Effects of Different Light Qualities on Proliferation and Physiological Characteristics of Aquilaria sinensis Tissue-Cultured Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Different Light Quality Treatments and Culture Conditions
2.3. Measurement Indexes and Methods
2.3.1. Determination of Growth Indexes
2.3.2. Methods of Measurement of Physiological Indicators
Determination of Pigment Content
Determination of Soluble Sugar Content (SS)
Determination of Soluble Protein (SP) Content
Determination of Free Proline (Pro) Content
Determination of Malondialdehyde (MDA) Content
Determination of Different Enzyme Activities
2.4. Data Analysis
3. Results
3.1. The Effect of Different Light Treatments on the Proliferation and Growth of A. sinensis Seedlings
3.2. Effects of Different Light Qualities on Photosynthetic Pigment Content of A. sinensis Histocultured Seedlings
3.3. Effect of Different Light Qualities on Soluble Solids Contents of A. sinensis Histocultured Seedlings
3.4. Effect of Different Light Qualities on Proline and Malondialdehyde in A. sinensis Histocultured Seedlings
3.5. Effect of Different Light Qualities on Enzyme Activities of A. sinensis Histocultured Seedlings
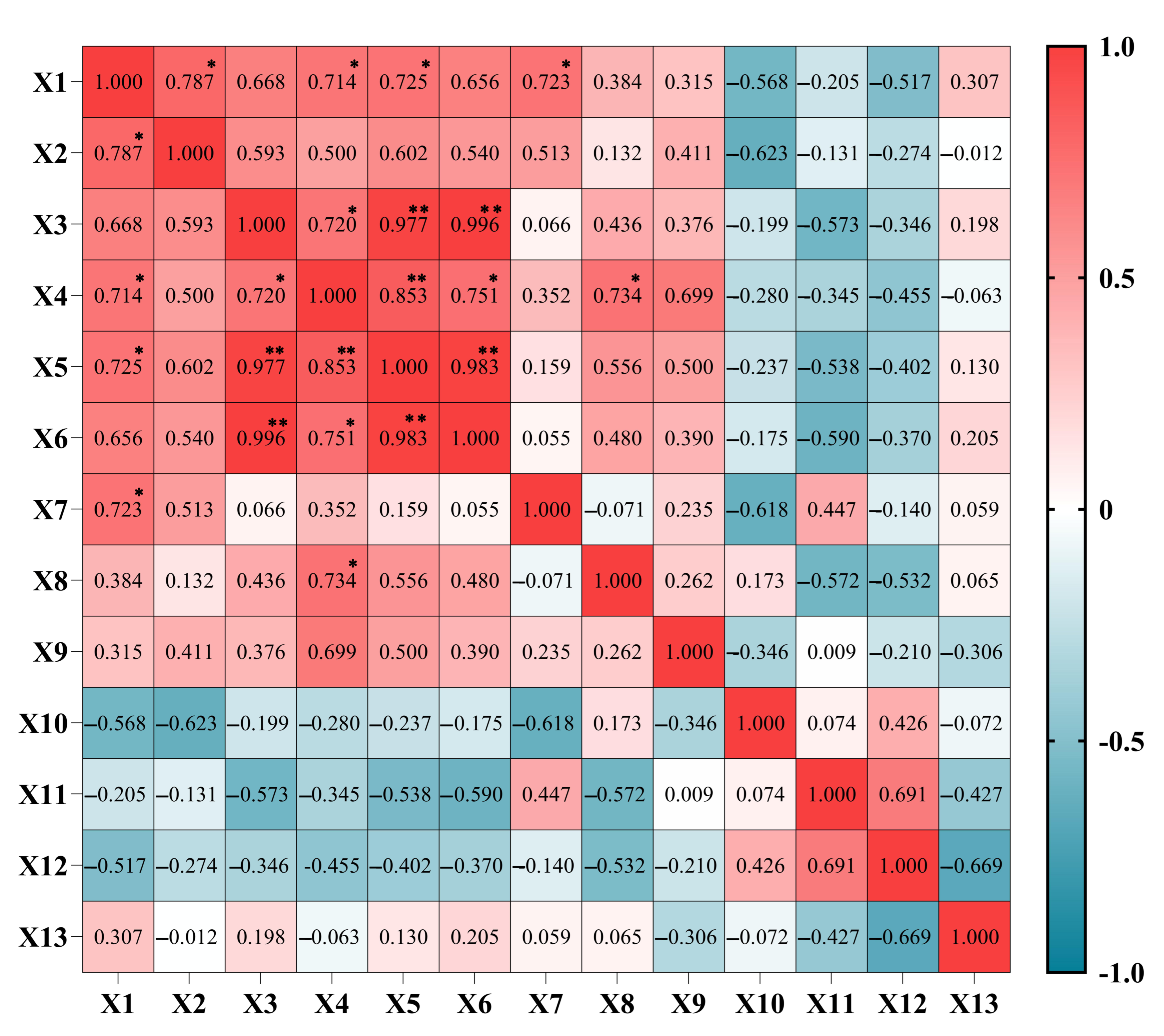
3.6. Correlation Analysis of Different Light Qualities on A. sinensis Histocultured Seedlings
3.7. Comprehensive Evaluation of Different Light Quality Adaptations of Group-Cultivated A. sinensis Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, F.-L.; Wu, S.-Z.; Xu, J.-H.; Sha, L.-H.; Li, Z.-J.; Fang, F.-Z. Effects of Different Canopy Densities of Rubber Forest on the Growth Characteristics of Interplanted, A. sinensis Saplings. For. Grassl. Resour. Res. 2024, 01, 143–150. [Google Scholar] [CrossRef]
