Gut Microbiota Modulation and Anti-Obesity Potential of Epigallocatechin-3-Gallate-Quercetin-Rutin Against High-Fat Diet-Induced Obesity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Composition
2.2. Animal Experimental Protocol
2.3. Lipid Analysis of Feces and Liver
2.4. Triglyceride and Total Cholesterol Analysis of Feces and Liver
2.5. Perirenal Adipocyte Morphology and Size Evaluation
2.6. 16S rRNA Microbial Gene Sequence Analysis
2.7. Short-Chain Fatty Acids Analysis of Feces
2.8. Lipid Metabolism Analysis of Liver, WAT, and BAT
2.9. Statistical Analysis
3. Results
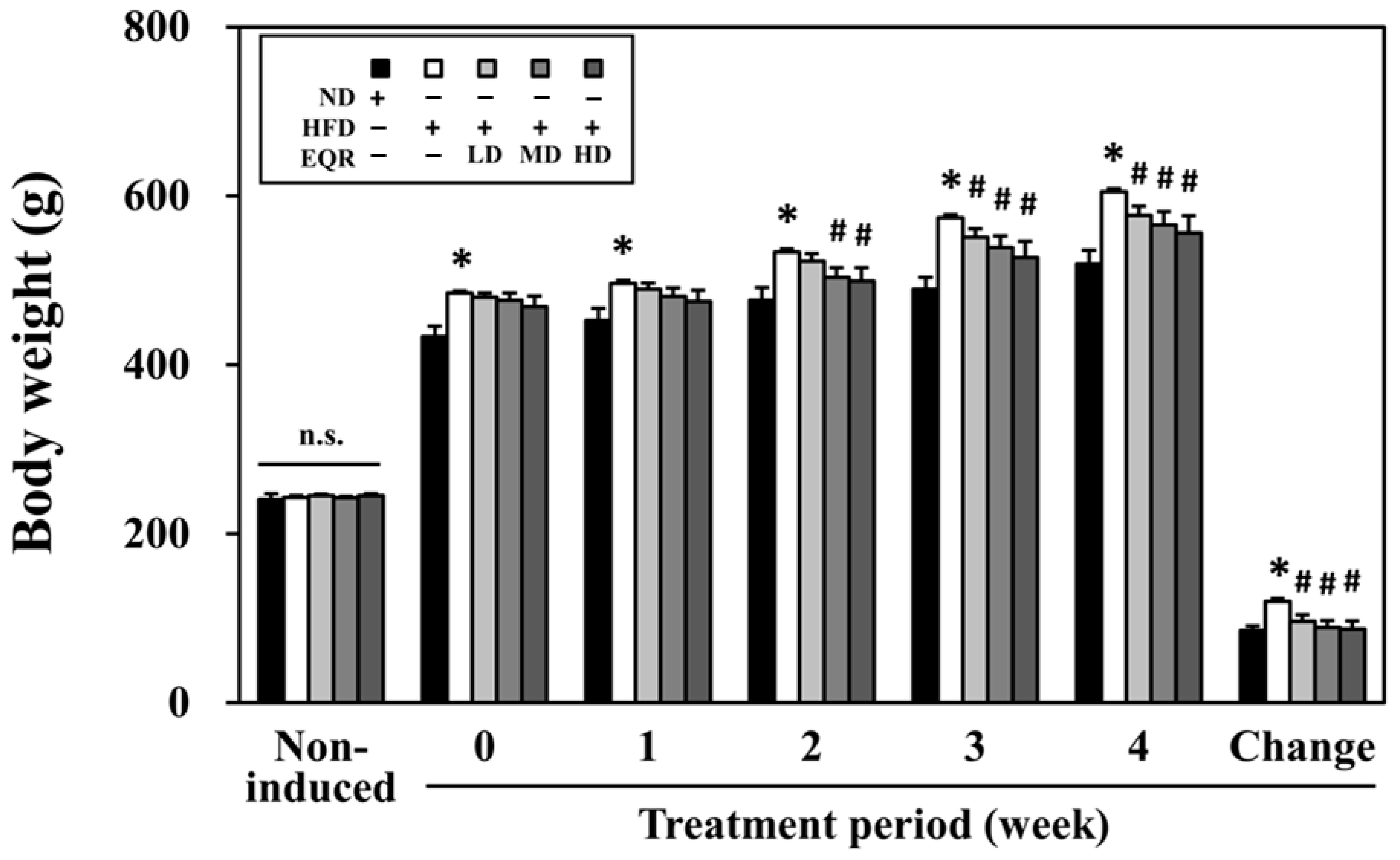
3.1. EQR’s Impact on Growth Metrics in Obese HFD-Fed Rats
3.2. Effects of EQR on Adipose Tissues in Obese HFD-Fed Rats
3.3. Effects of EQR on the Hepatic and Fecal Lipid Levels in Obese HFD-Fed Rats
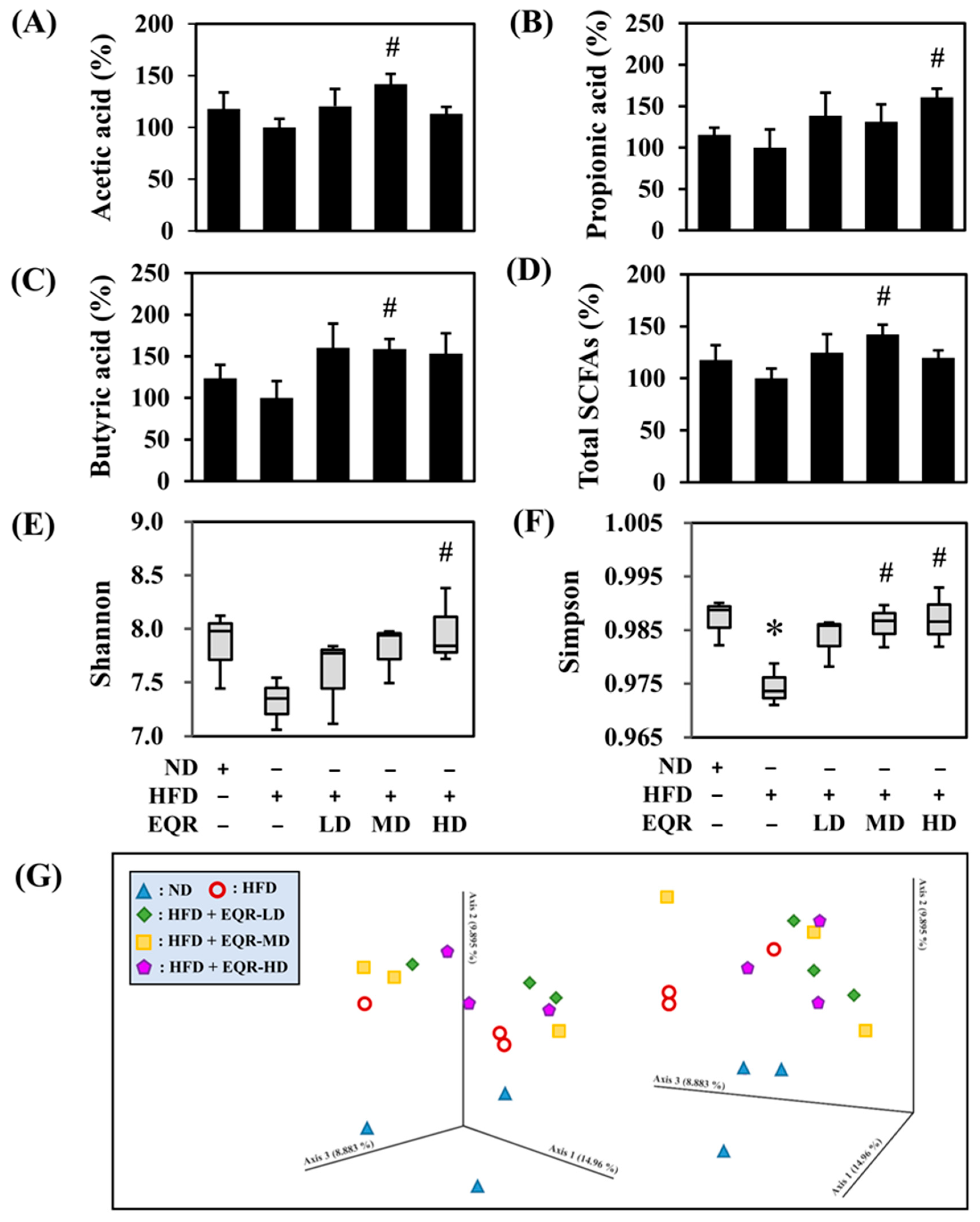
3.4. Effects of EQR on the Short-Chain Fatty Acids Contents in HFD-Induced Obese Rats
3.5. Effects of EQR on Gut Microbial Composition in Obese HFD-Fed Rats
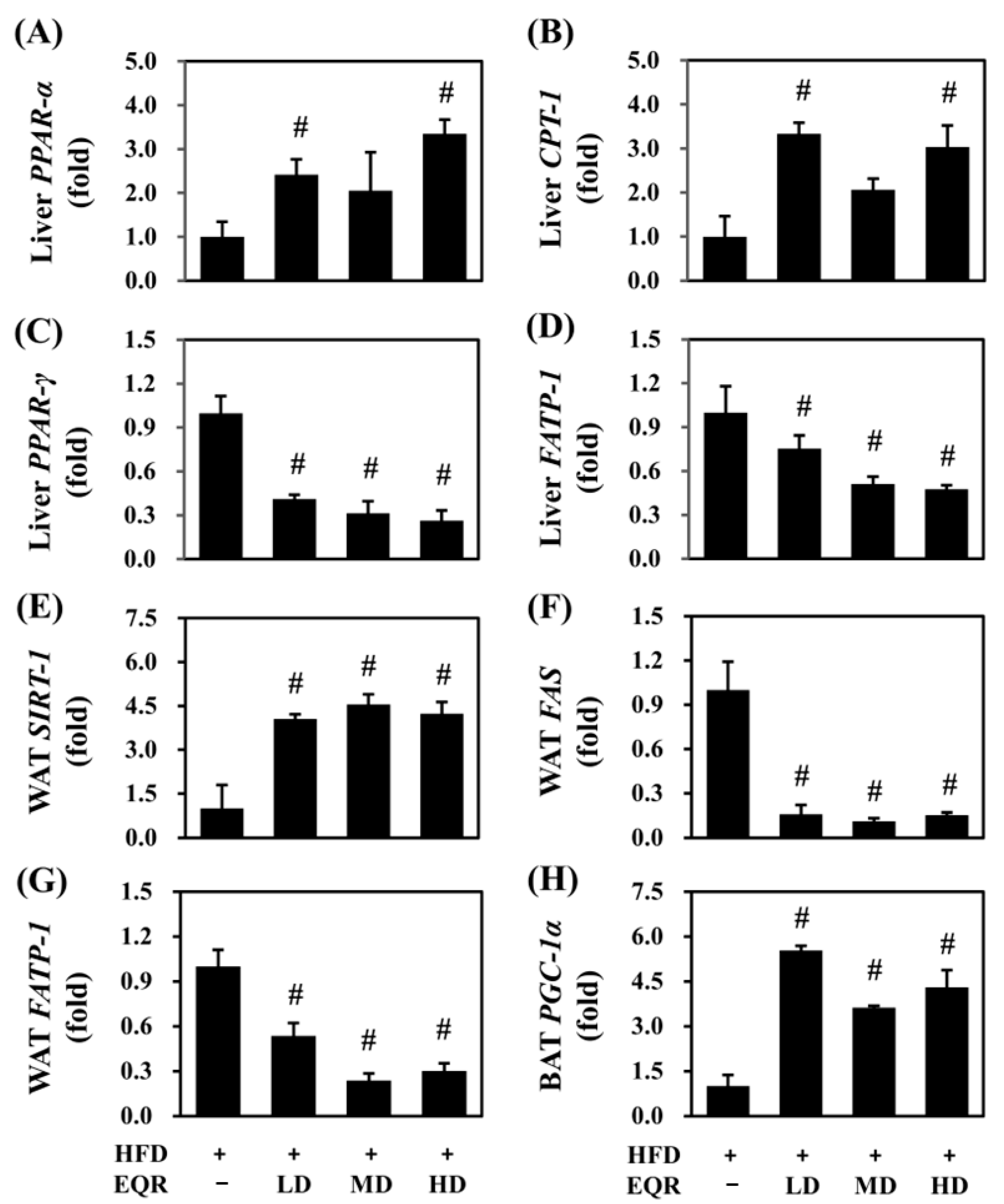
3.6. Effects of EQR on Lipid Metabolism in Obese HFD-Fed Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Yang, J.; Hu, J.; Zhu, C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 257–261. [Google Scholar] [CrossRef]
- Prendergast, H.; Tyo, C.; Colbert, C.; Kelley, M.; Pobee, R. Medical complications of obesity: Heightened importance in a COVID era. Int. J. Emerg. Med. 2022, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ge, S.; Li, S.; Lin, H.; Lin, S. Anti-obesity effect of trans-cinnamic acid on HepG2 cells and HFD-fed mice. Food Chem. Toxicol. 2020, 137, 111148. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Willcox, D.C.; Willcox, B.J.; Todoriki, H.; Suzuki, M. The Okinawan diet: Health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J. Am. Coll. Nutr. 2009, 28, 500S–516S. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Jing, N.; Zhao, Y.; Yang, X. EGCG regulates fatty acid metabolism of high-fat diet-fed mice in association with enrichment of gut Akkermansia muciniphila. J. Funct. Foods 2020, 75, 104261. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of tartary buckwheat, rutin, and quercetin on lipid metabolism in rats during high dietary fat intake. Food Sci. Nutr. 2020, 8, 199–213. [Google Scholar] [CrossRef]
- Diotallevi, C.; Fava, F.; Gobbetti, M.; Tuohy, K. Healthy dietary patterns to reduce obesity-related metabolic disease: Polyphenol-microbiome interactions unifying health effects across geography. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Hsu, C.L.; Yen, G.C. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007, 55, 8404–8410. [Google Scholar] [CrossRef]
- Ting, Y.; Chang, W.T.; Shiau, D.K.; Chou, P.H.; Wu, M.F.; Hsu, C.L. Antiobesity efficacy of quercetin-rich supplement on diet-induced obese rats: Effects on body composition, serum lipid profile, and gene expression. J. Agric. Food Chem. 2018, 66, 70–80. [Google Scholar] [CrossRef]
- Liu, M.E.; Chou, C.H.; Li, L.; Wu, Y.H.S.; Lin, Y.L.; Tu, D.G.; Chen, Y.C. Modulation effects of black-vinegar-based supplement against a high-fat dietary habit: Antiobesity/hypolipidemic, antioxidative, and energy-metabolism effects. J. Sci. Food Agric. 2020, 100, 2380–2388. [Google Scholar] [CrossRef]
- Tzang, B.S.; Yang, S.F.; Fu, S.G.; Yang, H.C.; Sun, H.L.; Chen, Y.C. Effects of dietary flaxseed oil on cholesterol metabolism of hamsters. Food Chem. 2009, 114, 1450–1455. [Google Scholar] [CrossRef]
- Galarraga, M.; Campión, J.; Muñoz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-de-Solórzano, C. Adiposoft: Automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef]
- Clarridge III, J.E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [PubMed]
