Biological Potential of Hypericum L. Sect. Drosocarpium Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Preparation of Extracts
2.3. Phytochemical Analysis of Plant Extracts
2.4. Biological Potential Evaluation
2.4.1. Antioxidant Potential Evaluation
2.4.2. Enzyme Inhibitory Activity
2.5. Data Processing
3. Results and Discussion
3.1. Chemical Characterization of the Sect. Drosocarpium Species
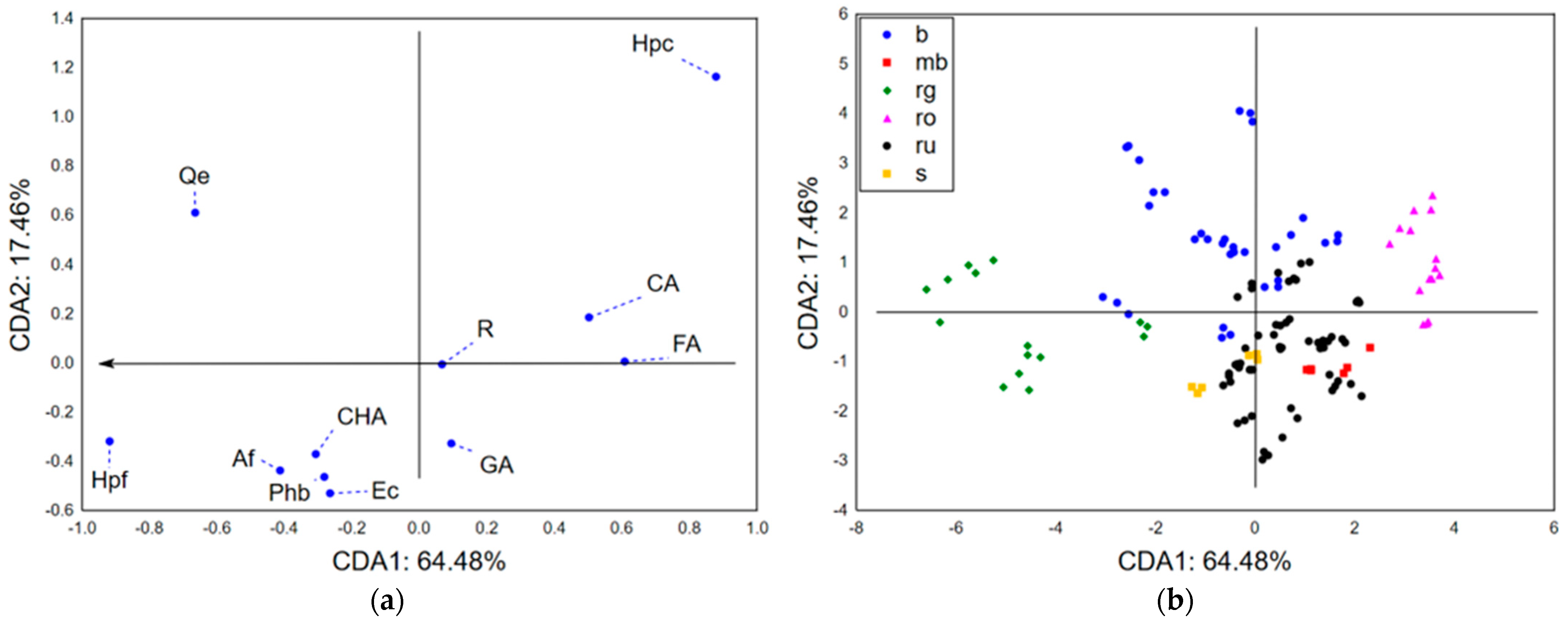
Chemometric Approach—Chemical Characterization
3.2. Biological Potential of the Sect. Drosocarpium Species
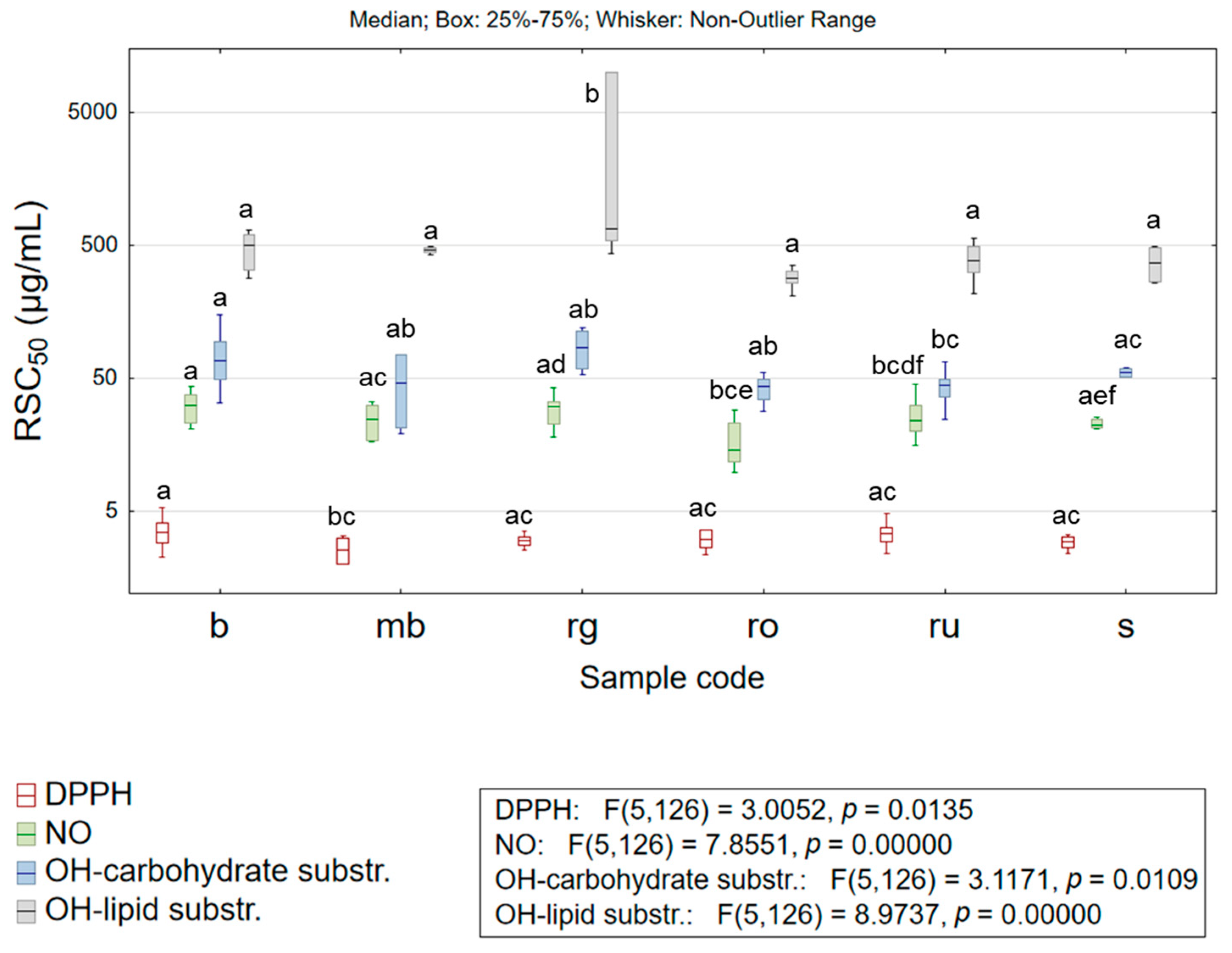
3.2.1. Antioxidant Potential
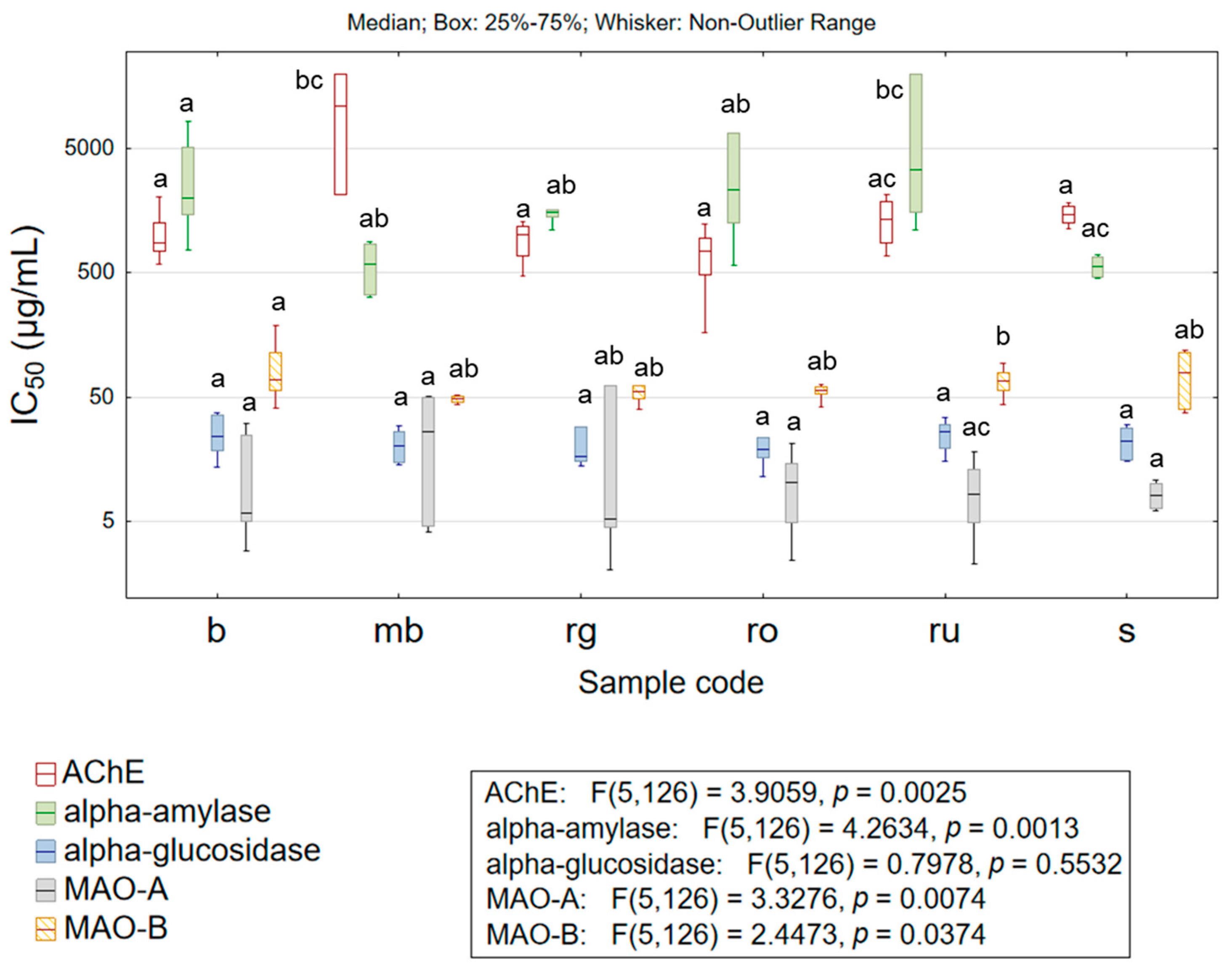
3.2.2. Inhibition of Biologically Important Enzymes
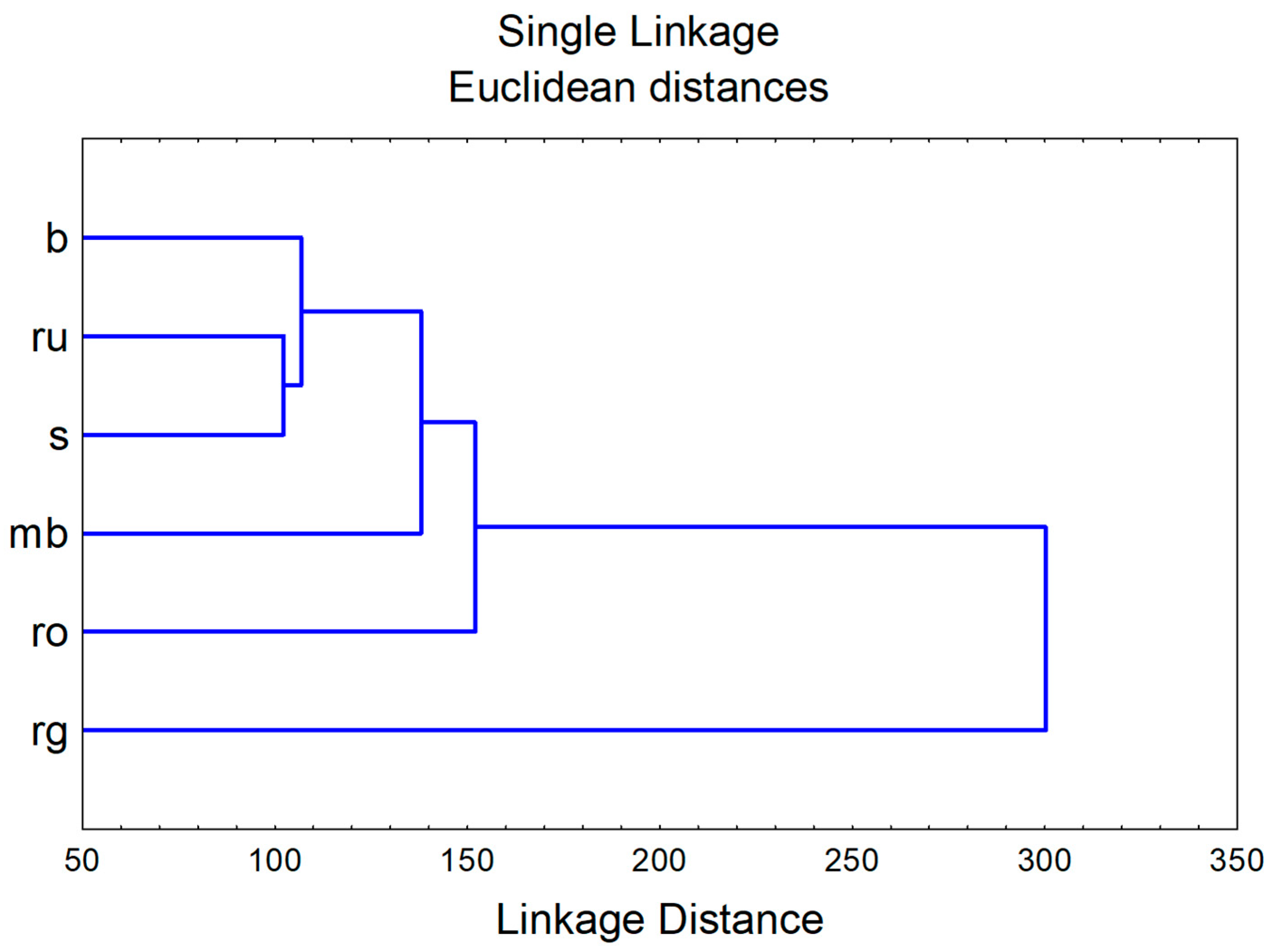
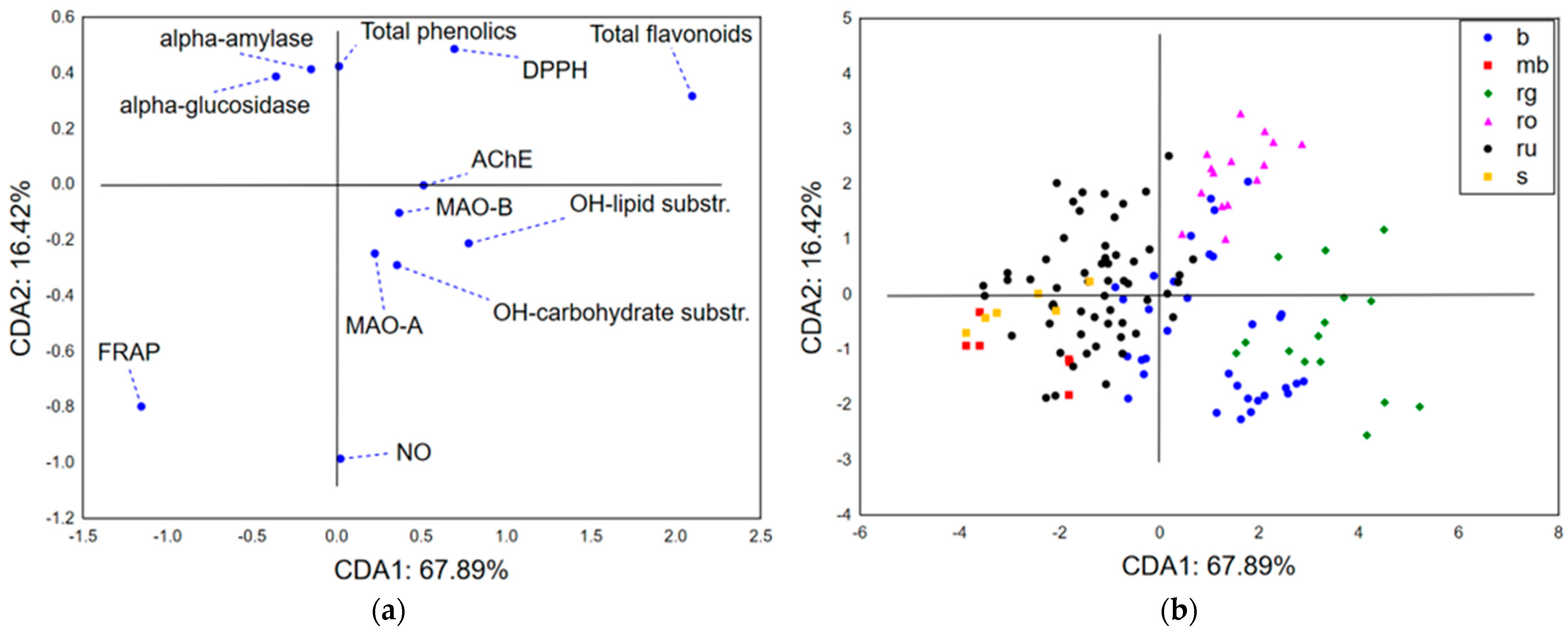
3.2.3. Chemometric Approach—Biological Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barciela, P.; Rodrigues, D.B.; Perez-Vazquez, A.; da Silveira, T.F.; Pires, T.C.; Mandim, F.; Carpena, M.; Pereira, C.; Ferreira, I.C.; Barros, L. Phytochemical diversity and biological activities of Hypericum japonicum and Hypericum sampsonii: Potential for natural product-based food applications. Food Chem. 2025, 484, 144355. [Google Scholar] [CrossRef]
- Caldeira, G.I.; Gouveia, L.P.; Serrano, R.; Silva, O.D. Hypericum genus as a natural source for biologically active compounds. Plants 2022, 11, 2509. [Google Scholar] [CrossRef]
- Kladar, N.; Božin, B.; Bijelić, K.; Bogavac, M.; Karaman, M.; Srđenović Čonić, B.; Rat, M.; Anačkov, G. Biological Activity of Genus Hypericum Sect. Hypericum Species—H. tetrapterum, H. maculatum subsp. immaculatum, H. triquetrifolium. Molecules 2023, 28, 6218. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, R.; Bensalel, J.; Morcol, T.; Gu, R.; Gallego-Delgado, J.; Kennelly, E.J.; Long, C. Metabolomic and chemometric analyses of St. John’s wort and related Asian Hypericum species linked to bioactivity. J. Ethnopharmacol. 2024, 329, 118163. [Google Scholar] [CrossRef]
- Radulović, N.; Stankov-Jovanović, V.; Stojanović, G.; Šmelcerović, A.; Spiteller, M.; Asakawa, Y. Screening of in vitro antimicrobial and antioxidant activity of nine Hypericum species from the Balkans. Food Chem. 2007, 103, 15–21. [Google Scholar] [CrossRef]
- Kitanov, G.M. Hypericin and pseudohypericin in some Hypericum species. Biochem. Syst. Ecol. 2001, 29, 171–178. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Mocan, A.; Tămaș, M.; Dias, M.I.; Pinela, J.; Stojković, D.; Soković, M.; Bădărău, A.S.; Crișan, G. Unravelling phytochemical and bioactive potential of three Hypericum species from Romanian spontaneous flora: H. Alpigenum, H. perforatum and H. Rochelii. Plants 2022, 11, 2773. [Google Scholar] [CrossRef]
- Vincent, O.M.; Nguta, J.M.; Mitema, E.S.; Musila, F.M.; Nyak, D.M.; Mohammed, A.H.; Gervason, M.A. Ethnopharmacology, pharmacological activities, and chemistry of the Hypericum genus. J. Phytopharm. 2021, 10, 105–113. [Google Scholar] [CrossRef]
- Nürk, N.M.; Crockett, S.L. Morphological and phytochemical diversity among Hypericum species of the Mediterranean Basin. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 14–28. [Google Scholar]
- Savikin, K.; Dobrić, S.; Tadić, V.; Zdunić, G. Antiinflammatory activity of ethanol extracts of Hypericum perforatum L., H. barbatum Jacq., H. hirsutum L., H. richeri Vill. and H. androsaemum L. in rats. Phytother. Res. 2007, 21, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Bianco, A. Secondary metabolites of Hypericum richeri Vill. collected in Central Italy: Chemotaxonomy and ethnomedicinal relevance. Trends Phytochem. Res. 2018, 2, 155–162. [Google Scholar]