- Liang, J.J.; Lv, T.M.; Xu, Z.Y.; Huang, X.X.; Song, S.J. Aquilaria sinensis (Lour.) Spreng: Phytochemical Review and Chemotaxonomic Values. Biochem. Syst. Ecol. 2022, 102, 104413. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Qian, S.-Z.; Liu, Y.-H.; Liu, M.; Zhong, R.-B.; Zhang, W.-X.; Jiang, Y.-M. Research Progress on Artificial Induction Methods and Resin Formation Mechanism in Agarwood. Chin. Wild Plant Resour. 2024, 43, 96–103. [Google Scholar]
- Wang, H.; Ding, X.-P.; Zeng, J.; Zhu, J.-H.; Dong, W.-H.; Chen, H.-Q.; Huang, S.-Z.; Li, W.; Mei, W.-L.; Dai, H.-F. Research Progress on Germplasm Resources, Quality Evaluation, and Formation Mechanism of Agarwood. Sci. China Life Sci. 2024, 54, 1885–1906. [Google Scholar]
- Wang, H.-J.; Sun, Y.-J.; Han, Q.-B.; Lin, S.-S. Large-scale Cultivation Techniques of Aquilaria sinensis in South China. Chin. Wild Plant Resour. 2016, 35, 67–69. [Google Scholar]
- Xu, Q.-X.; Wu, F.-H.; Zhou, L.-L. Study on Tissue Culture and Rapid Propagation of A. sinensis. Guangdong Agric. Sci. 2006, 8, 44–46. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Zhou, Z.-Z.; Qiu, Z.-F.; Liang, K.-N.; Zeng, B.-S.; Ma, H.-M.; Huang, G.-H. Study on Disinfection Methods of Explants in Tissue Culture of A. sinensis. J. Cent. South Univ. For. Technol. 2012, 32, 44–48. [Google Scholar] [CrossRef]
- Jia, X.; Jia, R.; Wang, Y.; Wu, K.; Chen, X. Optimization of Tissue Culture Regeneration System for Aquilaria sinensis Based on Uniform Design. North. Hortic. 2014, 8, 100–104. [Google Scholar]