- Preguica, I.; Alves, A.; Nunes, S.; Fernandes, R.; Gomes, P.; Viana, S.D.; Reis, F. Diet-induced rodent models of obesity-related metabolic disorders-A guide to a translational perspective. Obes. Rev. 2020, 21, e13081. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Paglicawan, L.; Soomro, S.; Abunofal, O.; Baig, S.; Vanarsa, K.; Hicks, J.; Mohan, C. Epigallocatechin-3-Gallate Dampens Non-Alcoholic Fatty Liver by Modulating Liver Function, Lipid Profile and Macrophage Polarization. Nutrients 2021, 13, 599. [Google Scholar] [CrossRef]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Liu, X.; Wang, W.; Song, J.; Zhao, L.; Ding, L.; Yang, L.; Zhou, M. Dose-Related Urinary Metabolic Alterations of a Combination of Quercetin and Resveratrol-Treated High-Fat Diet Fed Rats. Front. Pharmacol. 2021, 12, 655563. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Marques, L.; Cazarin, C.; Bicas, J.; Maróstica Junior, M.; Carrilho, E.; Bogusz Junior, S. Determination of Short Chain Fatty Acids in Mice Feces by Capillary Electrophoresis. J. Braz. Chem. Soc. 2019, 30, 1326–1334. [Google Scholar] [CrossRef]
- Mansoorian, B.; Combet, E.; Alkhaldy, A.; Garcia, A.L.; Edwards, C.A. Impact of Fermentable Fibres on the Colonic Microbiota Metabolism of Dietary Polyphenols Rutin and Quercetin. Int. J. Environ. Res. Public. Health 2019, 16, 292. [Google Scholar] [CrossRef]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Marčetić, M.; Samardžić, S.; Ilić, T.; Božić, D.D.; Vidović, B. Phenolic composition, antioxidant, anti-enzymatic, antimicrobial and prebiotic properties of Prunus spinosa L. fruits. Foods 2022, 11, 3289. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, X.; Hou, C.; Chen, L.; Zhang, Y.; Li, J. Effects of pomegranate peel polyphenols combined with inulin on gut microbiota and serum metabolites of high-fat-induced obesity rats. J. Agric. Food Chem. 2023, 71, 5733–5744. [Google Scholar] [CrossRef]
- Wang, R.; Yao, L.; Meng, T.; Li, C.; Wang, L. Rhodomyrtus tomentosa (Ait.) Hassk fruit phenolic-rich extract mitigates intestinal barrier dysfunction and inflammation in mice. Food Chem. 2022, 393, 133438. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef]
