Gene-Level Shift in Response to Synthetic Nitrogen Addition Promotes Larix olgensis (Ussurian Larch) Growth in a Short-Term Field Trial
Abstract
1. Introduction
2. Materials and Methods
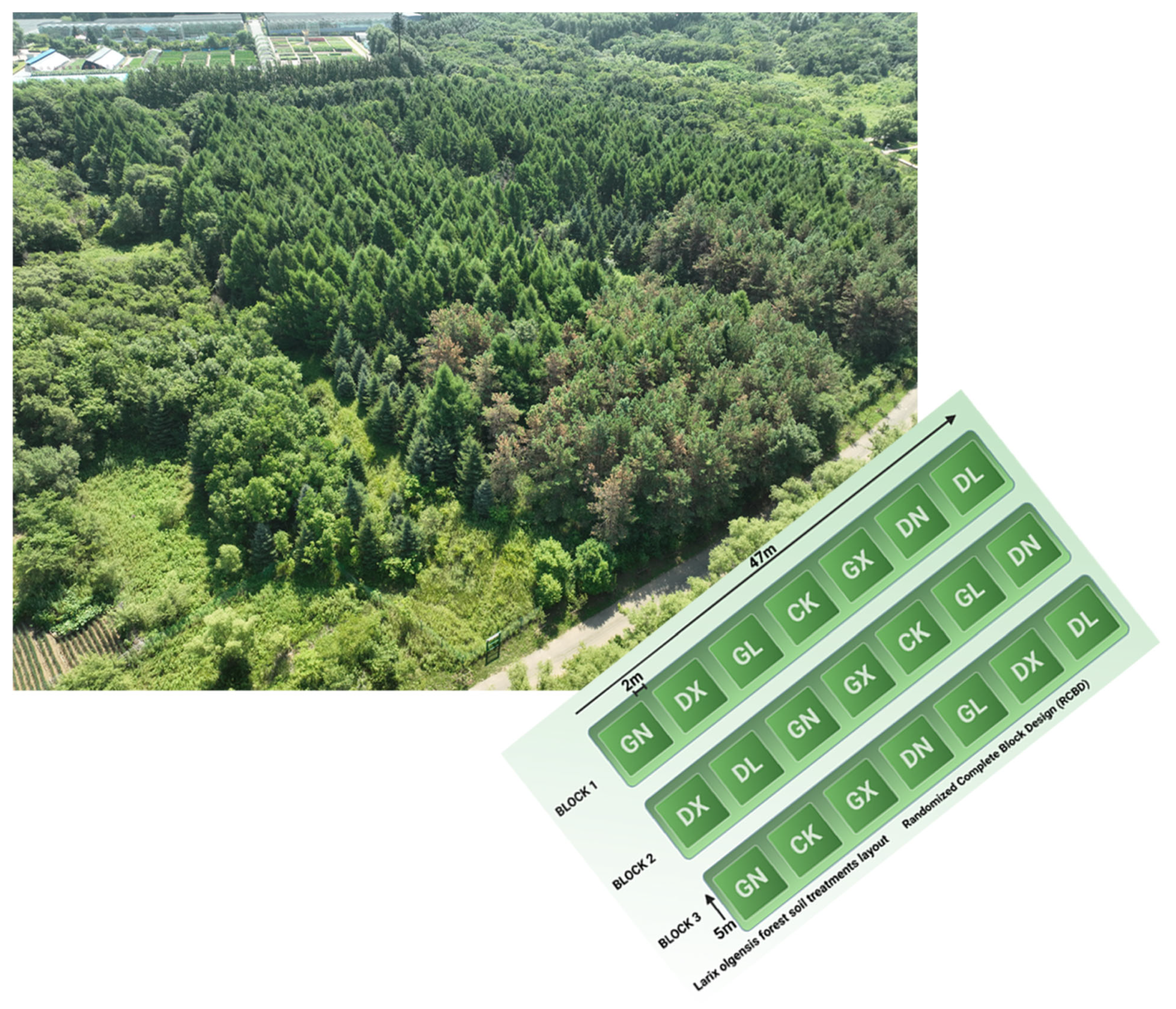
2.1. Experimental Site and Design
2.2. Chemical Test Methods of Soil Properties
2.3. Quantification of Functional Gene Abundances Using Quantitative PCR
2.4. Enzyme Assay
2.5. Statistical Analyses
3. Results
3.1. Effect of Nitrogen Addition on Soil Physico-Chemical Properties
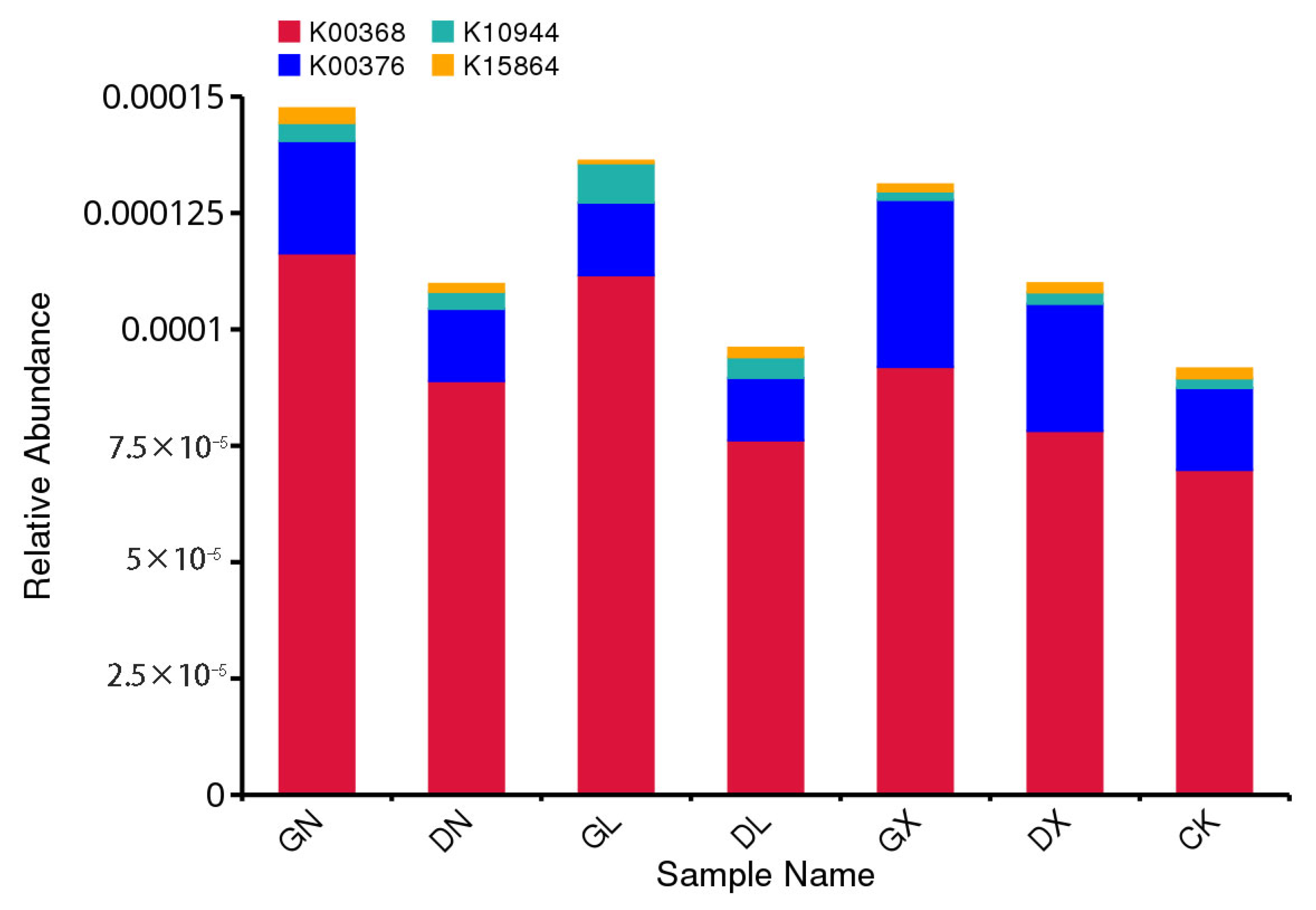
3.2. Effect of Nitrogen Addition on Ammonia Oxidizer and Denitrifier Abundances
3.3. Effect of Nitrogen Addition on Soil Enzymatic Activities
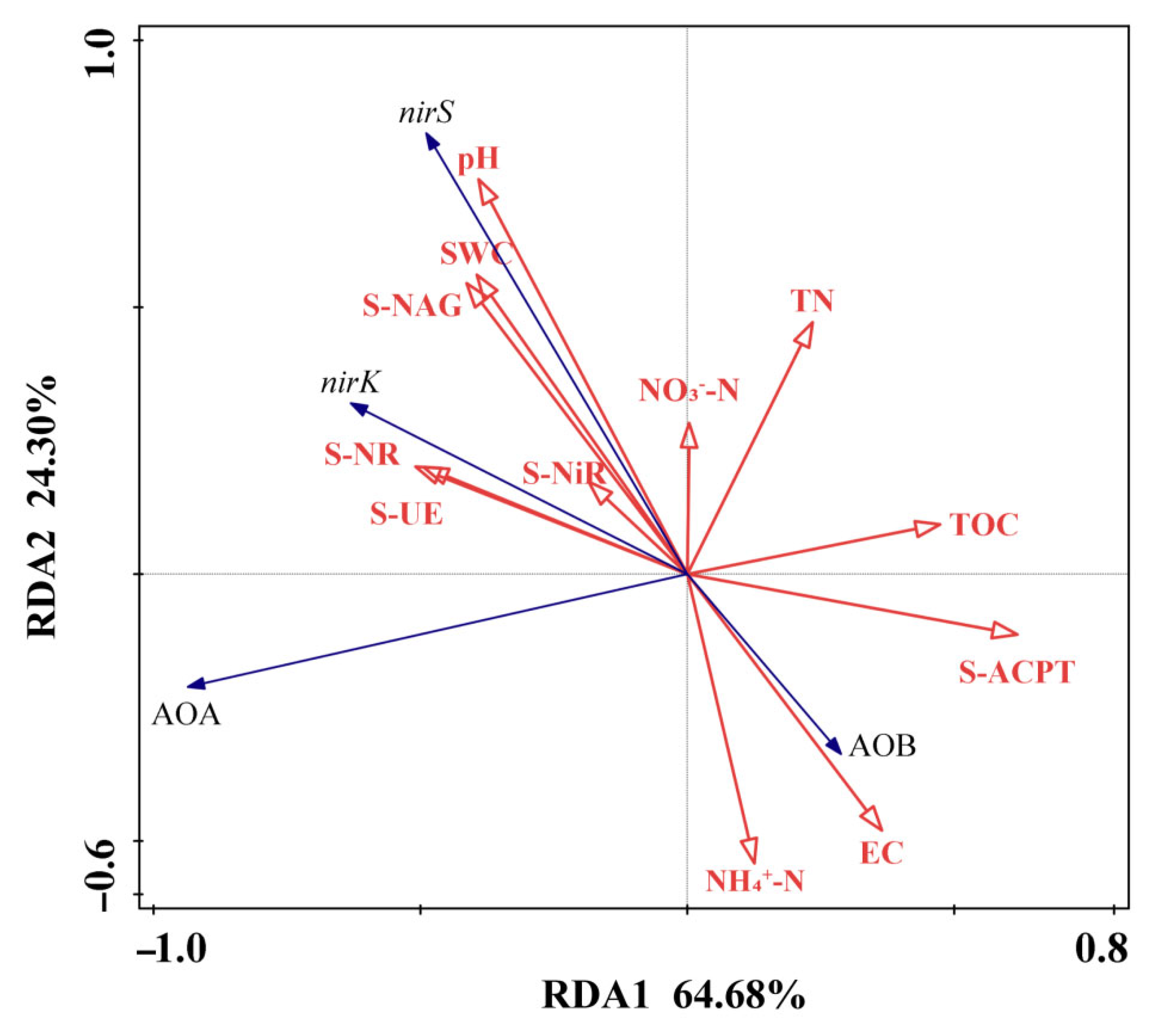
3.4. Relationship Between N Addition Effect on Gene Abundances, Soil Physico-Chemical Properties and Enzymes Activities
4. Discussion
4.1. Effect of Nitrogen Addition on Soil Properties and Enzymatic Activities
4.2. Effect of Nitrogen Addition on AOA and AOB Abundances
4.3. Effect of Nitrogen Addition on nirK and nirS Abundances
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rennenberg, H.; Dannenmann, M. Nitrogen Nutrition of Trees in Temperate Forests—The Significance of Nitrogen Availability in the Pedosphere and Atmosphere. Forests 2015, 6, 2820–2835. [Google Scholar] [CrossRef]
- Ameer, M.J.; Liu, Y.; Zhao, X.; Yan, S.; Qu, T. Effect of Different Synthetic Nitrogen Forms and Levels on Nitrification and Denitrification Key Genes Abundances: Implications for Oligotrophic Forest Soil Management. Nitrogen 2025, 6, 4. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, C.; Sainju, U.M.; Zhang, N.; Zhao, F.; Wang, W.; Wang, J. Differential Responses of Soil Microbial N-Cycling Functional Genes to 35 yr Applications of Chemical Fertilizer and Organic Manure in Wheat Field Soil on Loess Plateau. Agronomy 2023, 13, 1516. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Z.; Zhang, Y.; Shi, Y. Impacts of fertilization optimization on soil nitrogen cycling and wheat nitrogen utilization under water-saving irrigation. Front. Plant Sci. 2022, 13, 878424. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Hui, D.; Ray, A.; Kasrija, L.; Christian, J. Impacts of Climate Change and Agricultural Practices on Nitrogen Processes, Genes, and Soil Nitrous Oxide Emissions: A Quantitative Review of Meta-Analyses. Agriculture 2024, 14, 240. [Google Scholar] [CrossRef]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Rütting, T.; Schleusner, P.; Hink, L.; Prosser, J.I. The contribution of ammonia-oxidizing archaea and bacteria to gross nitrification under different substrate availability. Soil Biol. Biochem. 2021, 160, 108353. [Google Scholar] [CrossRef]
- Ramanathan, B.; Boddicker, A.M.; Roane, T.M.; Mosier, A.C. Nitrifier gene abundance and diversity in sediments impacted by acid mine drainage. Front. Microbiol. 2017, 8, 2136. [Google Scholar] [CrossRef]