- Yu, W.; Liu, Y.; Song, L.; Jacobs, D.F.; Du, X.; Ying, Y.; Shao, Q.; Wu, J. Effect of Differential Light Quality on Morphology, Photosynthesis, and Antioxidant Enzyme Activity in Camptotheca acuminata Seedlings. J. Plant Growth Regul. 2017, 36, 148–160. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Li, R.; Gao, M.-B.; Li, Z.; Huang, X.; Li, Y.; Li, G. Effects of Different LED Light Qualities on the Growth and Physiological Indexes of Tissue-cultured Solanum muricatum Plantlets. Trop. Agric. Sci. 2020, 40, 69–74. [Google Scholar]
- Peng, L.; Zhao, T.; Yang, B.-Y.; An, X.-R.; Huang, T. Effects of Different Light Qualities and Intensities on Growth, Related Enzyme Activities, and Components of Polygala tenuifolia. Chin. Tradit. Herb. Drugs 2018, 49, 5004–5009. [Google Scholar]
- Chen, M.-X.; Ye, Y.-Q.; Xu, S.-S.; Ling, K.-M. Optimization of Light Environment for Proliferation of Cunninghamia lanceolata Tissue Culture Seedlings. J. For. Environ. 2023, 43, 380–387. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zeng, B.-S. Proliferation Efficiency and Photoprotective Response of Qinan Agarwood Tissue Culture Seedlings under Different Light Qualities. Mol. Plant Breed. 2021, 19, 291–298. [Google Scholar] [CrossRef]
- Liao, D.-S.; Peng, L.-C.; Lin, K.-W.; Jie, W.-J.; Song, J. Effects of Different LED Light Qualities on Proliferation and Rooting of Two Yellow-Flowered Rhododendron Tissue Cultures. J. Yunnan Agric. Univ. (Nat. Sci.) 2019, 34, 826–832. [Google Scholar]
- Zhou, P.; Zhang, M.; Wu, S.-Z.; Huang, Q. Effects of LED Light Qualities on Stem Segment Proliferation and Rooting of Vaccinium bracteatum Tissue Culture Seedlings. J. Plant Res. 2018, 38, 697–703. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kang, Z.; Cai, H.; Guo, G.; Zeng, H.; Wang, W.; Tu, X. Physiological Response of Macadamia (Macadamia integrifolia) Seedlings to Drought Stress. Horticulturae 2025, 11, 347. [Google Scholar] [CrossRef]
