Correlations Between H. pylori Gastric Histopathology and NAFLD: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection Criteria
2.2. Endoscopy
2.3. H. pylori Diagnosis Using the Rapid Urease Test
2.4. Histopathology
2.5. Blood Tests
2.6. Statistical Analysis
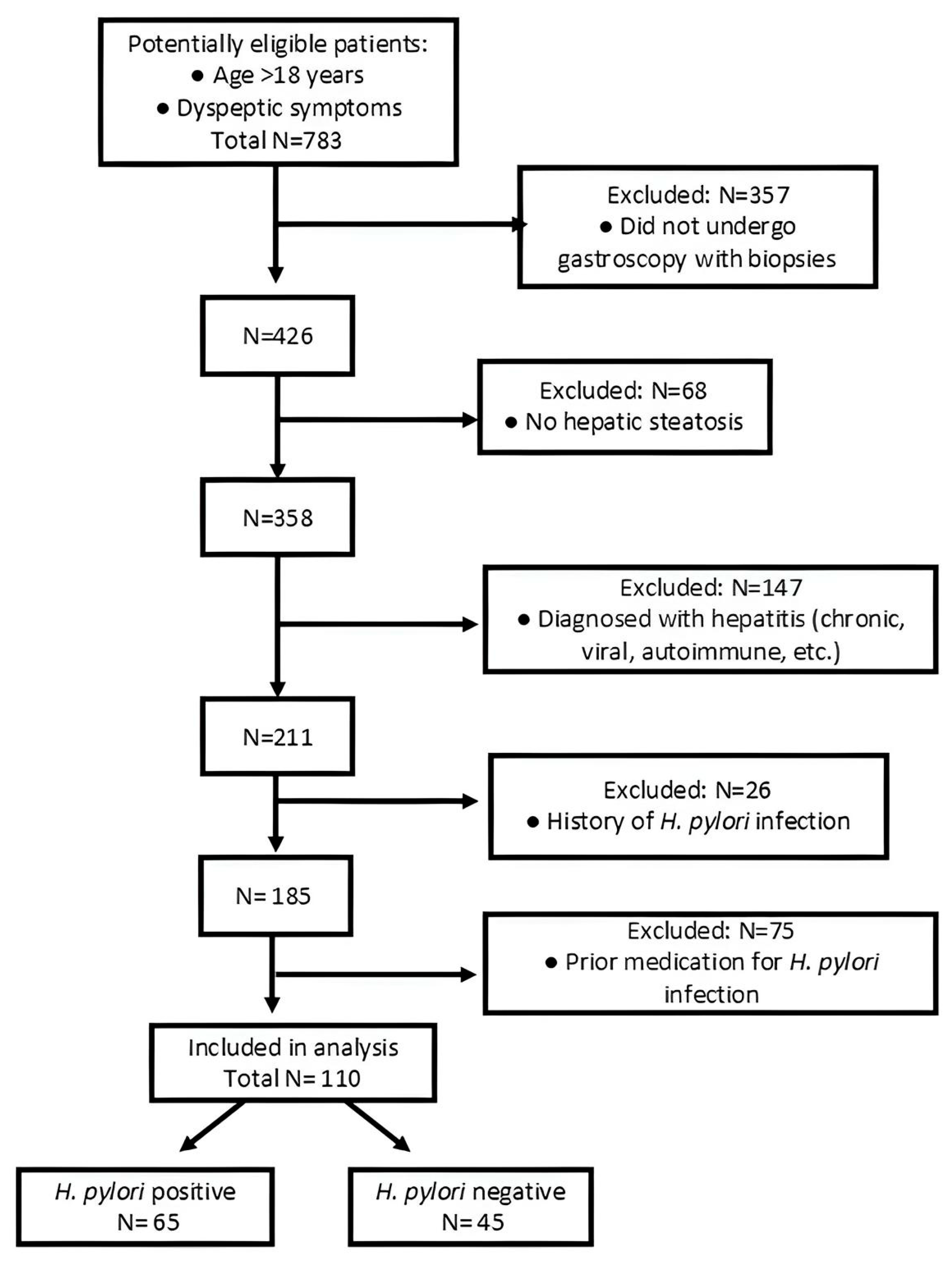
3. Results
3.1. Baseline Characteristics
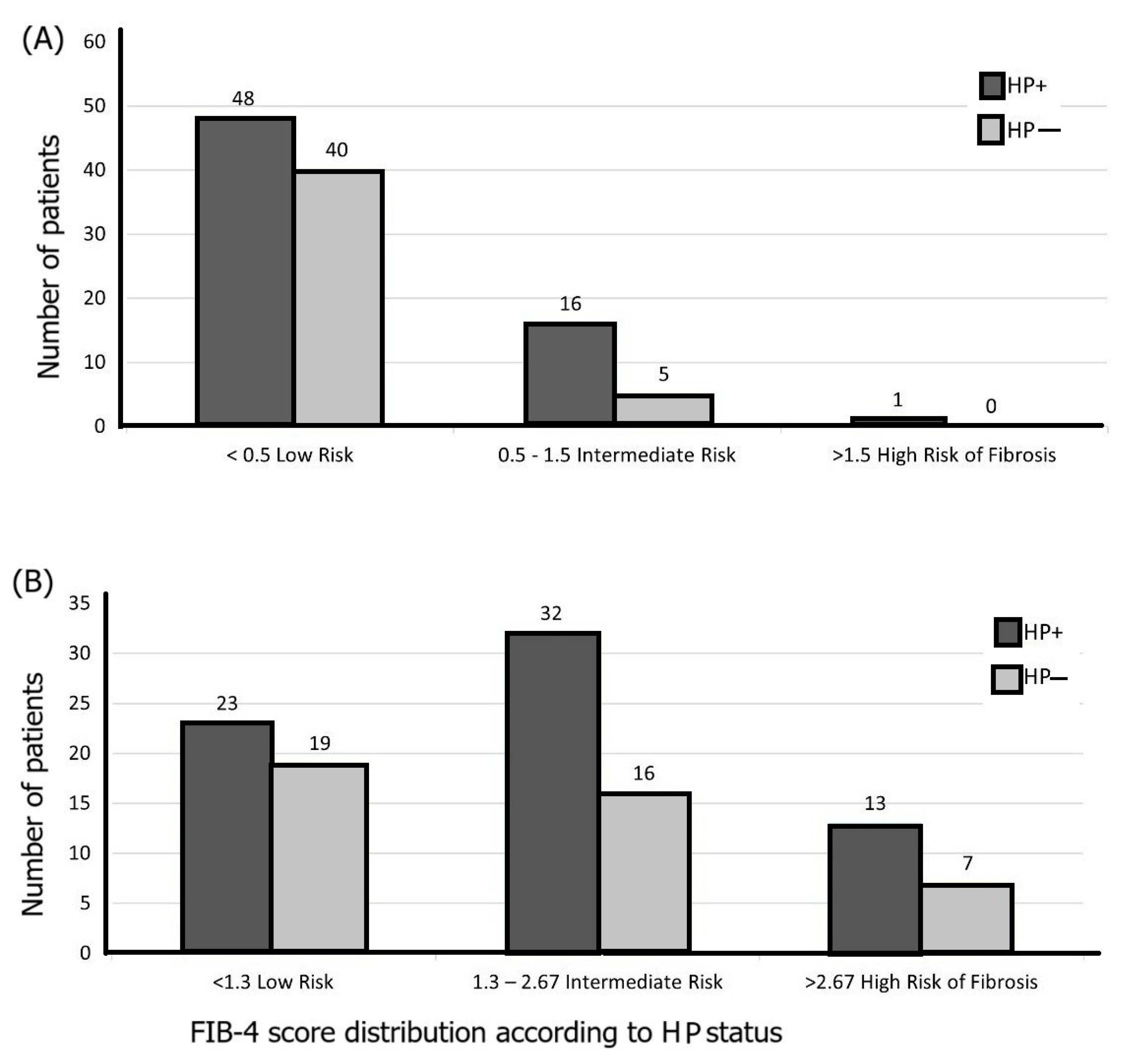
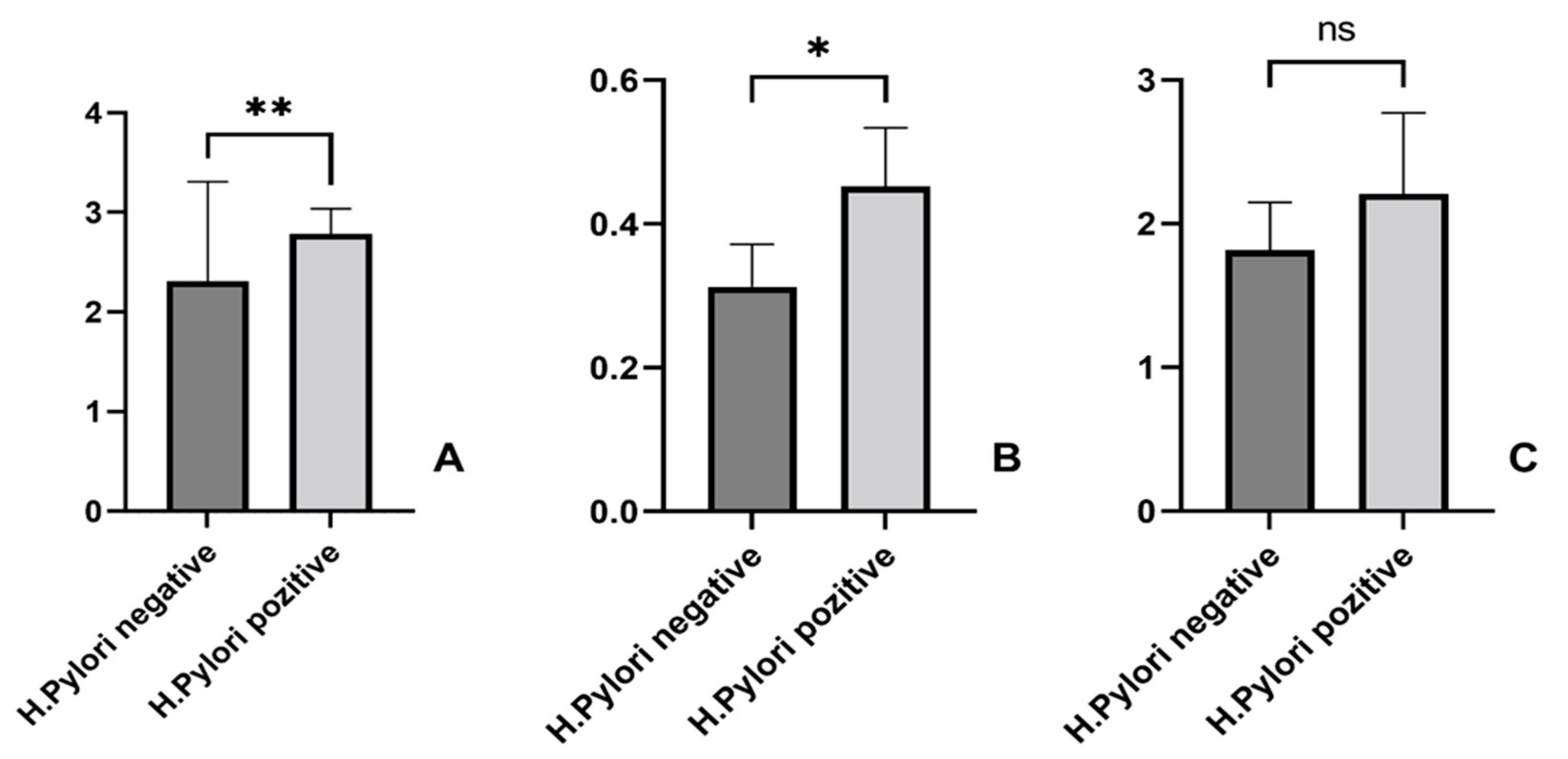
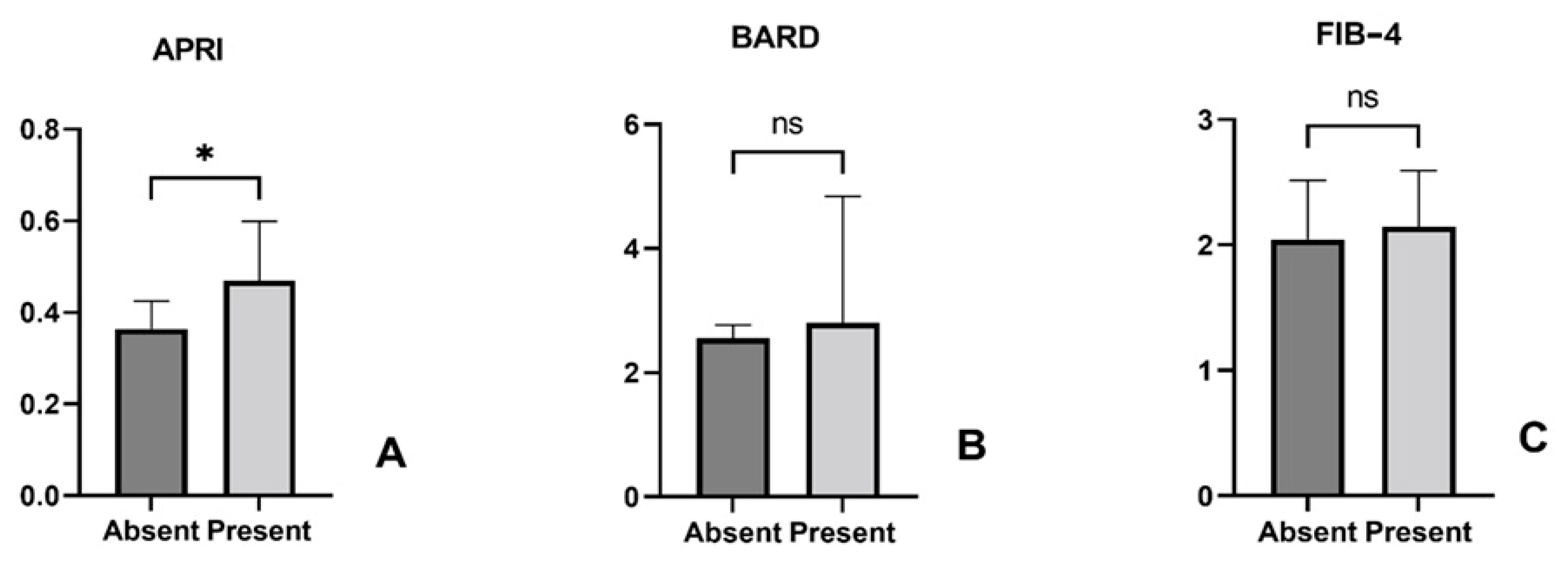
3.2. Association Between Liver Fibrosis Scores and H. pylori Infection
3.3. H. pylori Patients’ Features and Liver Fibrosis Scores
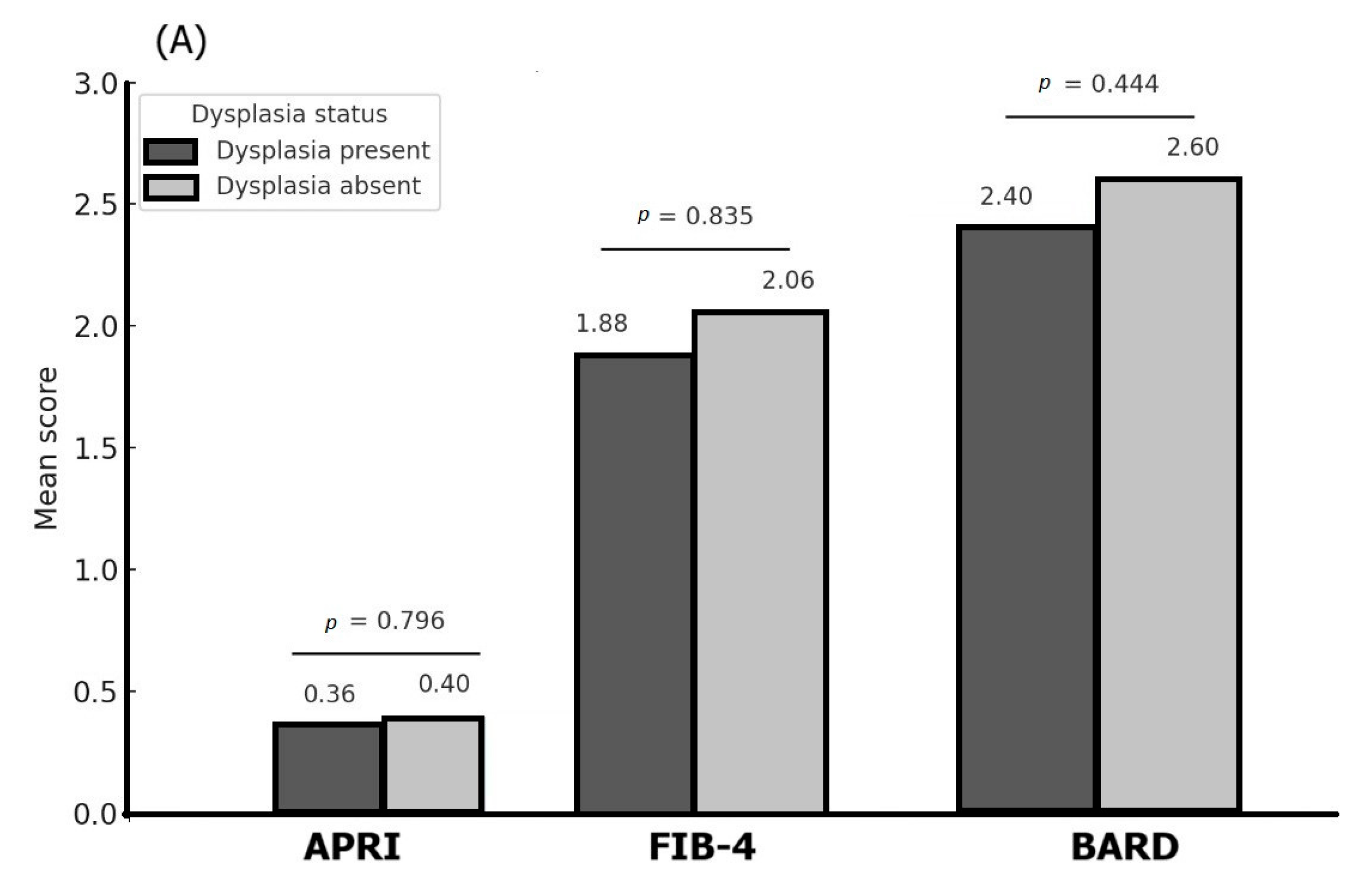
3.4. Associations Between Histopathological Features of the Gastric Lesions and Different Liver Fibrosis Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marshall, B.J.; Warren, J.R. Unidentified Curved Bacilli in the Stomach of Patients with Gastritis and Peptic Ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Watts, G. Nobel Prize Is Awarded to Doctors Who Discovered H Pylori. BMJ 2005, 331, 795. [Google Scholar] [CrossRef]
- Adadi, S.; Bennani, B.; Elabkari, M.; Ibrahimi, A.; Alaoui, S.; Elkhadir, M.; Harmouch, T.; Mahmoud, M.; Nejjari, C.; Benajah, D. Gastric Atrophy, Intestinal Metaplasia in Helicobacter pylori Gastritis: Prevalence and Predictors Factors. J. Biosci. Med. 2016, 4, 43–49. [Google Scholar] [CrossRef][Green Version]
- Stolte, M.; Bayerdörffer, E.; Morgner, A.; Alpen, B.; Wündisch, T.; Thiede, C.; Neubauer, A. Helicobacter and Gastric MALT Lymphoma. Gut 2002, 50 (Suppl. S3), iii19–iii24. [Google Scholar] [CrossRef] [PubMed]