- Ouyang, Y.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. Effect of nitrogen fertilization on the abundance of nitrogen cycling genes in agricultural soils: A meta-analysis of field studies. Soil Biol. Biochem. 2018, 127, 71–78. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Penttinen, P.; Chen, X.; Duan, P.; Fan, F.; Xiong, W.; Liu, M.; Tang, X.; Peng, D.; et al. Both AOA and AOB contribute to nitrification and show linear correlation with nitrate leaching in purple soils with a wide nitrogen gradient. Environ. Res. 2025, 264, 120403. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Petersen, D.G.; Blazewicz, S.J.; Firestone, M.; Herman, D.J.; Turetsky, M.; Waldrop, M. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ. Microbiol. 2012, 14, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Stopnišek, N.; Gubry-Rangin, C.; Höfferle, S.p.; Nicol, G.W.; Mandic-Mulec, I.; Prosser, J.I. Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment. Appl. Environ. Microbiol. 2010, 76, 7626–7634. [Google Scholar] [CrossRef]
- Levičnik-Höfferle, Š.; Nicol, G.W.; Ausec, L.; Mandić-Mulec, I.; Prosser, J.I. Stimulation of thaumarchaeal ammonia oxidation by ammonia derived from organic nitrogen but not added inorganic nitrogen. FEMS Microbiol. Ecol. 2012, 80, 114–123. [Google Scholar] [CrossRef]
- Zhaoming, C.; Qiang, W.; Yanli, L.; Jinping, Z.; Jiang, F.; Tao, L.; Qiaogang, Y.; Junwei, M. Effects of nitrogen levels on ammonia oxidizers and nitrification in fluvo-aquic soil. Acta Agric. Zhejiangensis 2022, 34, 2004. [Google Scholar]
- Abdo, A.I.; Xu, Y.; Shi, D.; Li, J.; Li, H.; El-Sappah, A.H.; Elrys, A.S.; Alharbi, S.A.; Zhou, C.; Wang, L. Nitrogen transformation genes and ammonia emission from soil under biochar and urease inhibitor application. Soil Tillage Res. 2022, 223, 105491. [Google Scholar] [CrossRef]
- Qin, W.; Wei, S.P.; Zheng, Y.; Choi, E.; Li, X.; Johnston, J.; Wan, X.; Abrahamson, B.; Flinkstrom, Z.; Wang, B. Ammonia-oxidizing bacteria and archaea exhibit differential nitrogen source preferences. Nat. Microbiol. 2024, 9, 524–536. [Google Scholar] [CrossRef]
- Sterngren, A.E.; Hallin, S.; Bengtson, P. Archaeal ammonia oxidizers dominate in numbers, but bacteria drive gross nitrification in N-amended grassland soil. Front. Microbiol. 2015, 6, 1350. [Google Scholar] [CrossRef]
- Sun, R.; Myrold, D.D.; Wang, D.; Guo, X.; Chu, H. AOA and AOB communities respond differently to changes of soil pH under long-term fertilization. Soil Ecol. Lett. 2019, 1, 126–135. [Google Scholar] [CrossRef]
- Gubry-Rangin, C.; Hai, B.; Quince, C.; Engel, M.; Thomson, B.C.; James, P.; Schloter, M.; Griffiths, R.I.; Prosser, J.I.; Nicol, G.W. Niche specialization of terrestrial archaeal ammonia oxidizers. Proc. Natl. Acad. Sci. USA 2011, 108, 21206–21211. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Uchiyama, I.; Toyoda, A.; Wang, Y.; Shimomura, Y.; Okubo, T.; Kurisu, F.; Hirono, Y.; Nonaka, K. An acid-tolerant ammonia-oxidizing γ-proteobacterium from soil. ISME J. 2017, 11, 1130–1141. [Google Scholar] [CrossRef]