- Li, M.Y.; Li, J.; Xie, F.J.; Zhou, J.; Sun, Y.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; Lin, Y.X.; et al. Combined evaluation of agronomic and quality traits to explore heat germplasm in celery (Apium graveolens L.). Sci. Hortic. 2023, 317, 112039. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of the free proline of water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, G.; Dominik, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkle, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, X.; Li, Y.; Peng, F.; Wang, L. Regulation of POD activity by pelargonidin during vegetative growth in radish (Raphanus sativus L.). Sci. Hortic. 2014, 174, 105111. [Google Scholar] [CrossRef]
- Wu, W.; Yang, C.; Lin, S.; Li, W.; Ou, S.; Guo, J.; Huang, X.; Liu, X.; Feng, F. Comprehensive Evaluation of Leaf Structure, Photosynthetic Characteristics, and Drought Resistance in Six Jackfruit (Artocarpus heterophyllus) Cultivars. Life 2025, 15, 1346. [Google Scholar] [CrossRef]
- Yang, J.L.; Jung, B.R. Lighting Enhances Morphophysiology by Inducing More Branching and Flowering in Chrysanthemum Grown in Controlled Environment. Int. J. Mol. Sci. 2021, 22, 12019. [Google Scholar] [CrossRef]
- Gu, A.-S.; Zhang, H.; Cui, J. Research Progress on the Application of Light Regulation in Plant Tissue Culture. Acta Bot. Boreali-Occident. Sin. 2011, 31, 2341–2346. [Google Scholar]
- Di, X.-R.; Jiao, X.-L.; Cui, J.; Liu, X.-Y.; Kong, Y. Effects of Different Light Quality Ratios from New Light Source LED on the Growth of Chrysanthemum Tissue Culture Seedlings. Plant Physiol. Commun. 2008, 4, 661–664. [Google Scholar]