- Hudak, L.; Jaraisy, A.; Haj, S.; Muhsen, K. An Updated Systematic Review and Meta-Analysis on the Association between Helicobacter pylori Infection and Iron Deficiency Anemia. Helicobacter 2017, 22, e12330. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, Y.-H.; Goh, K.-L.; Chang, L.-L. Helicobacter pylori and Systemic Disease. Gastroenterol. Res. Pract. 2014, 2014, 358494. [Google Scholar] [CrossRef]
- Zagari, R.M.; Romano, M.; Ojetti, V.; Stockbrugger, R.; Gullini, S.; Annibale, B.; Farinati, F.; Ierardi, E.; Maconi, G.; Rugge, M.; et al. Guidelines for the Management of Helicobacter pylori Infection in Italy: The III Working Group Consensus Report 2015. Dig. Liver Dis. 2015, 47, 903–912. [Google Scholar] [CrossRef]
- Wong, F.; Rayner-Hartley, E.; Byrne, M.F. Extraintestinal Manifestations of Helicobacter pylori: A Concise Review. World J. Gastroenterol. 2014, 20, 11950–11961. [Google Scholar] [CrossRef] [PubMed]
- Waluga, M.; Kukla, M.; Żorniak, M.; Bacik, A.; Kotulski, R. From the Stomach to Other Organs: Helicobacter pylori and the Liver. World J. Hepatol. 2015, 7, 2136–2146. [Google Scholar] [CrossRef]
- Bellentani, S.; Scaglioni, F.; Marino, M.; Bedogni, G. Epidemiology of Non-Alcoholic Fatty Liver Disease. Dig. Dis. 2010, 28, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Papatheodorou, A.; Patsiaoura, K.; Katsiki, E.; Zafeiriadou, E.; Zavos, C.; Anastasiadou, K.; Terpos, E. Helicobacter pylori Infection in Patients with Nonalcoholic Fatty Liver Disease. Metabolism 2013, 62, 121–126. [Google Scholar] [CrossRef]