- Keitel, S. Pharmacopoeial Standards: European Pharmacopoeia. Encycl. Pharm. Sci. Technol. 2013, 6, 2691–2703. [Google Scholar]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Goran, A.; Igic, R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008, 111, 925–929. [Google Scholar] [CrossRef]
- Božin, B.; Kladar, N.; Grujić, N.; Anačkov, G.; Samojlik, I.; Gavarić, N.; Čonić, B.S. Impact of origin and biological source on chemical composition, anticholinesterase and antioxidant properties of some St. John’s wort species (Hypericum spp., Hypericaceae) from the Central Balkans. Molecules 2013, 18, 11733–11750. [Google Scholar] [CrossRef]
- Ziaková, A.; Brandšteterová, E. Validation of HPLC determination of phenolic acids present in some Lamiaceae family plants. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 443–453. [Google Scholar] [CrossRef]
- Kladar, N.; Srđenović, B.; Grujić, N.; Bokić, B.; Rat, M.; Anačkov, G.; Božin, B. Ecologically and ontogenetically induced variations in phenolic compounds and biological activities of Hypericum maculatum subsp. maculatum, Hypericaceae. Braz. J. Bot. 2015, 38, 703–715. [Google Scholar] [CrossRef]
- Lesjak, M.M.; Beara, I.N.; Orčić, D.Z.; Anačkov, G.T.; Balog, K.J.; Francišković, M.M.; Mimica-Dukić, N.M. Juniperus sibirica Burgsdorf. as a novel source of antioxidant and anti-inflammatory agents. Food Chem. 2011, 124, 850–856. [Google Scholar] [CrossRef]
- Samoylenko, V.; Rahman, M.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.-H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–367. [Google Scholar] [CrossRef]