- Li, K.-L.; Wei, Y.-X.; Chong, Z.-L.; Meng, Z.-G.; Wang, Y. Red and Blue Light Promote Callus Induction and Proliferation in Upland Cotton. Sci. Agric. Sin. 2024, 57, 638–649. [Google Scholar]
- Miao, Y.-M.; Gao, Y.-J.; Gu, D.-Y.; Huang, C.-Q.; Jia, L. Effects of Light Quality on Growth, Physiology, and Secondary Metabolism of Pholidota cantonensis Tissue Culture Seedlings and Transcriptome Analysis. J. Plant Resour. Environ. 2025, 34, 21–32. [Google Scholar]
- Song, Y.; Xu, H.; Xiao, J.-L.; Wang, Q.; Yang, B.-Z. Research Progress on In Vitro Regeneration Culture of Pepper. J. Pepper 2023, 21, 1–6. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Zhao, X.-H.; Wei, Z.-F.; Cai, S.-S.; Fan, Y.-C. Effects of Different LED Light Qualities on Growth and Physiological Characteristics of Ornamental Crabapple Tissue Culture Seedlings. Shandong Agric. Sci. 2018, 50, 43–47. [Google Scholar] [CrossRef]
- Lopez-Figueroa, F.; Niell, F.X. Red-Light and Blue-Light Photoreceptors Controlling Chlorophyll a Synthesis in the Red Alga Porphyra umbilicalis and in the Green Alga Ulva rigida. Physiol. Plant. 1989, 76, 391–397. [Google Scholar] [CrossRef]
- Ren, G.-P.; Wang, X.-J.; Zhu, G.-F. Effects of Different LED Light Qualities on Proliferation and Rooting of Phalaenopsis Tissue Culture. Chin. Bull. Bot. 2016, 51, 81–88. [Google Scholar]
- Lin, H.-F.; Xu, K.-Z.; Zhou, H.-M.; Gan, W.-X.; Lin, F.-Z. Effects of Different LED Light on Growth and Physiological-Biochemical Characteristics of Cymbidium ran Luo Tissue Culture Seedlings. Jiangsu J. Agric. Sci. 2025, 41, 1302–1311. [Google Scholar]
- Zhang, Y.-Z.; Li, L.; Sun, X.-X.; Gu, H.; Cheng, D.-W. Research Progress on the Effects of Artificial Supplementary Lighting on Fruit Tree Growth and Development. J. Fruit Sci. 2024, 41, 2595–2605. [Google Scholar] [CrossRef]