- Taylor, N.S.; Fox, J.G.; Yan, L. In-Vitro Hepatotoxic Factor in Helicobacter Hepaticus, H. Pylori and Other Helicobacter Species. J. Med. Microbiol. 1995, 42, 48–52. [Google Scholar] [CrossRef]
- Fukuda, Y.; Bamba, H.; Okui, M.; Tamura, K.; Tanida, N.; Satomi, M.; Shimoyama, T.; Nishigami, T. Helicobacter pylori Infection Increases Mucosal Permeability of the Stomach and Intestine. Digestion 2001, 63 (Suppl. S1), 93–96. [Google Scholar] [CrossRef]
- Rockey, D.C.; Caldwell, S.H.; Goodman, Z.D.; Nelson, R.C.; Smith, A.D.; American Association for the Study of Liver Diseases. Liver Biopsy. Hepatology 2009, 49, 1017–1044. [Google Scholar] [CrossRef] [PubMed]
- Kazi, I.N.; Kuo, L.; Tsai, E. Noninvasive Methods for Assessing Liver Fibrosis and Steatosis. Gastroenterol. Hepatol. 2024, 20, 21–29. [Google Scholar]
- Wang, J.; Qin, T.; Sun, J.; Li, S.; Cao, L.; Lu, X. Non-Invasive Methods to Evaluate Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Front. Physiol. 2022, 13, 1046497. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and Validation of a Simple NAFLD Clinical Scoring System for Identifying Patients without Advanced Disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cui, H.; Li, N.; Wei, Y.; Lai, S.; Yang, Y.; Yin, X.; Chen, D.-F. Comparison of FIB-4 Index, NAFLD Fibrosis Score and BARD Score for Prediction of Advanced Fibrosis in Adult Patients with Non-Alcoholic Fatty Liver Disease: A Meta-Analysis Study. Hepatol. Res. 2016, 46, 862–870. [Google Scholar] [CrossRef]
