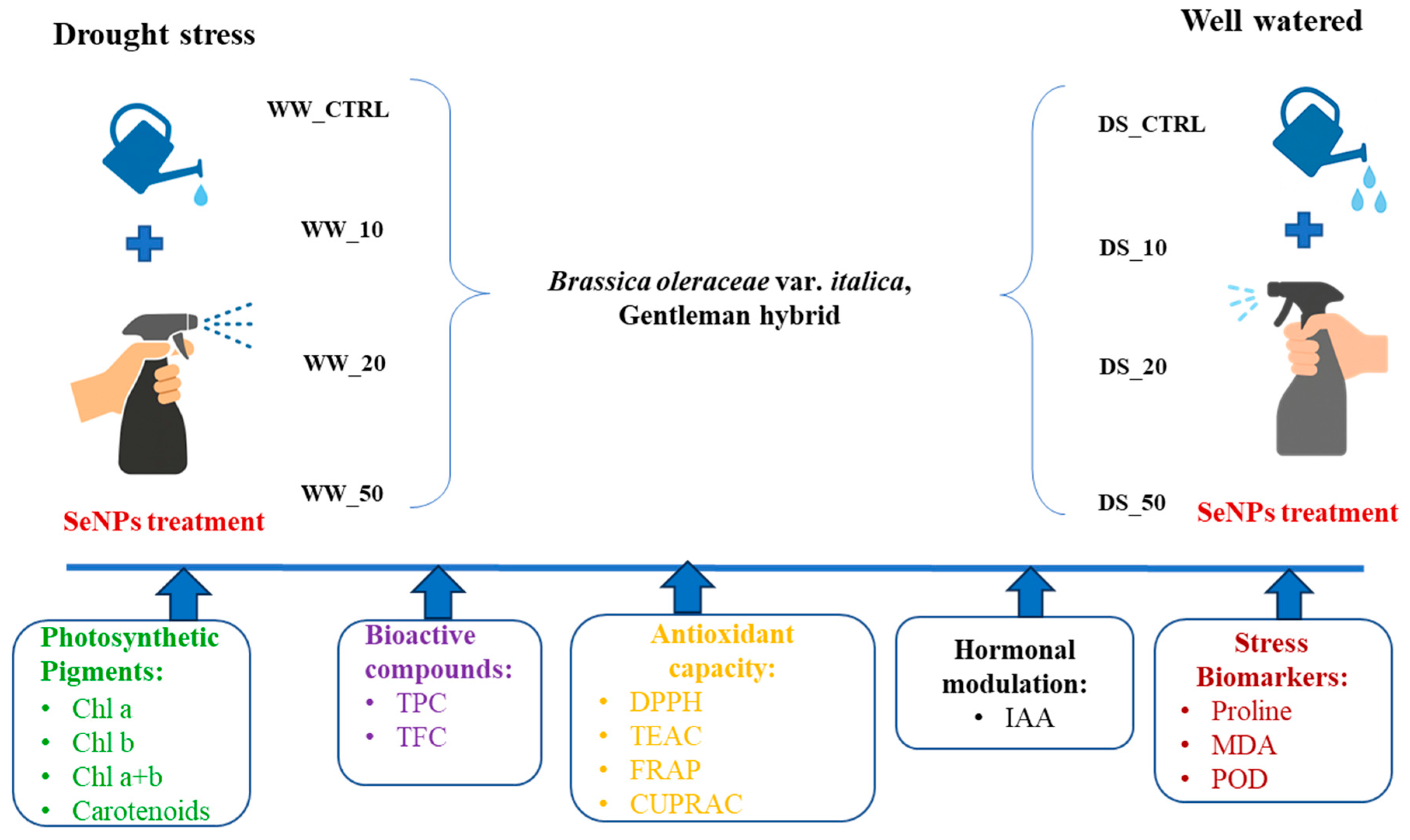
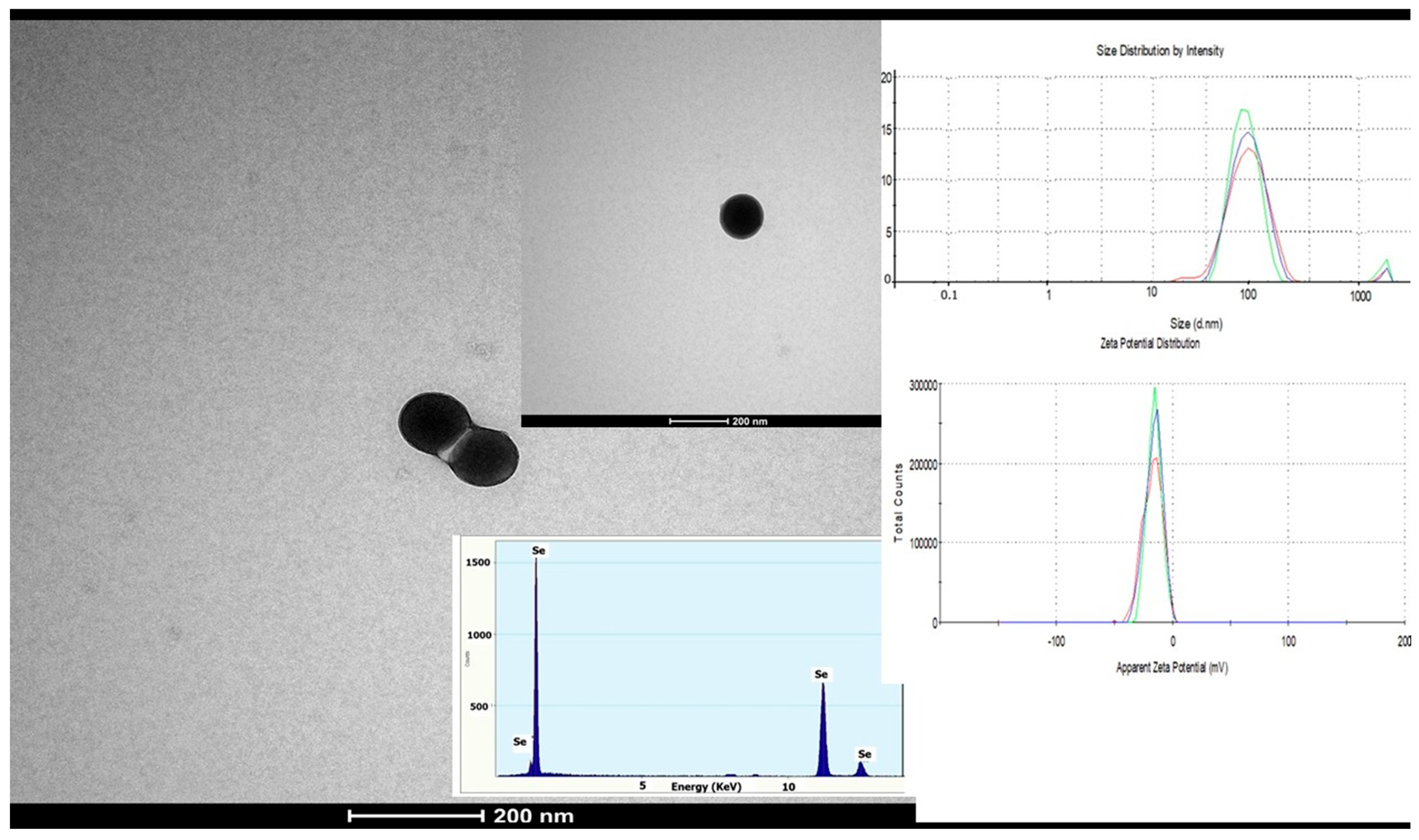
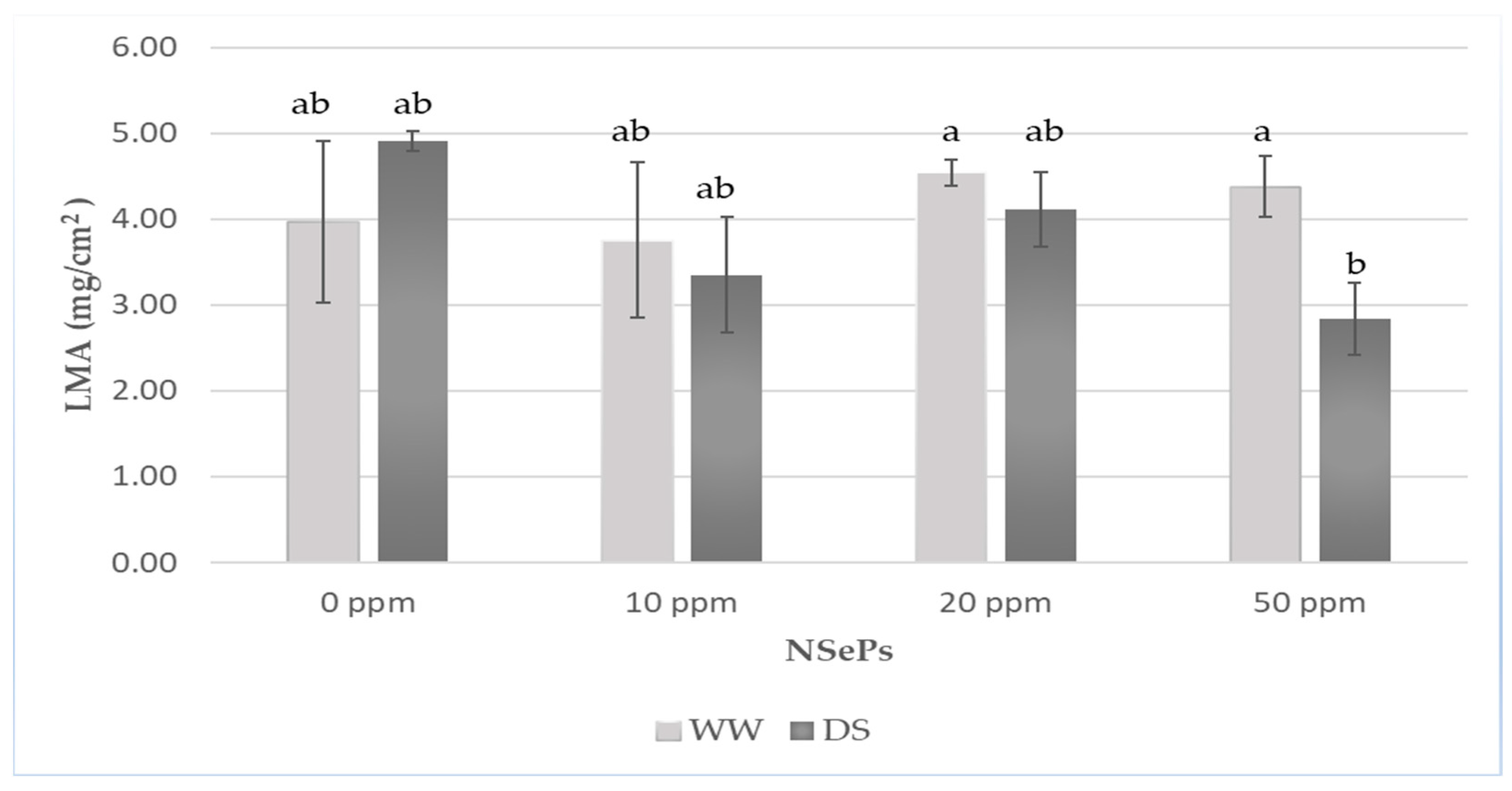
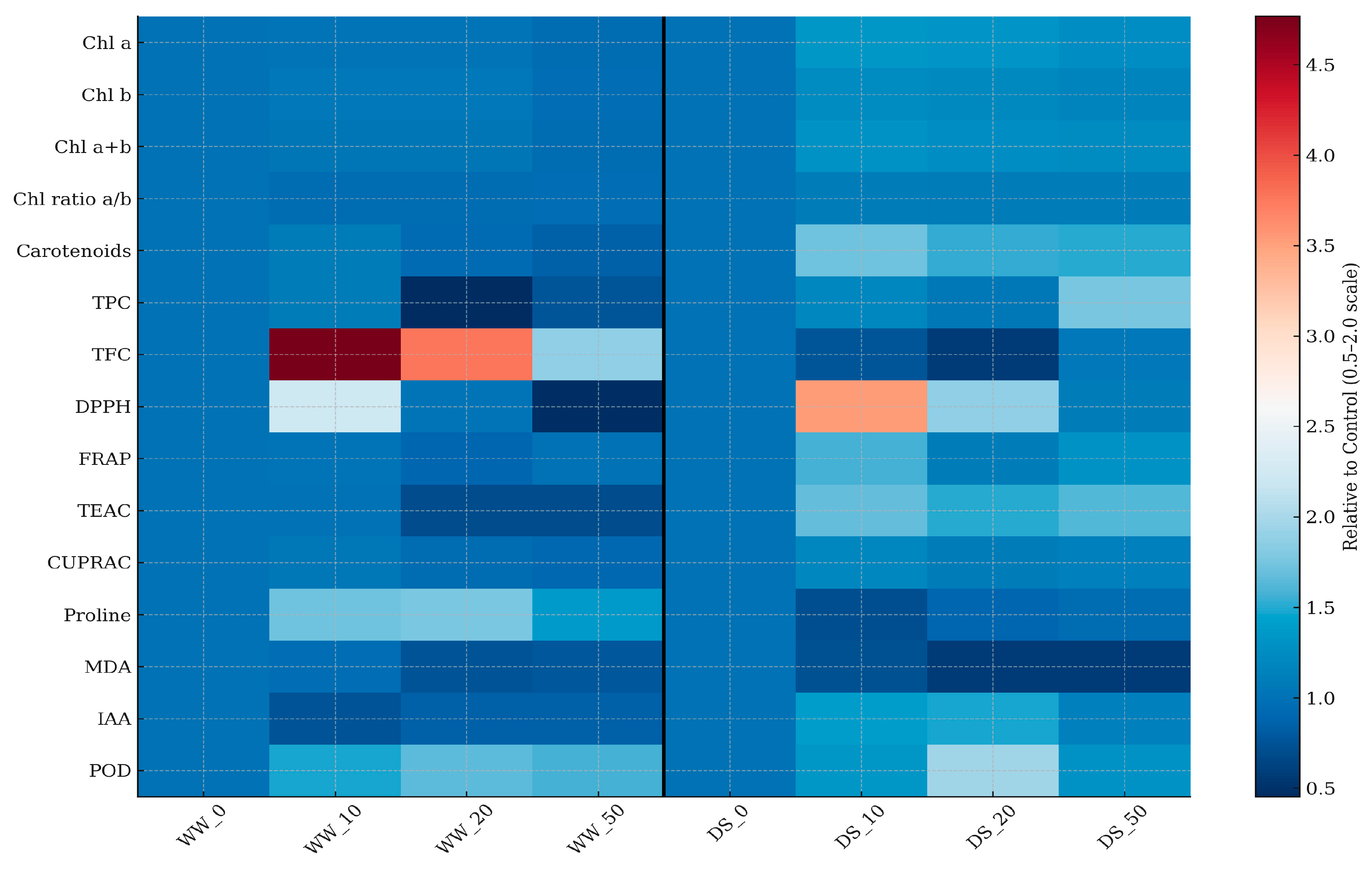
| Description | Label |
|---|---|
| Control under well-watered conditions without SeNP treatment | WW_0 |
| SeNP treatment at 10 ppm under well-watered conditions | WW_10 |
| SeNP treatment at 20 ppm under well-watered conditions | WW_20 |
| SeNP treatment at 50 ppm under well-watered conditions | WW_50 |
| Control under drought stress without SeNP treatment | DS_0 |
| SeNP treatment at 10 ppm under drought stress | DS_10 |
| SeNP treatment at 20 ppm under drought stress | DS_20 |
| SeNP treatment at 50 ppm under drought stress | DS_50 |
| Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DS_0 | DS_10 | DS_20 | DS_50 | WW_0 | WW_10 | WW_20 | WW_50 | ||
| Height (cm) | T1 | 87.53 ± 1.43 a | 86.25 ± 9.67 a | 84.80 ± 4.40 a | 87.53 ± 1.22 a | 84.88 ± 7.85 a | 84.40 ± 5.08 a | 85.34 ± 2.91 a | 88.50 ± 5.17 a |
| T2 | 92.12 ± 5.33 a | 92.16 ± 10.08 a | 86.10 ± 3.78 a | 91.90 ± 9.12 a | 98.04 ± 10.71 a | 86.78 ± 2.59 a | 87.82 ± 3.96 a | 91.78 ± 3.99 a | |