- Wu, P.-P.; Zhang, Z.; Li, R.; Ji, J.-H.; Mao, R. Impact of nitrogen addition on single and mixed tree leaf litter decomposition depends on N forms in subtropical China. Appl. Soil Ecol. 2023, 190, 104970. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Jiang, Y.; Hu, Y.; Zhang, M.; Zeng, Z. Response of bacteria harboring nirS and nirK genes to different N fertilization rates in an alkaline northern Chinese soil. Eur. J. Soil Biol. 2017, 82, 1–9. [Google Scholar] [CrossRef]
- Bárta, J.; Tahovská, K.; Kaåa, J. The effect of nitrate addition on abundance of nirK, nirS and gln genes in acidified Norway spruce forest soil. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 2–7 May 2010; p. 10333. [Google Scholar]
- Veraart, A.J.; Dimitrov, M.R.; Schrier-Uijl, A.P.; Smidt, H.; de Klein, J.J. Abundance, activity and community structure of denitrifiers in drainage ditches in relation to sediment characteristics, vegetation and land-use. Ecosystems 2017, 20, 928–943. [Google Scholar] [CrossRef]
- Wang, F.; Liang, X.; Ding, F.; Ren, L.; Liang, M.; An, T.; Li, S.; Wang, J.; Liu, L. The active functional microbes contribute differently to soil nitrification and denitrification potential under long-term fertilizer regimes in North-East China. Front. Microbiol. 2022, 13, 1021080. [Google Scholar] [CrossRef] [PubMed]
- Saleh-Lakha, S.; Shannon, K.E.; Henderson, S.L.; Zebarth, B.J.; Burton, D.L.; Goyer, C.; Trevors, J.T. Effect of nitrate and acetylene on nirS, cnorB, and nosZ expression and denitrification activity in Pseudomonas mandelii. Appl. Environ. Microbiol. 2009, 75, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Liu, Y.; Li, J.; Li, C.; Tu, B.; Yao, M.; Li, X. Patterns and drivers of nirK-type and nirS-type denitrifier community assembly along an elevation gradient. mSystems 2021, 6, e00667-00621. [Google Scholar] [CrossRef] [PubMed]
- Curtright, A.J.; Tiemann, L.K. Chemical identity of carbon substrates drives differences in denitrification and N2O reduction within agricultural soils. Soil Biol. Biochem. 2023, 184, 109078. [Google Scholar] [CrossRef]
- Saghaï, A.; Moore, O.C.; Jones, C.M.; Hallin, S. Microbial controls of nitrogen retention and N2O production in cropping systems supporting soil carbon accrual. Soil Biol. Biochem. 2025, 208, 109858. [Google Scholar] [CrossRef]
- Hallin, S.; Jones, C.M.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef]
- Nohrstedt, H.-Ö. Response of coniferous forest ecosystems on mineral soils to nutrient additions: A review of Swedish experiences. Scand. J. For. Res. 2001, 16, 555–573. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 2012, 173, 330–338. [Google Scholar] [CrossRef]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef]