- Raszeja-Wyszomirska, J.; Szymanik, B.; Ławniczak, M.; Kajor, M.; Chwist, A.; Milkiewicz, P.; Hartleb, M. Validation of the BARD Scoring System in Polish Patients with Nonalcoholic Fatty Liver Disease (NAFLD). BMC Gastroenterol. 2010, 10, 67. [Google Scholar] [CrossRef]
- Loaeza-del-Castillo, A.; Paz-Pineda, F.; Oviedo-Cárdenas, E.; Sánchez-Avila, F.; Vargas-Vorácková, F. AST to Platelet Ratio Index (APRI) for the Noninvasive Evaluation of Liver Fibrosis. Ann. Hepatol. 2008, 7, 350–357. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients with HIV/HCV Coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Blanco-Grau, A.; Gabriel-Medina, P.; Rodriguez-Algarra, F.; Villena, Y.; Lopez-Martínez, R.; Augustín, S.; Pons, M.; Cruz, L.-M.; Rando-Segura, A.; Enfedaque, B.; et al. Assessing Liver Fibrosis Using the FIB4 Index in the Community Setting. Diagnostics 2021, 11, 2236. [Google Scholar] [CrossRef]
- Ferraioli, G.; Soares Monteiro, L.B. Ultrasound-Based Techniques for the Diagnosis of Liver Steatosis. World J. Gastroenterol. 2019, 25, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic Accuracy and Reliability of Ultrasonography for the Detection of Fatty Liver: A Meta-Analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Joseph, R.; Lopez, R.; McCullough, A.J. Validity of Real Time Ultrasound in the Diagnosis of Hepatic Steatosis: A Prospective Study. J. Hepatol. 2009, 51, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The Utility of Radiological Imaging in Nonalcoholic Fatty Liver Disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef]