| T3 | 95.38 ± 6.83 ab | 101.58 ± 13.07 ab | 86.80 ± 5.07 b | 96.40 ± 14.93 ab | 111.78 ± 6.97 a | 105.64 ± 4.47 ab | 98.16 ± 6.52 ab | 103.26 ± 17.26 ab | |
| T4 | 100.10 ± 17.37 bc | 105.40 ± 14.38 bc | 92.20 ± 6.61 c | 103.94 ± 16.24 bc | 131.32 ± 9.13 a | 114.34 ± 2.13 ab | 102.46 ± 7.87 bc | 111.06 ± 18.32 bc | |
| T5 | 103.67 ± 19.13 bc | 110.60 ± 15.53 bc | 102.80 ± 9.83 c | 112.40 ± 17.92 bc | 135.20 ± 13.18 a | 123.20 ± 2.28 ab | 107.50 ± 11.15 bc | 121.60 ± 19.88 abc | |
| Stem (mm) | T1 | 7.27 ± 0.51 a | 7.49 ± 1.00 a | 7.27 ± 0.45 a | 7.23 ± 0.63 a | 7.48 ± 0.70 a | 7.32 ± 0.74 a | 7.23 ± 0.13 a | 7.40 ± 0.56 a |
| T2 | 7.66 ± 0.6 a | 7.70 ± 0.74 a | 7.77 ± 0.32 a | 7.53 ± 0.48 a | 7.96 ± 0.83 a | 7.57 ± 0.82 a | 7.41 ± 0.19 a | 7.98 ± 0.66 a | |
| T3 | 7.55 ± 1.02 a | 7.77 ± 0.75 a | 7.84 ± 0.33 a | 7.70 ± 0.45 a | 8.05 ± 0.85 a | 7.64 ± 0.81 a | 7.58 ± 0.18 a | 8.09 ± 0.62 a | |
| T4 | 9.20 ± 0.49 ab | 8.76 ± 0.91 ab | 8.67 ± 0.24 ab | 8.35 ± 0.34 b | 9.75 ± 0.85 a | 9.08 ± 1.05 ab | 8.36 ± 0.44 b | 8.61 ± 0.56 ab | |
| T5 | 9.61 ± 1.08 abc | 8.97 ± 0.71 bc | 9.21 ± 0.42 c | 8.78 ± 0.27 c | 10.74 ± 0.76 a | 10.40 ± 0.64 ab | 9.08 ± 1.05 c | 8.84 ± 0.55 c | |