- Wang, C.C.; Yen, J.H.; Cheng, Y.C.; Lin, C.Y.; Hsieh, C.T.; Gau, R.J.; Chiou, S.J.; Chang, H.Y. Polygala tenuifolia extract inhibits lipid accumulation in 3T3-L1 adipocytes and high-fat diet-induced obese mouse model and affects hepatic transcriptome and gut microbiota profiles. Food Nutr. Res. 2017, 61, 1379861. [Google Scholar] [CrossRef]
- Zhu, H.; Qiang, J.; He, J.; Tao, Y.; Bao, J.; Xu, P. Physiological parameters and gut microbiome associated with different dietary lipid levels in hybrid yellow catfish (Tachysurus fulvidracofemale symbolx Pseudobagrus vachelliimale symbol). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100777. [Google Scholar] [CrossRef]
- Saber, S.; Abd El-Fattah, E.E.; Yahya, G.; Gobba, N.A.; Maghmomeh, A.O.; Khodir, A.E.; Mourad, A.A.E.; Saad, A.S.; Mohammed, H.G.; Nouh, N.A.; et al. A Novel Combination Therapy Using Rosuvastatin and Lactobacillus Combats Dextran Sodium Sulfate-Induced Colitis in High-Fat Diet-Fed Rats by Targeting the TXNIP/NLRP3 Interaction and Influencing Gut Microbiome Composition. Pharmaceuticals 2021, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes 2021, 13, 1880221. [Google Scholar] [CrossRef] [PubMed]
- Lensu, S.; Pariyani, R.; Makinen, E.; Yang, B.; Saleem, W.; Munukka, E.; Lehti, M.; Driuchina, A.; Linden, J.; Tiirola, M.; et al. Prebiotic Xylo-Oligosaccharides Ameliorate High-Fat-Diet-Induced Hepatic Steatosis in Rats. Nutrients 2020, 12, 3225. [Google Scholar] [CrossRef]
- Piombino, P.; Genovese, A.; Esposito, S.; Moio, L.; Cutolo, P.P.; Chambery, A.; Severino, V.; Moneta, E.; Smith, D.P.; Owens, S.M.; et al. Saliva from obese individuals suppresses the release of aroma compounds from wine. PLoS ONE 2014, 9, e85611. [Google Scholar] [CrossRef]
