The Cortical Chlorenchyma Collaboration Gradient Dominates the Shoot Economics Space in Larix principis-rupprechtii
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
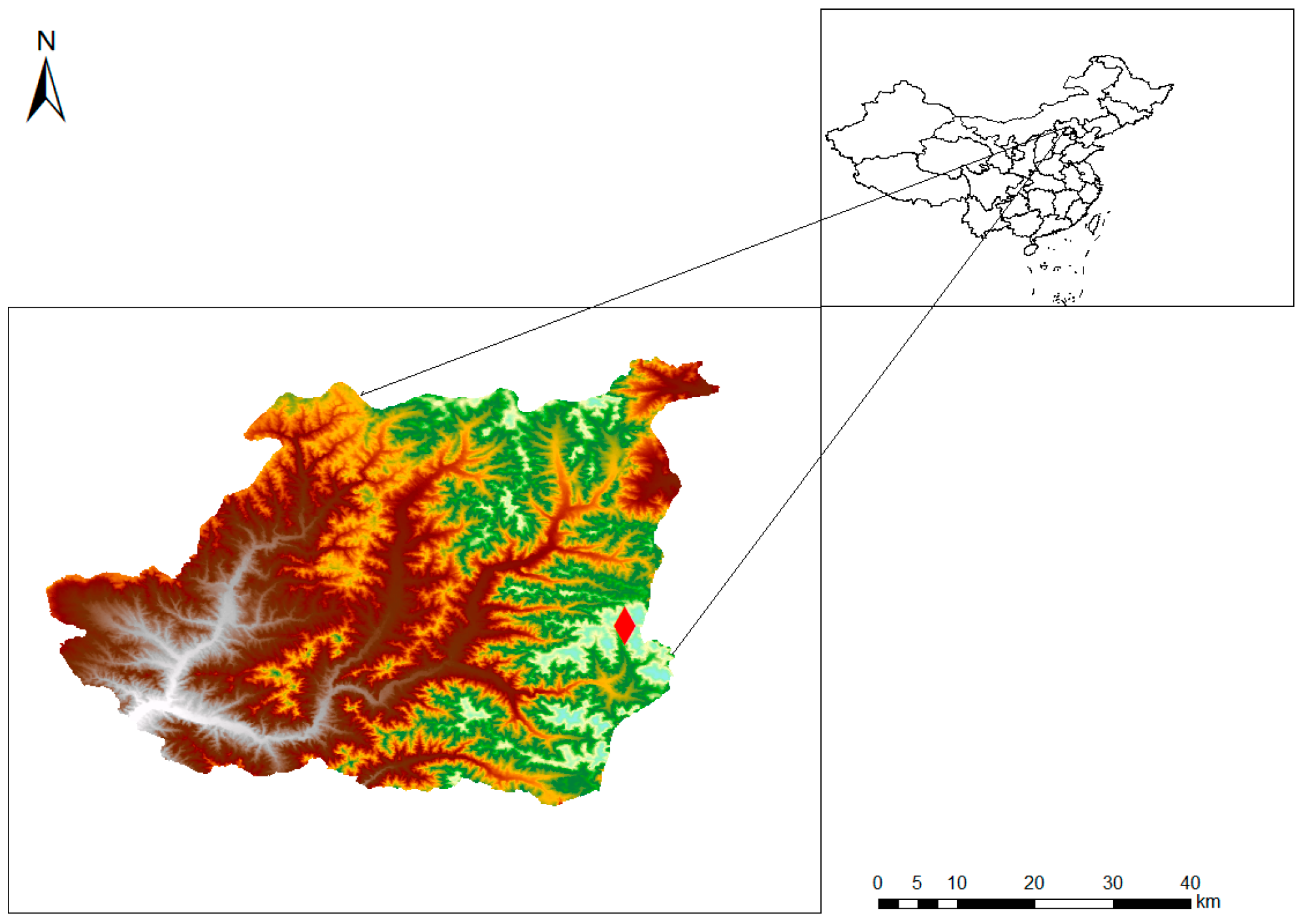