| SPAD index | T1 | 51.38 ± 7.22 a | 50.06 ± 5.76 a | 46.16 ± 2.87 a | 48.02 ± 5.84 a | 47.68 ± 5.21 a | 50.62 ± 2.70 a | 46.12 ± 1.46 a | 46.92 ± 3.99 a |
| T2 | 49.74 ± 2.69 a | 48.28 ± 5.21 a | 46.02 ± 3.23 a | 48.72 ± 2.06 a | 49.98 ± 1.72 a | 49.04 ± 2.11 a | 48.20 ± 2.28 a | 45.82 ± 3.04 a | |
| T3 | 56.84 ± 6.54 a | 54.66 ± 2.32 a | 54.76 ± 3.73 a | 55.98 ± 1.00 a | 56.00 ± 3.78 a | 55.68 ± 3.01 a | 52.22 ± 4.50 a | 52.56 ± 3.03 a | |
| T4 | 65.84 ± 7.48 ab | 57.02 ± 2.29 c | 58.40 ± 4.42 bc | 58.14 ± 1.23 bc | 68.08 ± 9.10 a | 57.66 ± 3.30 bc | 57.96 ± 4.08 bc | 55.54 ± 2.44 c | |
| T5 | 70.00 ± 8.09 ab | 64.66 ± 4.03 bc | 63.54 ± 4.93 bc | 63.14 ± 1.87 bc | 73.34 ± 8.07 a | 71.40 ± 1.32 ab | 67.48 ± 2.99 abc | 61.42 ± 5.37 c | |
| Treatments | Chl a (µg/mL) | Chl b (µg/mL) | Total Chl (µg/mL) | Chl a/b | CARs (µg/mL) | Total Chl/CARs |
|---|---|---|---|---|---|---|
| DS_0 | 8.18 ± 0.02 a | 5.13 ± 0.01 a | 13.31 ± 0.04 a | 1.59 ± 0.00 a | 1.26 ± 0.02 a | 10.54 ± 0.16 b |
| DS_10 | 11.09 ± 0.02 d | 6.31 ± 0.02 c | 17.40 ± 0.03 d | 1.76 ± 0.00 b | 2.18 ± 0.01 e | 7.97 ± 0.04 e |
| DS_20 | 10.76 ± 0.01 c | 6.24 ± 0.02 c | 17.00 ± 0.03 c | 1.73 ± 0.00 b | 2.00 ± 0.01 d | 8.50 ± 0.04 d |