- Tamura, M.; Hoshi, C.; Kobori, M.; Takahashi, S.; Tomita, J.; Nishimura, M.; Nishihira, J. Quercetin metabolism by fecal microbiota from healthy elderly human subjects. PLoS ONE 2017, 12, e0188271. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Chu, Q.; Zheng, X. Pomegranate fruit pulp polyphenols reduce diet-induced obesity with modulation of gut microbiota in mice. J. Sci. Food. Agric. 2021, 102, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Companys, J.; Gosalbes, M.J.; Pla-Paga, L.; Calderon-Perez, L.; Llaurado, E.; Pedret, A.; Valls, R.M.; Jimenez-Hernandez, N.; Sandoval-Ramirez, B.A.; Del Bas, J.M.; et al. Gut Microbiota Profile and Its Association with Clinical Variables and Dietary Intake in Overweight/Obese and Lean Subjects: A Cross-Sectional Study. Nutrients 2021, 13, 2032. [Google Scholar] [CrossRef]
- Calderon-Perez, L.; Llaurado, E.; Companys, J.; Pla-Paga, L.; Pedret, A.; Rubio, L.; Gosalbes, M.J.; Yuste, S.; Sola, R.; Valls, R.M. Interplay between dietary phenolic compound intake and the human gut microbiome in hypertension: A cross-sectional study. Food Chem. 2021, 344, 128567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hou, D.; Fu, Y.; Xue, Y.; Guan, X.; Shen, Q. Adzuki Bean Alleviates Obesity and Insulin Resistance Induced by a High-Fat Diet and Modulates Gut Microbiota in Mice. Nutrients 2021, 13, 3240. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, P.; Ramos-Lopez, O.; Cuevas-Sierra, A.; Martinez, J.A.; Milagro, F.I.; Riezu-Boj, J.I. A predictive regression model of the obesity-related inflammatory status based on gut microbiota composition. Int. J. Obes. 2021, 45, 2261–2268. [Google Scholar] [CrossRef]
- Wang, M.; Wang, B.; Wang, S.; Lu, H.; Wu, H.; Ding, M.; Ying, L.; Mao, Y.; Li, Y. Effect of Quercetin on Lipids Metabolism Through Modulating the Gut Microbial and AMPK/PPAR Signaling Pathway in Broilers. Front. Cell Dev. Biol. 2021, 9, 616219. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Gao, H.; Wang, Z.; Lin, Y.; Liao, X.; Zhang, H.; Chi, Y.; Zhu, H.; Dong, S. PPARalpha and PPARgamma activation attenuates total free fatty acid and triglyceride accumulation in macrophages via the inhibition of Fatp1 expression. Cell Death Dis. 2019, 10, 39. [Google Scholar] [CrossRef]