2.2. Study Site
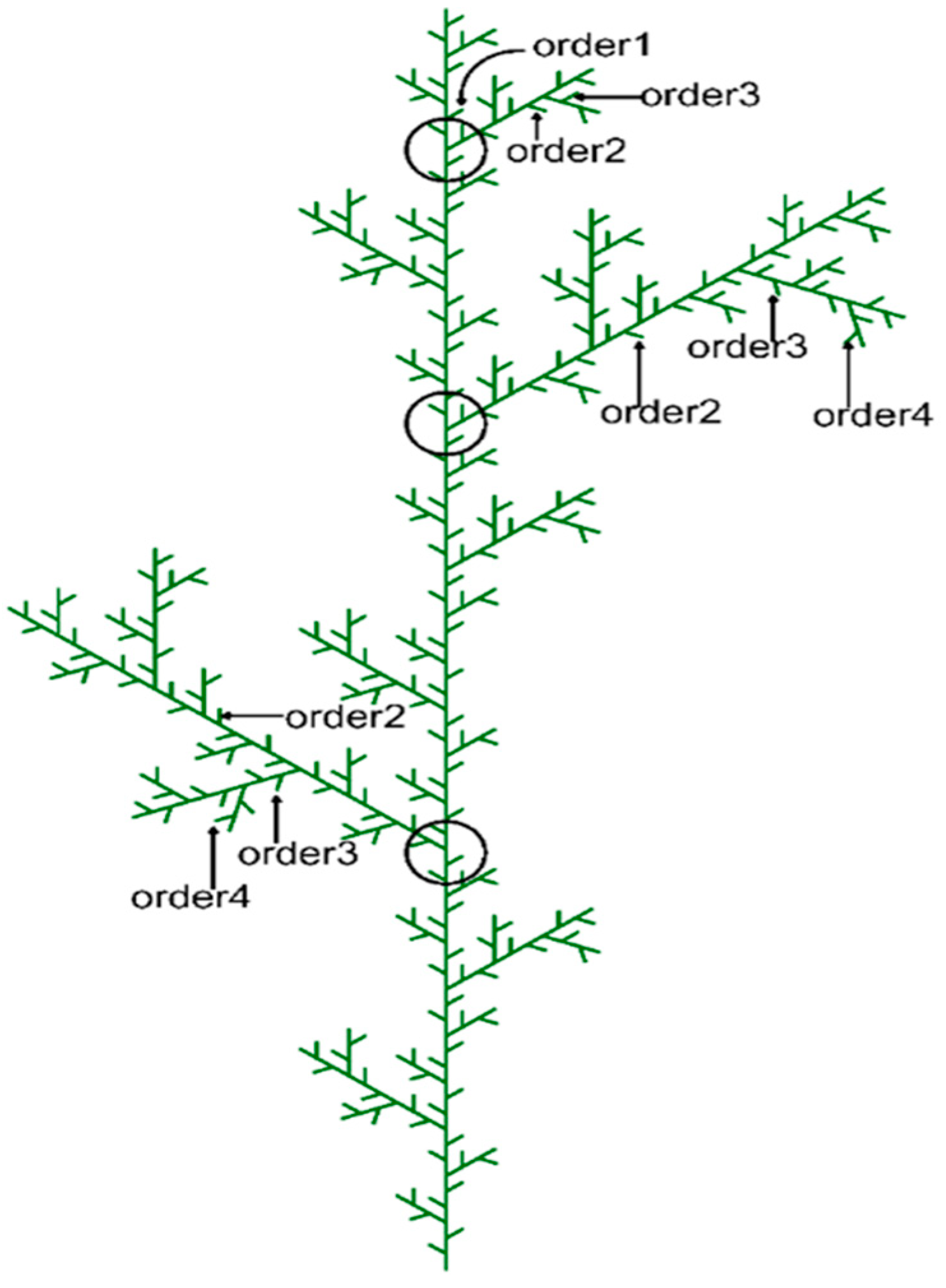
2.3. Sampling Procedures
2.4. Trait Measurements
2.5. Data Analysis
3. Results
3.1. Trait Correlations in the Shoot
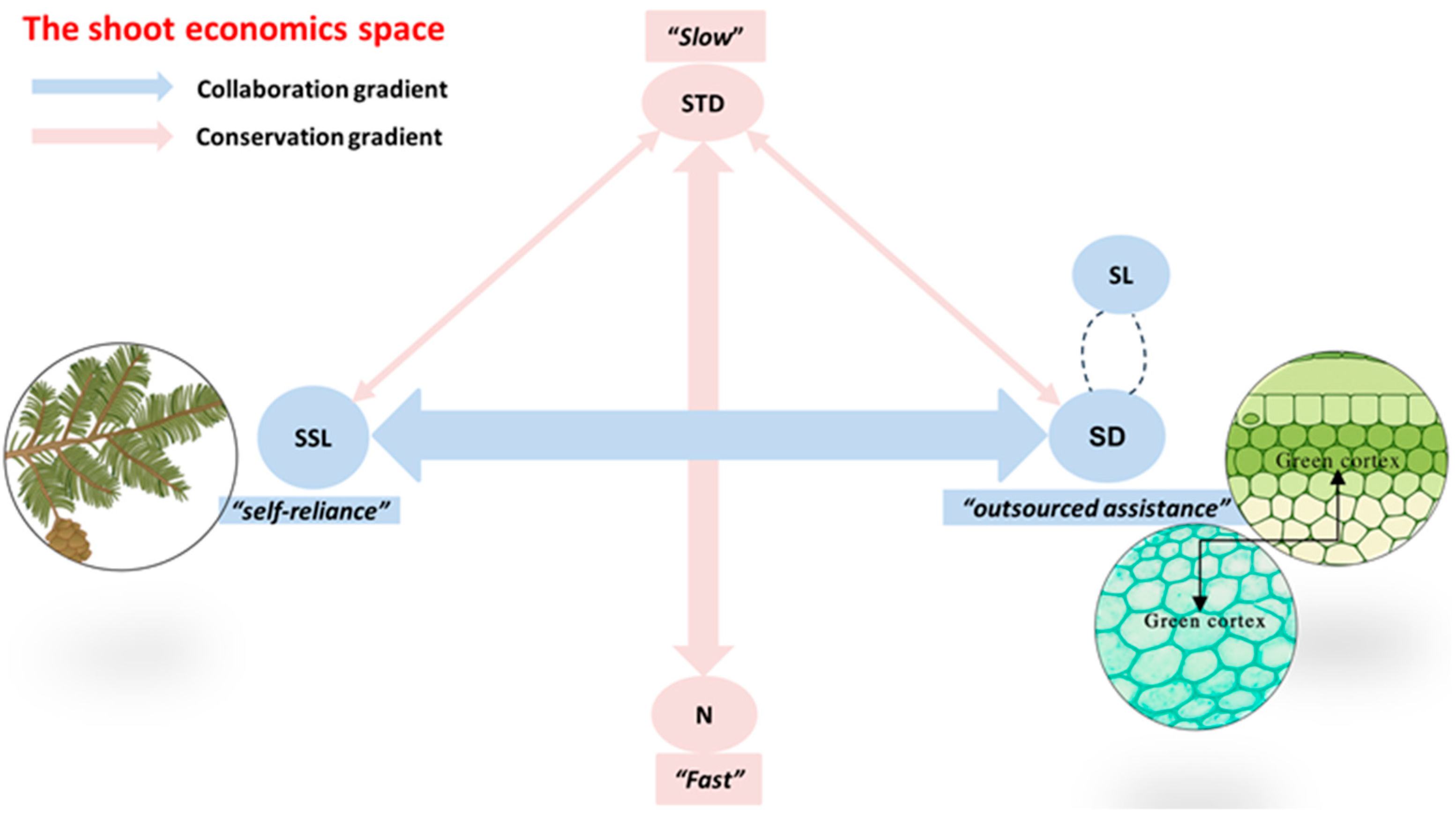