- Rugge, M.; Correa, P.; Dixon, M.F.; Fiocca, R.; Hattori, T.; Lechago, J.; Leandro, G.; Price, A.B.; Sipponen, P.; Solcia, E.; et al. Gastric Mucosal Atrophy: Interobserver Consistency Using New Criteria for Classification and Grading. Aliment. Pharmacol. Ther. 2002, 16, 1249–1259. [Google Scholar] [CrossRef]
- Cardos, A.I.; Maghiar, A.; Zaha, D.C.; Pop, O.; Fritea, L.; Miere (Groza), F.; Cavalu, S. Evolution of Diagnostic Methods for Helicobacter pylori Infections: From Traditional Tests to High Technology, Advanced Sensitivity and Discrimination Tools. Diagnostics 2022, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and Grading of Gastritis. The Updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Cardos, I.A.; Zaha, D.C.; Sindhu, R.K.; Cavalu, S. Revisiting Therapeutic Strategies for H. Pylori Treatment in the Context of Antibiotic Resistance: Focus on Alternative and Complementary Therapies. Molecules 2021, 26, 6078. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Malfertheiner, P.; Yu, H.-T.; Kuo, C.-L.; Chang, Y.-Y.; Meng, F.-T.; Wu, Y.-X.; Hsiao, J.-L.; Chen, M.-J.; Lin, K.-P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef]
- Kawai, S.; Wang, C.; Lin, Y.; Sasakabe, T.; Okuda, M.; Kikuchi, S. Lifetime Incidence Risk for Gastric Cancer in the Helicobacter pylori-Infected and Uninfected Population in Japan: A Monte Carlo Simulation Study. Int. J. Cancer 2022, 150, 18–27. [Google Scholar] [CrossRef]
- Cheng, D.-D.; He, C.; Ai, H.-H.; Huang, Y.; Lu, N.-H. The Possible Role of Helicobacter pylori Infection in Non-Alcoholic Fatty Liver Disease. Front. Microbiol. 2017, 8, 743. [Google Scholar] [CrossRef]