- Abid, M.; Zhang, Y.J.; Li, Z.; Bai, D.F.; Zhong, Y.P.; Fang, J.B. Effect of Salt Stress on Growth, Physiological and Biochemical Characters of Four Kiwifruit Genotypes. Sci. Hortic. 2020, 271, 109473. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative Stress and Antioxidant Activity as the Basis of Senescence in Maize Leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Dai, Y.-H.; Liu, Z.-B.; Yang, S.; Mao, L.-Z.; Zou, X.-X. Effects of Different Light Qualities on Photosynthetic Physiological Characteristics of Pepper Leaf Color Yellow Mutant. Acta Bot. Boreali-Occident. Sin. 2023, 43, 601–610. [Google Scholar]
- Wang, Z.-Y.; Zhang, Y.-P.; Qiao, Y.-X.; Zhang, W.-H.; He, J.-X. Effects of Light Quality on Growth and Physiological Characteristics of Diploid and Tetraploid Anthurium Seedlings. Chin. J. Trop. Crops 2025, 46, 1903–1911. [Google Scholar]
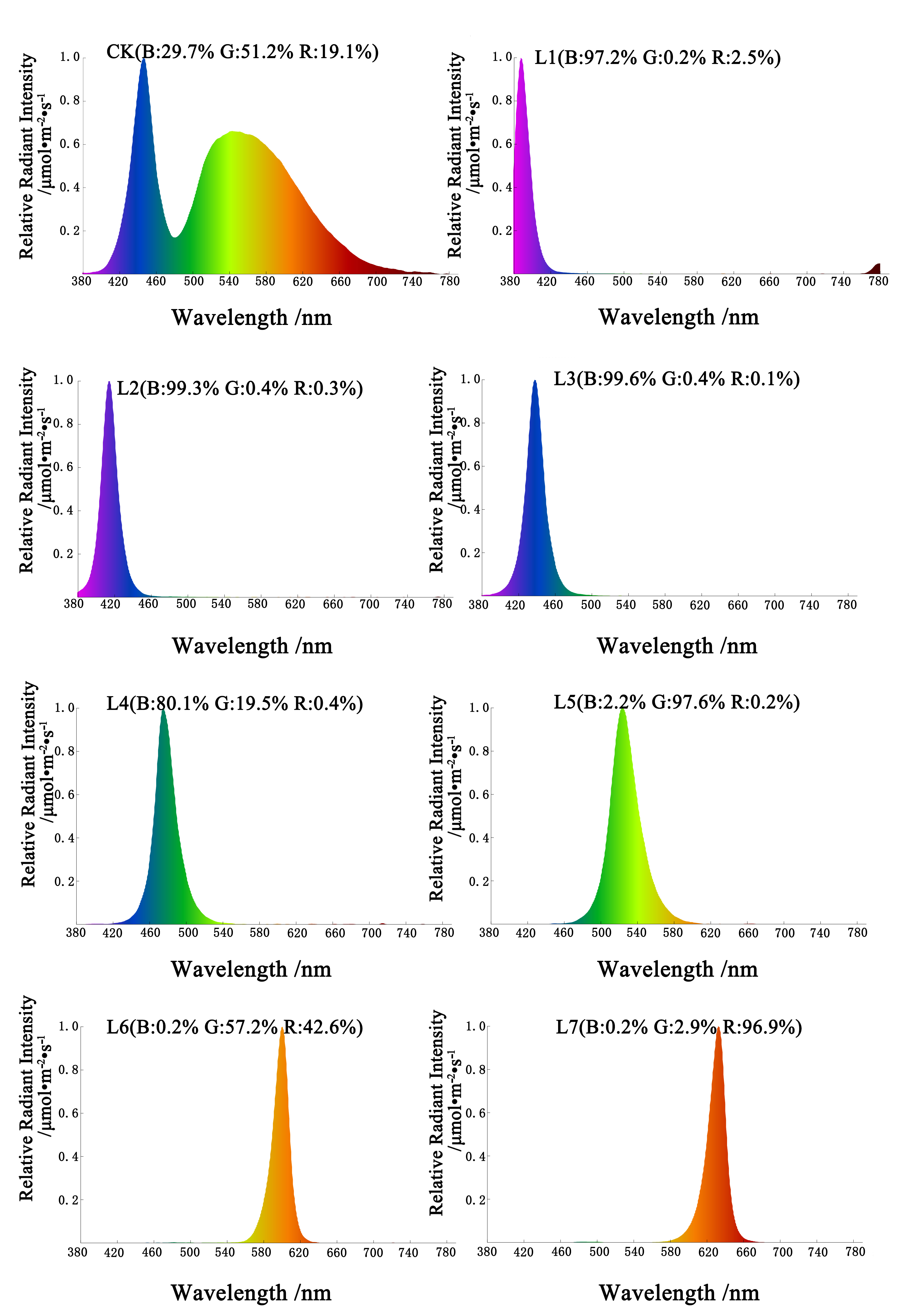

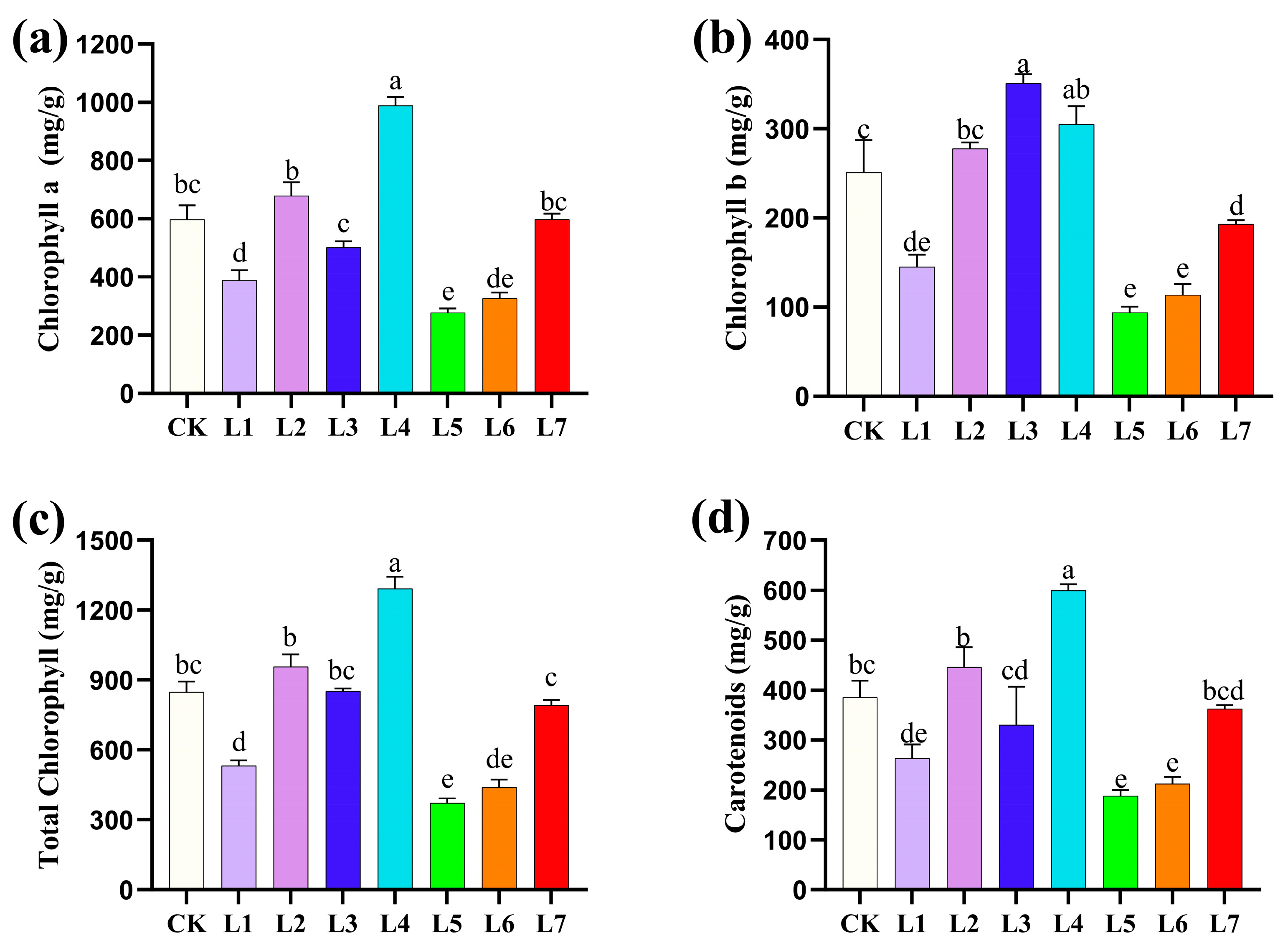
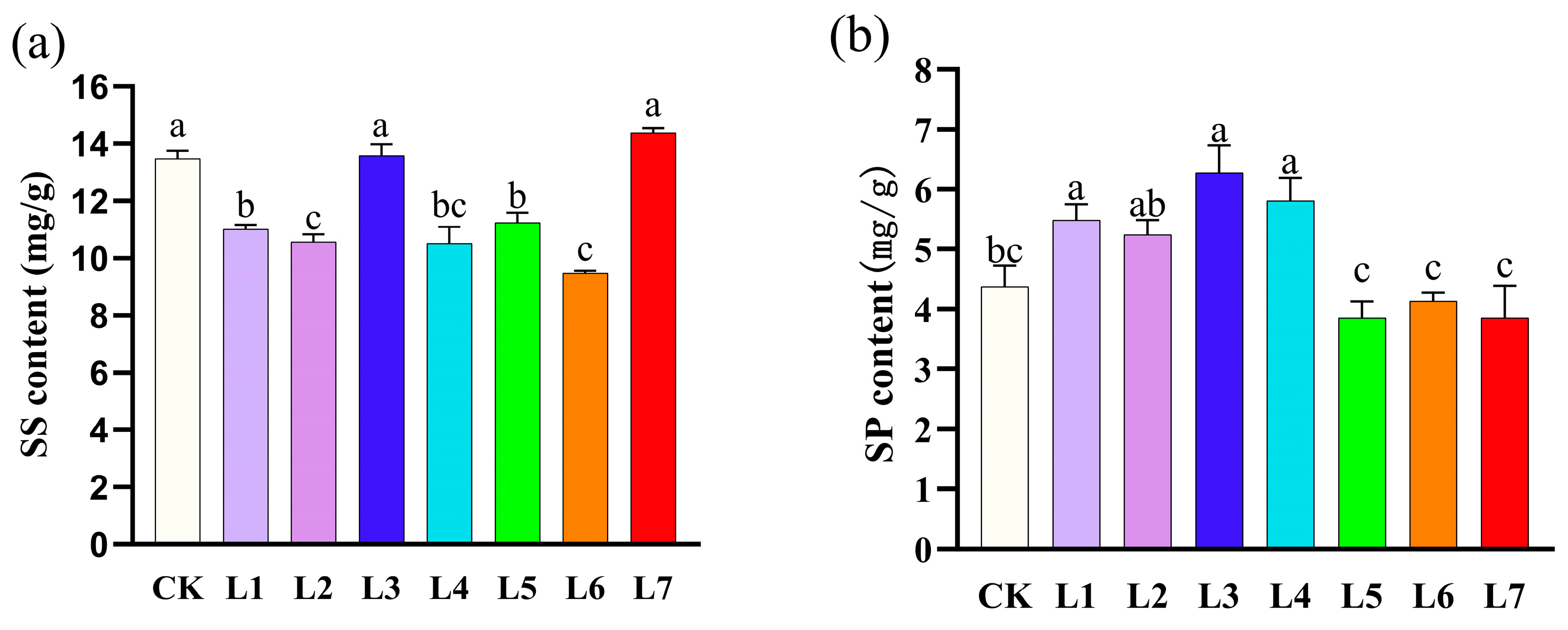

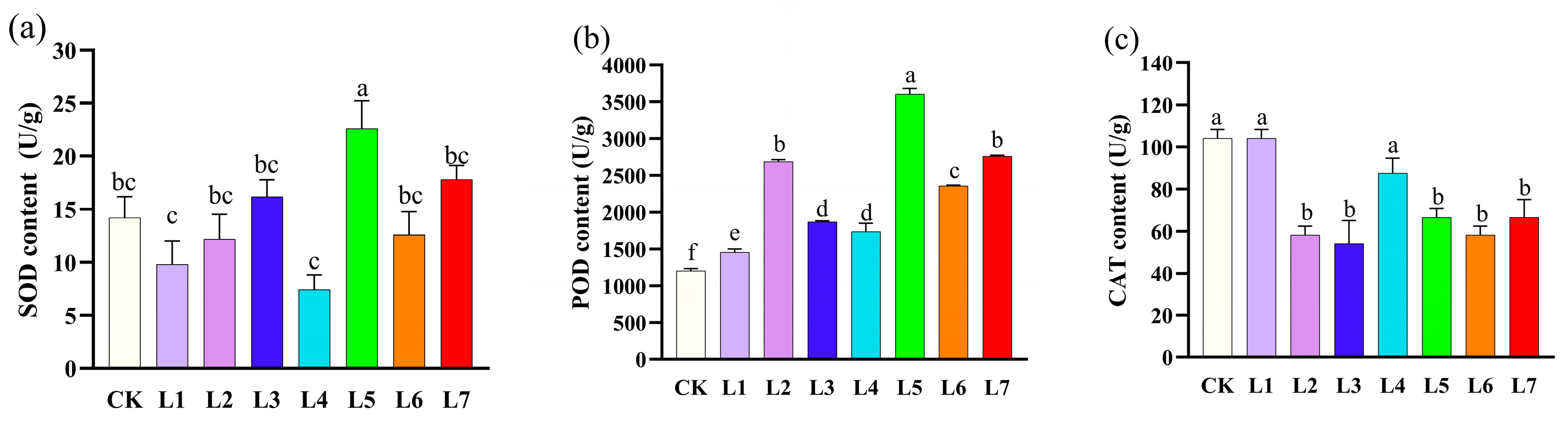
| Light Quality Treatments | Multiplication rate (Number) | Plant Height (cm) | Shoot Growth |
|---|---|---|---|
| CK | 3.13 ± 0.09 c | 2.49 ± 0.03 b | Leaf blades light green, good length, small amount of healing tissue at base |
| L1 | 2.1 ± 0.09 e | 2.08 ± 0.03 c | Leaf blades intensely green, poorly growing, with more healing tissue at the base |
| L2 | 2.03 ± 0.11 e | 1.94 ± 0.01 d | Leaf blades yellowish, poorly grown, with small amounts of healing tissue at base |
| L3 | 3.53 ± 0.09 b | 2.52 ± 0.02 b | Leaf blades light green, good growth, few healing groups at base |