| DS_50 | 10.41 ± 0.01 b | 5.96 ± 0.02 b | 16.37 ± 0.03 b | 1.75 ± 0.00 b | 1.91 ± 0.02 c | 8.58 ± 0.06 d |
| WW_0 | 11.80 ± 0.02 e | 7.18 ± 0.02 e | 18.98 ± 0.02 f | 1.65 ± 0.01 a | 1.98 ± 0.03 d | 9.58 ± 0.11 c |
| WW_10 | 12.01 ± 0.28 e | 7.63 ± 0.18 f | 19.64 ± 0.09 g | 1.58 ± 0.07 a | 2.14 ± 0.06 e | 9.18 ± 0.23 c |
| WW_20 | 12.01 ± 0.28 e | 7.61 ± 0.17 f | 19.55 ± 0.19 g | 1.57 ± 0.08 a | 1.84 ± 0.06 c | 10.63 ± 0.24 b |
| WW_50 | 11.15 ± 0.04 d | 6.95 ± 0.03 d | 18.11 ± 0.02 e | 1.60 ± 0.01 a | 1.63 ± 0.04 b | 11.09 ± 0.28 a |
| Mean | 10.93 ± 0.19 | 6.63 ± 0.13 | 17.55 ± 0.31 | 1.65 ± 0.01 | 1.86 ± 0.05 | 9.51 ± 1.10 |
| MS (Treatment) | 7.859 ** | 3.800 ** | 21.715 ** | 0.031 ** | 0.448 ** | 4.091 ** |
| MS (Error) | 0.020 | 0.008 | 0.006 | 0.001 | 0.001 | 0.033 |
| Treatments | TPC (mg GAE/g FW) | TFC (mg QE/g FW) | DPPH (µmol TE/g FW) | FRAP (µmol TE/g FW) | TEAC (µmol TE/g FW) | CUPRAC (µmol TE/g FW) |
|---|---|---|---|---|---|---|
| DS_0 | 3.41 ± 0.20 bc | 7.26 ± 0.77 d | 5.60 ± 0.60 b | 18.47 ± 0.35 a | 11.64 ± 1.41 a | 221.30 ± 5.38 a |
| DS_10 | 4.08 ± 0.24 c | 5.59 ± 0.27 c | 19.75 ± 0.26 ef | 29.16 ± 3.63 c | 19.48 ± 1.54 b | 266.30 ± 36.25 ef |