- Mun’im, A.; Katrin, K.; Azizahwati, A.; Andriani, A.; Mahmudah, K.; Mashita, M. Screening of α-glucosidase inhibitory activity of some Indonesian medicinal plants. Int. J. Med. Aromat. Plants 2013, 3, 144–150. [Google Scholar]
- Güzey, G.; Ibadova, S.; Öztürk, Y.; Öztürk, N.; Maggi, F.; Sagratini, G.; Ricciutelli, M.; Vittori, S. Antiproliferative and antioxidant effects of three Hypericum species of Turkish origin: H. perforatum, H. montbretii and H. origanifolium. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 91–99. [Google Scholar]
- Yaman, C.; Onlu, S.; Ahmed, H.; Erenler, R. Comparison of phytochemicals and antioxidant capacity of Hypericum perforatum; wild plant parts and in vitro samples. J. Anim. Plant Sci. 2022, 32, 596–603. [Google Scholar] [CrossRef]
- Baljak, J.; Bogavac, M.; Karaman, M.; Srđenović Čonić, B.; Vučković, B.; Anačkov, G.; Kladar, N. Chemical Composition and Biological Activity of Hypericum Species—H. hirsutum, H. barbatum, H. rochelii. Plants 2024, 13, 2905. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, Y.; Morcol, T.; Lin, F.; Gu, R.; Kennelly, E.J.; Long, C. UPLC-QTof-MS chemical profiling and characterization of antiproliferative and anti-inflammatory compounds from seven Hypericum species in China. Ind. Crops Prod. 2021, 173, 114156. [Google Scholar] [CrossRef]
- Maurya, S.K.; Divakar, S.; Rathee, S.; Patil, U.K. Diverse Therapeutic Potentials of Hypericin: An In-Depth Review. Curr. Top. Med. Chem. 2025, 25, 2478–2512. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Verma, V.; Spiteller, M.; Ahmad, S.M.; Puri, S.C.; Qazi, G.N. Phytochemical analysis and genetic characterization of six Hypericum species from Serbia. Phytochemistry 2006, 67, 171–177. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Spiteller, M. Phytochemical analysis of nine Hypericum L. species from Serbia and the FYR Macedonia. Die Pharm.-Int. J. Pharm. Sci. 2006, 61, 251–252. [Google Scholar]