- Liou, C.J.; Wu, S.J.; Shen, S.C.; Chen, L.C.; Chen, Y.L.; Huang, W.C. Phloretin ameliorates hepatic steatosis through regulation of lipogenesis and Sirt1/AMPK signaling in obese mice. Cell Biosci. 2020, 10, 114. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Martins, F.F.; Santos, L.P.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Administration of eicosapentaenoic and docosahexaenoic acids may improve the remodeling and browning in subcutaneous white adipose tissue and thermogenic markers in brown adipose tissue in mice. Mol. Cell Endocrinol. 2019, 482, 18–27. [Google Scholar] [CrossRef]


| Items | ND | HFD | |||
|---|---|---|---|---|---|
| Control | EQR-LD | EQR-MD | EQR-HD | ||
| Growth parameters | |||||
| Feed intake (g/rat/day) | 33.31 ± 0.94 | 23.22 ± 0.37 * | 22.43 ± 0.49 | 22.40 ± 0.68 | 21.83 ± 0.74 |
| Energy intake (kcal/rat/day) | 100.07 ± 2.80 | 117.26 ± 1.84 * | 113.27 ± 2.49 | 112.05 ± 3.42 | 110.13 ± 3.80 |
| Feed efficiency (%) | 13.47 ± 0.57 | 27.19 ± 0.55 * | 22.51 ± 1.49 # | 20.76 ± 1.45 # | 20.65 ± 1.78 # |
| Water intake (mL/rat/day) | 36.79 ± 2.39 | 39.48 ± 1.99 | 47.61 ± 4.22 | 44.66 ± 3.39 | 42.02 ± 3.07 |
| Adipose tissue weight (mg/g rat) | |||||
| Total body fat | 98.72 ± 6.19 | 138.99 ± 4.98 * | 114.36 ± 7.59 # | 104.53 ± 6.79 # | 106.04 ± 8.89 # |
| Subcutaneous | 27.39 ± 1.94 | 42.24 ± 2.48 * | 34.15 ± 3.40 | 30.00 ± 2.46 # | 31.27 ± 3.26 # |
| Visceral | 71.16 ± 4.93 | 96.99 ± 3.83 * | 79.94 ± 4.58 # | 74.54 ± 5.52 # | 74.45 ± 6.08 # |
| Mesenteric | 17.39 ± 1.55 | 22.85 ± 1.56 * | 19.63 ± 1.52 | 16.18 ± 1.56 # | 16.54 ± 1.32 # |
| Retroperitoneal | 15.99 ± 1.48 | 22.99 ± 1.17 * | 20.81 ± 2.74 | 18.50 ± 2.48 | 19.06 ± 2.58 |
| Inguinal | 11.45 ± 1.19 | 19.12 ± 2.38 * | 13.53 ± 1.46 | 11.52 ± 1.32 # | 12.41 ± 1.91 # |
| Perirenal | 30.03 ± 2.72 | 42.51 ± 1.99 * | 32.61 ± 2.59 # | 32.16 ± 2.95 # | 31.54 ± 3.26 # |
| Epididymal | 23.53 ± 1.64 | 31.78 ± 1.70 * | 27.52 ± 1.41 | 26.27 ± 2.31 | 26.12 ± 1.86 # |