- Stephen, J.R.; Kowalchuk, G.A.; Bruns, M.-A.V.; McCaig, A.E.; Phillips, C.J.; Embley, T.M.; Prosser, J.I. Analysis of β-Subgroup Proteobacterial Ammonia Oxidizer Populations in Soil by Denaturing Gradient Gel Electrophoresis Analysis and Hierarchical Phylogenetic Probing. Appl. Environ. Microbiol. 1998, 64, 2958–2965. [Google Scholar] [CrossRef]
- Rösch, C.; Bothe, H. Improved assessment of denitrifying, N2-fixing, and total-community bacteria by terminal restriction fragment length polymorphism analysis using multiple restriction enzymes. Appl. Environ. Microbiol. 2005, 71, 2026–2035. [Google Scholar] [CrossRef]
- Braker, G.; Fesefeldt, A.; Witzel, K.-P. Development of PCR Primer Systems for Amplification of Nitrite Reductase Genes (nirK and nirS) To Detect Denitrifying Bacteria in Environmental Samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar] [CrossRef]
- Saiya-Cork, K.; Sinsabaugh, R.; Zak, D. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Hidalgo-García, A.; Torres, M.J.; Salas, A.; Bedmar, E.J.; Girard, L.; Delgado, M.J. Rhizobium etli produces nitrous oxide by coupling the assimilatory and denitrification pathways. Front. Microbiol. 2019, 10, 980. [Google Scholar] [CrossRef]
- Ming, Y.; Abdullah Al, M.; Zhang, D.; Zhu, W.; Liu, H.; Cai, L.; Yu, X.; Wu, K.; Niu, M.; Zeng, Q.; et al. Insights into the evolutionary and ecological adaption strategies of nirS- and nirK-type denitrifying communities. Mol. Ecol. 2024, 33, e17507. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Li, M.; Zhao, X.; Luo, H.; Zhao, L. Nitrogen Deposition May Benefit to Larix olgensis Root Soils. Forests 2023, 14, 1014. [Google Scholar] [CrossRef]
- Fang, H.; Cheng, S.; Yu, G.; Yang, X.; Xu, M.; Wang, Y.; Li, L.; Dang, X.; Wang, L.; Li, Y. Nitrogen deposition impacts on the amount and stability of soil organic matter in an alpine meadow ecosystem depend on the form and rate of applied nitrogen. Eur. J. Soil Sci. 2014, 65, 510–519. [Google Scholar] [CrossRef]
- Du, Y.; Guo, P.; Liu, J.; Wang, C.; Yang, N.; Jiao, Z. Different types of nitrogen deposition show variable effects on the soil carbon cycle process of temperate forests. Glob. Change Biol. 2014, 20, 3222–3228. [Google Scholar] [CrossRef]
- Qu, T.; Zhao, X.; Yan, S.; Liu, Y.; Ameer, M.J.; Zhao, L. Interruption after Short-Term Nitrogen Additions Improves Ecological Stability of Larix olgensis Forest Soil by Affecting Bacterial Communities. Microorganisms 2024, 12, 969. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, S.; Fang, H.; Yang, Y.; Guo, Y.; Li, Y.; Zhou, Y. Contrasting response of soil N2O release to ammonium, nitrate, and urea addition rates is determined by substrate availability and microbial community abundance and composition. Eur. J. Soil Biol. 2022, 109, 103393. [Google Scholar] [CrossRef]