- Zdunic, G.; Godjevac, D.; Savikin, K.; Petrovic, S. Comparative analysis of phenolic compounds in seven Hypericum species and their antioxidant properties. Nat. Prod. Commun. 2017, 12, 1934578X1701201140. [Google Scholar] [CrossRef]
- Kladar, N.; Mrđanović, J.; Anačkov, G.; Šolajić, S.; Gavarić, N.; Srđenović, B.; Božin, B. Hypericum perforatum: Synthesis of active principles during flowering and fruitification—Novel aspects of biological potential. Evid.-Based Complement. Altern. Med. 2017, 2017, 2865610. [Google Scholar] [CrossRef]
- Kakouri, E.; Trigas, P.; Daferera, D.; Skotti, E.; Tarantilis, P.A.; Kanakis, C. Chemical characterization and antioxidant activity of nine Hypericum species from Greece. Antioxidants 2023, 12, 899. [Google Scholar] [CrossRef]
- Zdunić, G.; Gođevac, D.; Šavikin, K.; Novaković, M.; Milosavljević, S.; Petrović, S. Isolation and identification of phenolic compounds from Hypericum richeri Vill. and their antioxidant capacity. Nat. Prod. Res. 2011, 25, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Çirak, C.; Radušienė, J.; Arslan, B. Variation of bioactive substances in Hypericum montbretii during plant growth. Nat. Prod. Res. 2008, 22, 246–252. [Google Scholar] [CrossRef]
- Choi, S.-S.; Park, H.-R.; Lee, K.-A. A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.D.; Hillwig, M.L.; Neighbors, J.D.; Sim, Y.