| Items | ND | HFD | |||
|---|---|---|---|---|---|
| Control | EQR-LD | EQR-MD | EQR-HD | ||
| Liver (mg/g tissue) | |||||
| Total lipid | 72.50 ± 1.94 | 119.92 ± 10.01 * | 124.12 ± 10.57 | 92.85 ± 5.35 # | 92.33 ± 5.68 # |
| Triglyceride | 14.29 ± 1.39 | 33.15 ± 3.13 * | 39.92 ± 4.48 | 28.65 ± 2.94 | 27.36 ± 3.09 |
| Total cholesterol | 6.45 ± 0.05 | 7.53 ± 0.85 | 6.48 ± 0.06 | 6.44 ± 0.03 | 6.49 ± 0.04 |
| Feces (mg/g dried feces) | |||||
| Total lipid | 28.93 ± 2.28 | 40.44 ± 2.63 * | 37.39 ± 1.77 | 44.01 ± 2.59 | 44.43 ± 2.02 |
| Triglyceride | 3.75 ± 0.21 | 5.00 ± 1.23 | 5.23 ± 1.21 | 6.69 ± 1.68 | 5.67 ± 1.30 |
| Total cholesterol | 3.05 ± 0.10 | 3.95 ± 0.23 * | 4.08 ± 0.38 | 4.27 ± 0.34 | 3.60 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, Y.-J.; Cho, C.-C.; Hung, Y.-T.; Chen, L.-Y.; Wong, Y.-C.; Chen, S.-C.; Hsu, C.-L. Gut Microbiota Modulation and Anti-Obesity Potential of Epigallocatechin-3-Gallate-Quercetin-Rutin Against High-Fat Diet-Induced Obesity in Rats. Life 2025, 15, 1331. https://doi.org/10.3390/life15081331
Chien Y-J, Cho C-C, Hung Y-T, Chen L-Y, Wong Y-C, Chen S-C, Hsu C-L. Gut Microbiota Modulation and Anti-Obesity Potential of Epigallocatechin-3-Gallate-Quercetin-Rutin Against High-Fat Diet-Induced Obesity in Rats. Life. 2025; 15(8):1331. https://doi.org/10.3390/life15081331
Chicago/Turabian StyleChien, Yu-Jou, Ching-Chang Cho, Yu-Ting Hung, Li-You Chen, Yue-Ching Wong, Shiuan-Chih Chen, and Chin-Lin Hsu. 2025. "Gut Microbiota Modulation and Anti-Obesity Potential of Epigallocatechin-3-Gallate-Quercetin-Rutin Against High-Fat Diet-Induced Obesity in Rats" Life 15, no. 8: 1331. https://doi.org/10.3390/life15081331
APA StyleChien, Y.-J., Cho, C.-C., Hung, Y.-T., Chen, L.-Y., Wong, Y.-C., Chen, S.-C., & Hsu, C.-L. (2025). Gut Microbiota Modulation and Anti-Obesity Potential of Epigallocatechin-3-Gallate-Quercetin-Rutin Against High-Fat Diet-Induced Obesity in Rats. Life, 15(8), 1331. https://doi.org/10.3390/life15081331