| DS_20 | 3.57 ± 0.04 bc | 4.19 ± 0.26 b | 10.54 ± 0.59 c | 20.49 ± 0.56 ab | 17.55 ± 0.38 b | 244.43 ± 1.50 bc |
| DS_50 | 6.01 ± 0.67 d | 7.75 ± 0.75 d | 22.14 ± 0.26 f | 24.13 ± 1.12 bc | 18.96 ± 0.25 b | 252.43 ± 6.50 cd |
| WW_0 | 3.15 ± 0.64 bc | 0.78 ± 0.61 a | 15.23 ± 2.22 d | 20.22 ± 0.56 ab | 18.06 ± 1.15 b | 260.18 ± 0.50de |
| WW_10 | 3.43 ± 0.60 bc | 3.73 ± 0.21 b | 18.81 ± 0.17 e | 20.63 ± 2.10 ab | 18.06 ± 0.38 b | 273.73 ± 3.02 f |
| WW_20 | 1.43 ± 0.16 a | 5.77 ± 0.13 c | 2.70 ± 0.09 a | 18.12 ± 0.28 a | 12.66 ± 0.38 a | 245.68 ± 0.75 bc |
| WW_50 | 2.41 ± 0.64 ab | 1.47 ± 0.43 a | 7.39 ± 1.02 b | 20.08 ± 3.49 ab | 12.54 ± 0.51 a | 239.93 ± 1.25 b |
| Mean | 3.44 ± 1.32 | 4.57 ± 2.45 | 12.77 ± 6.95 | 21.41 ± 3.83 | 16.12 ± 3.19 | 250.49 ± 16.06 |
| MS (Treatment) | 5.228 ** | 19.137 ** | 156.873 ** | 39.219 ** | 31.643 ** | 819.170 ** |
| MS (Error) | 0.218 | 0.238 | 0.855 | 3.982 | 0.812 | 12.255 |
| Treatments | MDA (µmol/g FW) | Proline (µmol/g FW) | POD (U/g FW) | IAA (nmol/g FW) |
|---|---|---|---|---|
| DS_0 | 26.22 ± 3.36 e | 3.83 ± 0.07 e | 52.46 ± 1.15 b | 145.00 ± 4.59 a |