-J.; Kohut, M.L.; Wiemer, D.F.; Wurtele, E.S.; Birt, D.F. Pseudohypericin is necessary for the light-activated inhibition of prostaglandin E2 pathways by a 4 component system mimicking an Hypericum perforatum fraction. Phytochemistry 2008, 69, 2354–2362. [Google Scholar] [CrossRef]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.-G.; Scheper, T. Identification of major constituents of Hypericum perforatum L. extracts in Syria by development of a rapid, simple, and reproducible HPLC-ESI-Q-TOF MS analysis and their antioxidant activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef]
- Singh, A.K.; Singla, R.K.; Pandey, A.K. Chlorogenic acid: A dietary phenolic acid with promising pharmacotherapeutic potential. Curr. Med. Chem. 2023, 30, 3905–3926. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Singh, R.; Sadiq, N.M. Cholinesterase Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Dong, Q.; Hu, N.; Yue, H.; Wang, H.; Wei, Y. Rapid screening of α-glucosidase inhibitors in Hypericum perforatum L. using bio-affinity chromatography coupled with UPLC/MS. Biomed. Chromatogr. 2023, 37, e5536. [Google Scholar] [CrossRef] [PubMed]
| Taxon | H. barbatum (n = 11 Samples) | H. montbretii (n = 2 Samples) | H. richerii subsp. grisebachii (n = 5 Samples) | H. rochelii (n = 5 Samples) | H. rumeliacum (n = 19 Samples) | H. spruneri (n = 2 Samples) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MV | Mdn | SD | MV | Mdn | SD | MV | Mdn | SD | MV | Mdn | SD | MV | Mdn | SD | MV | Mdn | SD | |
| Total phenolics (mg GAE/g d. e.) | 119.57 a | 119.42 | 35.08 | 152.25 ac | 149.99 | 66.56 | 142.71 ad | 141.28 | 31.76 | 164.90 bcd | 166.95 | 28.92 | 145.31 bcde | 142.97 | 29.49 | 110.43 ae | 109.62 | 35.70 |
| Total flavonoids (mg QE/g d. e.) | 33.76 a | 34.19 | 11.11 | 25.43 a | 25.49 | 2.86 | 44.92 b | 42.00 | 10.34 | 46.24 b | 47.86 | 11.43 | 33.27 a | 32.25 | 7.70 | 22.91 a | 23.19 | 2.50 |