- Izhari, M.A.; Al Mutawa, O.A.; Mahzari, A.; Alotaibi, E.A.; Almashary, M.A.; Alshahrani, J.A.; Gosady, A.R.A.; Almutairi, A.M.; Dardari, D.M.M.; AlGarni, A.K.A. Helicobacter pylori (H. pylori) Infection-Associated Dyslipidemia in the Asir Region of Saudi Arabia. Life 2023, 13, 2206. [Google Scholar] [CrossRef]
- Cardos, I.A.; Danila, C.; Ghitea, T.C.; Pop, O.; Pascalau, A.; Cavalu, S. Histopathology Features of H. Pylori Gastritis Associated with Altered Lipid Profile: An Observational Study from a Tertiary Healthcare Center in North West Romania. Vivo 2024, 38, 1421–1428. [Google Scholar] [CrossRef]
- Adenote, A.; Dumic, I.; Madrid, C.; Barusya, C.; Nordstrom, C.W.; Rueda Prada, L. NAFLD and Infection, a Nuanced Relationship. Can. J. Gastroenterol. Hepatol. 2021, 2021, 5556354. [Google Scholar] [CrossRef]
- Ji, D.; Qin, E.; Xu, J.; Zhang, D.; Cheng, G.; Wang, Y.; Lau, G. Non-Alcoholic Fatty Liver Diseases in Patients with COVID-19: A Retrospective Study. J. Hepatol. 2020, 73, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.B.; Mograbi, J.M.; Amara, A.E.; Abu Elheja, O.H.; Mahamid, M.N. Non-Alcoholic Fatty Liver Disease and 30-Day All-Cause Mortality in Adult Patients with Community-Acquired Pneumonia. QJM 2019, 112, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Roytman, M.; Lin, J.; McGrath, M.; Klar, A.; Boone, K.; Higa, K.; Ma, P. Association between Helicobacter pylori Infection, MASLD, and Liver Fibrosis in Patients with Severe Obesity: A Single-Center Experience. Surg. Endosc. 2024, 38, 6873–6879. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.M.; Kumar, S. The Association Between Helicobacter pylori Infection and Nonalcoholic Fatty Liver Disease. Curr. Gastroenterol. Rep. 2017, 19, 5. [Google Scholar] [CrossRef]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e7. [Google Scholar] [CrossRef] [PubMed]