- Yan, B.; Sun, Y.; He, G.; He, R.; Zhang, M.; Fang, H.; Shi, L. Nitrogen enrichment affects soil enzymatic stoichiometry via soil acidification in arid and hot land. Pedobiologia 2020, 81, 150663. [Google Scholar] [CrossRef]
- Weng, B.; Xie, X.; Yang, J.; Liu, J.; Lu, H.; Yan, C. Research on the nitrogen cycle in rhizosphere of Kandeliaobovata under ammonium and nitrate addition. Mar. Pollut. Bull. 2013, 76, 227–240. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Gao, S.; Wang, P.; Qiu, J.; Shang, S. Impacts of simulated nitrogen deposition on soil enzyme activity in a northern temperate forest ecosystem depend on the form and level of added nitrogen. Eur. J. Soil Biol. 2021, 103, 103287. [Google Scholar] [CrossRef]
- Su, J.-Q.; Li, X.-R.; Bao, J.-T. Effects of nitrogen addition on soil physico-chemical properties and enzyme activities in desertified steppe. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2014, 25, 664–670. [Google Scholar]
- Kang, H.; Lee, D. Inhibition of extracellular enzyme activities in a forest soil by additions of inorganic nitrogen. Commun. Soil Sci. Plant Anal. 2005, 36, 2129–2135. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.; Yuan, M.; Zhang, Z.; Chen, S.; Ruan, Y.; Huang, Q. Impacts of urea and 3, 4-dimethylpyrazole phosphate on nitrification, targeted ammonia oxidizers, non-targeted nitrite oxidizers, and bacteria in two contrasting soils. Front. Microbiol. 2022, 13, 952967. [Google Scholar] [CrossRef]
- Wertz, S.; Leigh, A.K.; Grayston, S.J. Effects of long-term fertilization of forest soils on potential nitrification and on the abundance and community structure of ammonia oxidizers and nitrite oxidizers. FEMS Microbiol. Ecol. 2012, 79, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Sauder, L.A.; Peterse, F.; Schouten, S.; Neufeld, J.D. Low-ammonia niche of ammonia-oxidizing archaea in rotating biological contactors of a municipal wastewater treatment plant. Environ. Microbiol. 2012, 14, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Offre, P.; Prosser, J.I.; Nicol, G.W. Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol. Ecol. 2009, 70, 99–108. [Google Scholar] [CrossRef]
- Boyle-Yarwood, S.A.; Bottomley, P.J.; Myrold, D.D. Community composition of ammonia-oxidizing bacteria and archaea in soils under stands of red alder and Douglas fir in Oregon. Environ. Microbiol. 2008, 10, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C.; Shen, J.-P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.-Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef]
- Lehtovirta-Morley, L.E.; Stoecker, K.; Vilcinskas, A.; Prosser, J.I.; Nicol, G.W. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc. Natl. Acad. Sci. USA 2011, 108, 15892–15897. [Google Scholar] [CrossRef]
- Hink, L.; Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. The consequences of niche and physiological differentiation of archaeal and bacterial ammonia oxidisers for nitrous oxide emissions. ISME J. 2018, 12, 1084–1093. [Google Scholar] [CrossRef]