| Dry extract yield (%) | 14.43 | 15.25 | 3.03 | 19.21 | 19.08 | 1.84 | 17.48 | 18.45 | 2.96 | 18.46 | 19.08 | 3.62 | 16.64 | 16.30 | 3.28 | 19.05 | 18.71 | 2.11 |
| H. barbatum (n = 11 Samples) | H. montbretii (n = 2 Samples) | H. richerii subsp. grisebachii (n = 5 Samples) | H. rochelii (n = 5 Samples) | H. rumeliacum (n = 19 Samples) | H. spruneri (n = 2 Samples) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MV | Mdn | SD | MV | Mdn | SD | MV | Mdn | SD | MV | Mdn | SD | MV | Mdn | SD | MV | Mdn | SD | |
| µg/g of dry herb | ||||||||||||||||||
| Hpc | 689.02 a | 573.44 | 415.42 | 215.42 bc | 208.45 | 159.65 | 575.32 acde | 560.54 | 331.60 | 738.41 a | 774.12 | 158.09 | 366.60 bd | 291.16 | 229.74 | 311.49 be | 308.69 | 80.87 |
| Hpf | 998.65 a | 953.08 | 481.14 | 311.76 bc | 309.31 | 196.65 | 2020.72 b | 1773.03 | 969.13 | 702.84 ac | 630.59 | 181.10 | 487.28 bc | 446.31 | 223.66 | 833.70 ac | 823.17 | 237.88 |
| Af | 51.65 a | 45.14 | 43.87 | 27.36 a | 26.75 | 6.12 | 95.58 bc | 120.37 | 55.81 | 28.49 a | 29.72 | 21.41 | 47.14 a | 51.03 | 26.50 | 67.21 ac | 65.61 | 36.33 |
| R | 216.29 a | 147.81 | 152.05 | 115.99 ac | 114.87 | 77.75 | 812.08 b | 784.73 | 405.48 | 97.86 ac | 109.72 | 19.93 | 208.03 ac | 100.60 | 331.07 | 110.05 ac | 107.39 | 24.56 |
| Qe | 148.52 a | 163.62 | 60.87 | 89.59 bc | 86.52 | 66.88 | 210.25 b | 194.44 | 55.00 | 32.75 bcd | n.d. | 41.60 | 78.06 bc | 73.18 | 33.43 | 75.42 bcd | 76.52 | 8.57 |
| Ec | 32.64 a | n.d. | 71.82 | 1268.07 b | 1207.34 | 1391.63 | 131.42 a | n.d. | 272.52 | 95.82 a | n.d. | 198.56 | 223.19 a | n.d. | 482.77 | n.d. a | n.d. | / |