| Variable | Overall (M ± SD) | H. pylori+ (M ± SD) | H. pylori − (M ± SD) | p-Value (HP− vs. HP+) |
|---|---|---|---|---|
| Age (years) | 62.3 ± 12.7 | 61.9 ± 12.0 | 63.0 ± 13.8 | 0.663 |
| BMI (kg/m2) | 28.8 ± 8.2 | 29.9 ± 7.8 | 25.9 ± 8.4 | 0.058 |
| ALT (U/L) | 28.4 ± 21.2 | 32.1 ± 21.4 | 23.0 ± 20.0 | 0.025 |
| AST (U/L) | 33.7 ± 21.1 | 38.1 ± 23.4 | 27.5 ± 15.6 | 0.005 |
| Platelet count (103/µL) | 244.9 ± 92.5 | 248.8 ± 103.9 | 239.3 ± 73.8 | 0.575 |
| Fasting glucose (mg/dL) | 110.4 ± 44.0 | 110.8 ± 34.6 | 110.0 ± 55.1 | 0.928 |
| Total cholesterol (mg/dL) | 181.1 ± 50.1 | 188.9 ± 52.7 | 169.0 ± 43.8 | 0.038 |
| Triglycerides (mg/dL) | 139.5 ± 83.4 | 150.8 ± 98.4 | 122.3 ± 49.4 | 0.052 |
| HDL cholesterol (mg/dL) | 44.4 ± 20.4 | 43.7 ± 23.7 | 45.9 ± 10.9 | 0.545 |
| LDL cholesterol (mg/dL) | 107.1 ± 35.6 | 108.6 ± 33.6 | 104.4 ± 39.5 | 0.606 |
| Diabetes mellitus (n, %) | 41 (45.1%) | 27 (42.2%) | 14 (51.9%) | 0.538 |
| Parameter Estimates | Variable | Estimate | Standard Error | 95% CI (Asymptotic) | p-Value |
|---|---|---|---|---|---|
| β0 | Intercept | 1.874 | 0.487 | 0.907–2.841 | 0.0002 |
| β1 | Age | 0.0155 | 0.007 | 0–0.03 | 0.0389 |
| β2 | Gender (female = 0) | 0.2794 | 0.188 | −0.09–0.653 | 0.141 |
| β3 | H. pylori (negative = 0) | −0.4561 | 0.191 | −0.839–−0.0 | 0.019 |
| β4 | Environment (urban = 0) | −0.330 | 0.191 | −0.710–0.05 | 0.088 |
| Parameter Estimates | Variable | Estimate | Standard Error | 95% CI (Asymptotic) | p-Value |