| DS_10 | 19.20 ± 0.95 cd | 2.75 ± 0.03 c | 71.38 ± 0.29 f | 203.30 ± 8.51 d |
| DS_20 | 15.38 ± 0.31 abc | 3.23 ± 0.03 d | 102.48 ± 1.58 g | 212.80 ± 6.22 de |
| DS_50 | 15.22 ± 0.45 ab | 3.03 ± 0.09 cd | 68.22 ± 0.86 ef | 164.70 ± 3.28 ab |
| WW_0 | 19.56 ± 0.84 d | 1.32 ± 0.21 a | 40.13 ± 0.29 a | 229.90 ± 4.91 e |
| WW_10 | 18.99 ± 0.39 bcd | 2.84 ± 0.08 b | 59.34 ± 0.00 c | 172.90 ± 0.33 bc |
| WW_20 | 14.75 ± 1.16 a | 2.34 ± 0.18 b | 66.50 ± 2.29 de | 196.10 ± 1.97 cd |
| WW_50 | 15.24 ± 0.54 ab | 1.23 ± 0.21 a | 63.21 ± 1.00 d | 194.80 ± 22.27 cd |
| Mean | 18.07 ± 0.79 | 2.50 ± 0.18 | 65.47 ± 3.51 | 189.96 ± 5.59 |
| MS (Treatment) | 45.082 ** | 2.441 ** | 971.692 ** | 2273.934 ** |
| MS (Error) | 1.873 | 0.018 | 1.371 | 83.405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Vicas, S.I.; Rosan, C.A.; Padilla-Contreras, D.; Cavalu, S.D.; Zsiros, R.; Borza, I.M.; Gitea, D.; Iancu, C.V.; Yildirim, E.; Aydin, M.; et al. Foliar Application of Nanoselenium Enhances Drought Tolerance in Brassica oleracea var. italica Through Antioxidant Reinforcement and Pigment Stabilization. Life 2026, 16, 70. https://doi.org/10.3390/life16010070