| FA | 6.82 a | n.d. | 21.87 | 304.14 b | 290.64 | 260.18 | n.d. a | n.d. | / | 247.00 b | 264.04 | 233.38 | 143.84 bc | 96.22 | 180.11 | n.d. ac | n.d. | / |
| GA | 50.90 a | 46.35 | 47.55 | n.d. a | n.d. | / | 111.14 bc | 127.13 | 41.54 | 40.55 a | 34.22 | 40.89 | 55.55 a | 30.10 | 75.43 | 73.75 ac | 73.22 | 24.37 |
| CHA | 227.78 a | 167.32 | 228.34 | 21.75 a | 20.85 | 23.84 | 655.57 b | 205.51 | 618.24 | 94.00 a | 114.19 | 87.96 | 192.93 a | 140.52 | 309.37 | 178.42 a | 180.63 | 12.31 |
| CA | 62.52 a | 62.50 | 24.49 | 106.68 ab | 103.02 | 100.18 | 69.88 a | 58.65 | 28.55 | 88.39 ab | 93.15 | 37.85 | 51.66 ac | 48.89 | 45.42 | 71.02 a | 69.52 | 15.04 |
| Phb | 293.86 a | 118.34 | 351.90 | 258.30 ad | 256.17 | 84.47 | 124.94 abd | 105.03 | 74.44 | 208.26 ac | 288.42 | 152.69 | 1187.06 bcd | 946.61 | 1976.97 | 923.92 ad | 898.64 | 284.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kladar, N.; Srđenović Čonić, B.; Anačkov, G.; Hitl, M.; Bokić, B.; Radak, B.; Rat, M. Biological Potential of Hypericum L. Sect. Drosocarpium Species. Life 2025, 15, 1332. https://doi.org/10.3390/life15081332
Kladar N, Srđenović Čonić B, Anačkov G, Hitl M, Bokić B, Radak B, Rat M. Biological Potential of Hypericum L. Sect. Drosocarpium Species. Life. 2025; 15(8):1332. https://doi.org/10.3390/life15081332
Chicago/Turabian StyleKladar, Nebojša, Branislava Srđenović Čonić, Goran Anačkov, Maja Hitl, Bojana Bokić, Boris Radak, and Milica Rat. 2025. "Biological Potential of Hypericum L. Sect. Drosocarpium Species" Life 15, no. 8: 1332. https://doi.org/10.3390/life15081332
APA StyleKladar, N., Srđenović Čonić, B., Anačkov, G., Hitl, M., Bokić, B., Radak, B., & Rat, M. (2025). Biological Potential of Hypericum L. Sect. Drosocarpium Species. Life, 15(8), 1332. https://doi.org/10.3390/life15081332