|---|---|---|---|---|---|
| β0 | Intercept | 0.189 | 0.138 | −0.085–0.464 | 0.175 |
| β1 | Age | 0.004 | 0.002 | 0–0.008 | 0.036 |
| β2 | Gender (female = 0) | −0.047 | 0.053 | −0.153–0.058 | 0.378 |
| β3 | H. pylori (negative = 0) | −0.146 | 0.054 | −0.254–−0.038 | 0.0084 |
| β4 | Environment (urban= 0) | 0.014 | 0.054 | −0.094–0.122 | 0.797 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardos, I.A.; Dănilă, C.; Pop, O.L.; Pop-Crisan, A.; Pavel Burta, O.; Camarasan, A.; Marc, F.; Cavalu, S.D. Correlations Between H. pylori Gastric Histopathology and NAFLD: A Retrospective Observational Study. Life 2025, 15, 1309. https://doi.org/10.3390/life15081309
Cardos IA, Dănilă C, Pop OL, Pop-Crisan A, Pavel Burta O, Camarasan A, Marc F, Cavalu SD. Correlations Between H. pylori Gastric Histopathology and NAFLD: A Retrospective Observational Study. Life. 2025; 15(8):1309. https://doi.org/10.3390/life15081309
Chicago/Turabian StyleCardos, Ioana Alexandra, Cătălina Dănilă, Ovidiu Laurean Pop, Andrea Pop-Crisan, Ovidiu Pavel Burta, Andreea Camarasan, Felicia Marc, and Simona Daniela Cavalu. 2025. "Correlations Between H. pylori Gastric Histopathology and NAFLD: A Retrospective Observational Study" Life 15, no. 8: 1309. https://doi.org/10.3390/life15081309
APA StyleCardos, I. A., Dănilă, C., Pop, O. L., Pop-Crisan, A., Pavel Burta, O., Camarasan, A., Marc, F., & Cavalu, S. D. (2025). Correlations Between H. pylori Gastric Histopathology and NAFLD: A Retrospective Observational Study. Life, 15(8), 1309. https://doi.org/10.3390/life15081309