| L4 | 4.07 ± 0.13 a | 2.57 ± 0.04 ab | Leaf blade intensely green, good growth, small amount of healing tissue at base |
| L5 | 2.07 ± 0.13 e | 1.63 ± 0.02 e | Leaf blade light green, poor growth, more healing tissue at base |
| L6 | 2.77 ± 0.14 d | 1.53 ± 0.02 f | Leaf blade light green, poor growth, more healing tissue at base |
| L7 | 4.1 ± 0.13 a | 2.63 ± 0.03 a | Leaf blade intensely green, good growth, small amount of healing tissue at base |
| Index | Principal Component Coefficient | |||
|---|---|---|---|---|
| C1 | C2 | C3 | C4 | |
| Value-added coefficient | 0.138 | 0.14 | −0.133 | 0.055 |
| Plant height | 0.114 | 0.189 | −0.022 | 0.236 |
| Chlorophyll a | 0.143 | −0.074 | 0.097 | 0.38 |
| Chlorophyll b | 0.143 | 0.02 | 0.177 | −0.289 |
| Total chlorophyll | 0.152 | −0.049 | 0.128 | 0.196 |
| Carotenoids | 0.143 | −0.088 | 0.104 | 0.327 |
| Soluble sugar | 0.055 | 0.328 | −0.148 | −0.118 |
| Soluble protein | 0.097 | −0.184 | 0.111 | −0.483 |
| Proline | 0.086 | 0.125 | 0.258 | −0.36 |
| Malondialdehyde | −0.073 | −0.244 | 0.22 | 0.052 |
| Superoxide dismutase (SOD) | −0.089 | 0.289 | 0.115 | −0.005 |
| Peroxidase (POD) | −0.102 | 0.108 | 0.325 | 0.402 |
| Catalase (CAT) | 0.039 | −0.140 | −0.454 | 0.100 |
| Characteristic root | 6.195 | 2.484 | 1.787 | 1.017 |
| Contribution rate% | 47.652 | 19.11 | 13.749 | 7.826 |
| Cumulative contribution% | 47.652 | 66.762 | 80.511 | 88.338 |
| Light Quality Treatments | Principal Component Score | Value of the Affiliation Function | Consolidated Assessed Value | Rankings | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | |||
| CK | 0.67 | 0.49 | −1.24 | −0.42 | 0.74 | 0.65 | 0.11 | 0.45 | 0.53 | 4 |
| L1 | −0.51 | −1.23 | −1.56 | −0.44 | 0.35 | 0.00 | 0.00 | 0.45 | 0.20 | 8 |