Vicas SI, Rosan CA, Padilla-Contreras D, Cavalu SD, Zsiros R, Borza IM, Gitea D, Iancu CV, Yildirim E, Aydin M, et al. Foliar Application of Nanoselenium Enhances Drought Tolerance in Brassica oleracea var. italica Through Antioxidant Reinforcement and Pigment Stabilization. Life. 2026; 16(1):70. https://doi.org/10.3390/life16010070
Chicago/Turabian StyleVicas, Simona Ioana, Cristina Adriana Rosan, Daniela Padilla-Contreras, Simona Daniela Cavalu, Richard Zsiros, Ioana Maria Borza, Daniela Gitea, Carmen Violeta Iancu, Ertan Yildirim, Murat Aydin, and et al. 2026. "Foliar Application of Nanoselenium Enhances Drought Tolerance in Brassica oleracea var. italica Through Antioxidant Reinforcement and Pigment Stabilization" Life 16, no. 1: 70. https://doi.org/10.3390/life16010070
APA StyleVicas, S. I., Rosan, C. A., Padilla-Contreras, D., Cavalu, S. D., Zsiros, R., Borza, I. M., Gitea, D., Iancu, C. V., Yildirim, E., Aydin, M., Ekinci, M., Yigider, E., & Gitea, M. A. (2026). Foliar Application of Nanoselenium Enhances Drought Tolerance in Brassica oleracea var. italica Through Antioxidant Reinforcement and Pigment Stabilization. Life, 16(1), 70. https://doi.org/10.3390/life16010070