- Jia, Z.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J.M.; Stark, J.M. Ammonium availability and temperature control contributions of ammonia oxidizing bacteria and archaea to nitrification in an agricultural soil. Soil Biol. Biochem. 2017, 113, 161–172. [Google Scholar] [CrossRef]
- Lu, X.; Bottomley, P.J.; Myrold, D.D. Contributions of ammonia-oxidizing archaea and bacteria to nitrification in Oregon forest soils. Soil Biol. Biochem. 2015, 85, 54–62. [Google Scholar] [CrossRef]
- Hu, L.; Dong, Z.; Wang, Z.; Xiao, L.; Zhu, B. The contributions of ammonia oxidizing bacteria and archaea to nitrification-dependent N2O emission in alkaline and neutral purple soils. Sci. Rep. 2022, 12, 19928. [Google Scholar] [CrossRef] [PubMed]
- Xingchen, D.; Zhang, J.; Huizhen, Q.; Zhang, H.; Chaoyue, L.; Delei, D.; Qirong, S.; Zhongjun, J. Chronic nitrogen fertilization modulates competitive interactions among microbial ammonia oxidizers in a loess soil. Pedosphere 2019, 29, 24–33. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, S.; Jiang, Z.; Wu, Y.; Cui, L.; Huang, X.; Macreadie, P.I. Nitrogen purification potential limited by nitrite reduction process in coastal eutrophic wetlands. Sci. Total Environ. 2019, 694, 133702. [Google Scholar] [CrossRef]
- Pold, G.; Bonilla-Rosso, G.; Saghaï, A.; Strous, M.; Jones, C.M.; Hallin, S. Phylogenetics and environmental distribution of nitric oxide-forming nitrite reductases reveal their distinct functional and ecological roles. ISME Commun. 2024, 4, ycae020. [Google Scholar] [CrossRef]
- Graf, D.R.; Jones, C.M.; Hallin, S. Intergenomic comparisons highlight modularity of the denitrification pathway and underpin the importance of community structure for N2O emissions. PLoS ONE 2014, 9, e114118. [Google Scholar] [CrossRef]
- Herold, M.B.; Giles, M.E.; Alexander, C.J.; Baggs, E.M.; Daniell, T.J. Variable response of nirK and nirS containing denitrifier communities to long-term pH manipulation and cultivation. FEMS Microbiol. Lett. 2018, 365, fny035. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; González-López, J.; Bedmar, E.J. Distinct effect of nitrogen fertilisation and soil depth on nitrous oxide emissions and nitrifiers and denitrifiers abundance. Biol. Fertil. Soils 2018, 54, 829–840. [Google Scholar] [CrossRef]
- Szukics, U.; Hackl, E.; Zechmeister-Boltenstern, S.; Sessitsch, A. Contrasting response of two forest soils to nitrogen input: Rapidly altered NO and N 2 O emissions and nirK abundance. Biol. Fertil. Soils 2009, 45, 855–863. [Google Scholar] [CrossRef]
- Ligi, T.; Truu, M.; Truu, J.; Nõlvak, H.; Kaasik, A.; Mitsch, W.J.; Mander, Ü. Effects of soil chemical characteristics and water regime on denitrification genes (nirS, nirK, and nosZ) abundances in a created riverine wetland complex. Ecol. Eng. 2014, 72, 47–55. [Google Scholar] [CrossRef]