| L2 | 0.12 | −0.75 | 1.25 | 0.11 | 0.56 | 0.18 | 1.00 | 0.62 | 0.49 | 5 |
| L3 | 0.52 | 1.30 | 1.00 | −1.87 | 0.69 | 0.95 | 0.91 | 0.00 | 0.64 | 2 |
| L4 | 1.44 | −0.89 | 0.48 | 1.07 | 1.00 | 0.13 | 0.72 | 0.92 | 0.67 | 1 |
| L5 | −1.53 | −0.13 | 0.51 | 0.49 | 0.00 | 0.41 | 0.74 | 0.74 | 0.24 | 7 |
| L6 | −0.95 | −0.22 | 0.10 | −0.26 | 0.20 | 0.38 | 0.59 | 0.51 | 0.29 | 6 |
| L7 | 0.25 | 1.43 | −0.54 | 1.32 | 0.60 | 1.00 | 0.36 | 1.00 | 0.61 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Chen, C.; Yang, C.; Li, W.; Lai, J.; Min, X.; Gan, S.; Yi, R.; Lin, C.; Feng, F. Effects of Different Light Qualities on Proliferation and Physiological Characteristics of Aquilaria sinensis Tissue-Cultured Seedlings. Life 2025, 15, 1770. https://doi.org/10.3390/life15111770
Feng L, Chen C, Yang C, Li W, Lai J, Min X, Gan S, Yi R, Lin C, Feng F. Effects of Different Light Qualities on Proliferation and Physiological Characteristics of Aquilaria sinensis Tissue-Cultured Seedlings. Life. 2025; 15(11):1770. https://doi.org/10.3390/life15111770
Chicago/Turabian StyleFeng, Le, Chuqi Chen, Chongcheng Yang, Wei Li, Jiapeng Lai, Xiaoyun Min, Siting Gan, Runhua Yi, Chenjun Lin, and Feng Feng. 2025. "Effects of Different Light Qualities on Proliferation and Physiological Characteristics of Aquilaria sinensis Tissue-Cultured Seedlings" Life 15, no. 11: 1770. https://doi.org/10.3390/life15111770
APA StyleFeng, L., Chen, C., Yang, C., Li, W., Lai, J., Min, X., Gan, S., Yi, R., Lin, C., & Feng, F. (2025). Effects of Different Light Qualities on Proliferation and Physiological Characteristics of Aquilaria sinensis Tissue-Cultured Seedlings. Life, 15(11), 1770. https://doi.org/10.3390/life15111770