- Xu, M.; Li, T.; Liu, W.; Ding, J.; Gao, L.; Han, X.; Zhang, X. Sensitivity of soil nitrifying and denitrifying microorganisms to nitrogen deposition on the Qinghai–Tibetan plateau. Ann. Microbiol. 2021, 71, 6. [Google Scholar] [CrossRef]
- Shang, S.; Song, M.; Wang, C.; Dou, X.; Wang, J.; Liu, F.; Zhu, C.; Wang, S. Decrease of nitrogen cycle gene abundance and promotion of soil microbial-N saturation restrain increases in N2O emissions in a temperate forest with long-term nitrogen addition. Chemosphere 2023, 338, 139378. [Google Scholar] [CrossRef] [PubMed]
- Cuhel, J.; Šimek, M.; Laughlin, R.J.; Bru, D.; Cheneby, D.; Watson, C.J.; Philippot, L. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef]
- Wittorf, L.; Jones, C.M.; Bonilla-Rosso, G.; Hallin, S. Expression of nirK and nirS genes in two strains of Pseudomonas stutzeriharbouring both types of NO-forming nitrite reductases. Res. Microbiol. 2018, 169, 343–347. [Google Scholar] [CrossRef]
- Deng, M.; Dai, Z.; Senbati, Y.; Li, L.; Song, K.; He, X. Aerobic denitrification microbial community and function in zero-discharge recirculating aquaculture system using a single biofloc-based suspended growth reactor: Influence of the carbon-to-nitrogen ratio. Front. Microbiol. 2020, 11, 1760. [Google Scholar] [CrossRef]
- Saarenheimo, J.; Rissanen, A.J.; Arvola, L.; Nykänen, H.; Lehmann, M.F.; Tiirola, M. Genetic and environmental controls on nitrous oxide accumulation in lakes. PLoS ONE 2015, 10, e0121201. [Google Scholar] [CrossRef]
- Azziz, G.; Monza, J.; Etchebehere, C.; Irisarri, P. nirS-and nirK-type denitrifier communities are differentially affected by soil type, rice cultivar and water management. Eur. J. Soil Biol. 2017, 78, 20–28. [Google Scholar] [CrossRef]
- Lennon, E.F.; Houlton, B.Z. Coupled molecular and isotopic evidence for denitrifier controls over terrestrial nitrogen availability. ISME J. 2017, 11, 727–740. [Google Scholar] [CrossRef]
- Cantarel, A.A.; Rouifed, S.; Simon, L.; Bourg, J.; Gervaix, J.; Blazère, L.; Poussineau, S.; Creuzé des Châtelliers, C.; Piola, F. In nitrate-rich soil, fallopia x bohemica modifies functioning of N cycle compared to native monocultures. Diversity 2020, 12, 156. [Google Scholar] [CrossRef]
- Helen, D.; Kim, H.; Tytgat, B.; Anne, W. Highly diverse nirK genes comprise two major clades that harbour ammonium-producing denitrifiers. BMC Genom. 2016, 17, 155. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Li, Q.; Zhang, L.; Qin, Y.; Sun, B.; Liu, H. Changes of Soil Nitrogen Fractions and nirS-Type Denitrifier Microbial Community in Response to N Fertilizer in the Semi-Arid Area of Northeast China. Agronomy 2023, 13, 2212. [Google Scholar] [CrossRef]
- Fudjoe, S.K.; Li, L.; Anwar, S.; Shi, S.; Xie, J.; Wang, L.; Xie, L.; Yongjie, Z. Nitrogen fertilization promoted microbial growth and N2O emissions by increasing the abundance of nirS and nosZdenitrifiers in semiarid maize field. Front. Microbiol. 2023, 14, 1265562. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, S.A.; Roco, C.A.; Debenport, S.J.; Anderson, T.R.; Hofmeister, K.L.; Walter, M.T.; Shapleigh, J.P. Metagenomic analysis reveals distinct patterns of denitrification gene abundance across soil moisture, nitrate gradients. Environ. Microbiol. 2019, 21, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Saghaï, A.; Hallin, S. Diversity and ecology of NrfA-dependent ammonifying microorganisms. Trends Microbiol. 2024, 32, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Nizzoli, D.; Carraro, E.; Nigro, V.; Viaroli, P. Effect of organic enrichment and thermal regime on denitrification and dissimilatory nitrate reduction to ammonium (DNRA) in hypolimnetic sediments of two lowland lakes. Water Res. 2010, 44, 2715–2724. [Google Scholar] [CrossRef]


| Variable | pH | EC (ds m−1) | WC (%) | TC (g kg−1) | TN (g kg−1) | NH4+ (mg kg−1) | NO3− (mg kg−1) |
|---|---|---|---|---|---|---|---|
| CK | 5.613 ± 0.050 b | 44.300 ± 14.123 b | 9.467 ± 4.407 b | 37 ± 2 c | 3.1 ± 0.2 c | 1.230 ± 0.649 bc | 8.645 ± 3.522 cd |
| GN | 5.237 ± 0.051 c | 50.033 ± 15.130 b | 8.433 ± 0.493 ab | 39 ± 3 c | 3.7 ± 0.3 bc | 1.605 ± 0.366 bc | 6.075 ± 1.699 d |
| DN | 4.997 ± 0.129 c | 49.200 ± 9.350 b | 10.967 ± 3.465 b | 41 ± 1 bc | 4.8 ± 1 ab | 2.380 ± 1.036 a | 9.112 ± 2.648 bc |
| GL | 4.563 ± 0.170 d | 42.367 ± 4.842 a | 10.500 ± 0.794 ab | 45 ± 3 a | 5.2 ± 2 ab | 1.549 ± 0.527 b | 7.618 ± 3.609 b |
| DL | 5.107 ± 0.055 c | 45.500 ± 5.556 ab | 12.333 ± 4.989 b | 40 ± 5 a | 5.0 ± 0.1 a | 1.084 ± 0.443 bc | 7.621 ± 1.130 bcd |
| GX | 5.837 ± 0.093 ab | 89.833 ± 18.095 b | 8.533 ± 2.301 b | 49 ± 1 ab | 5.6 ± 5 a | −0.020 ± 0.133 d | 16.381 ± 4.323 a |
| DX | 5.990 ± 0.017 a | 53.300 ± 5.129 b | 10.433 ± 5.105 a | 29 ± 4 bc | 4.8 ± 0.2 ab | 1.237 ± 0.145 cd | 7.553 ± 0.730 bcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameer, M.J.; Liu, Y.; Yan, S.; Qu, T. Gene-Level Shift in Response to Synthetic Nitrogen Addition Promotes Larix olgensis (Ussurian Larch) Growth in a Short-Term Field Trial. Life 2025, 15, 1403. https://doi.org/10.3390/life15091403
Ameer MJ, Liu Y, Yan S, Qu T. Gene-Level Shift in Response to Synthetic Nitrogen Addition Promotes Larix olgensis (Ussurian Larch) Growth in a Short-Term Field Trial. Life. 2025; 15(9):1403. https://doi.org/10.3390/life15091403
Chicago/Turabian StyleAmeer, Muhammad Jamal, Yushan Liu, Siyu Yan, and Tongbao Qu. 2025. "Gene-Level Shift in Response to Synthetic Nitrogen Addition Promotes Larix olgensis (Ussurian Larch) Growth in a Short-Term Field Trial" Life 15, no. 9: 1403. https://doi.org/10.3390/life15091403
APA StyleAmeer, M. J., Liu, Y., Yan, S., & Qu, T. (2025). Gene-Level Shift in Response to Synthetic Nitrogen Addition Promotes Larix olgensis (Ussurian Larch) Growth in a Short-Term Field Trial. Life, 15(9), 1403. https://doi.org/10.3390/life15091403