3.2. The Proposal of the Conceptual Framework of the Shoot Economics Space
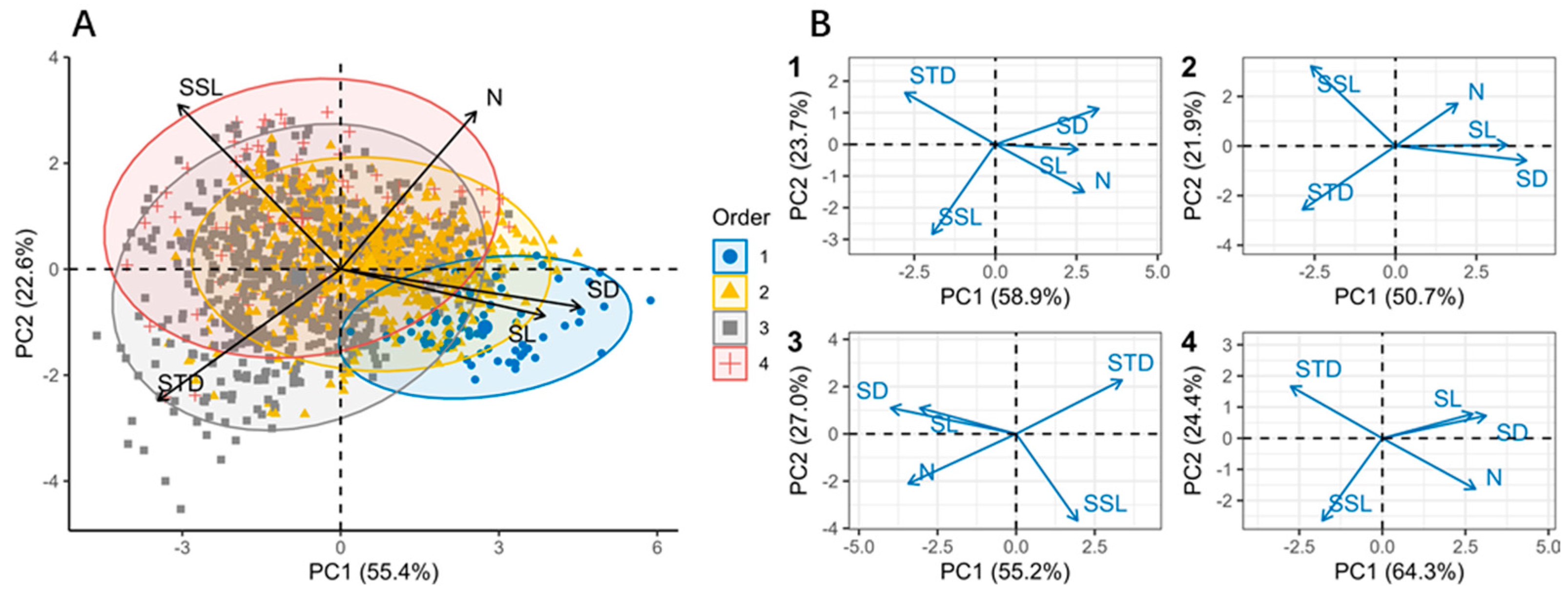
3.3. Multidimensional Coordination of Shoot Traits
4. Discussion
4.1. Correlations in Shoot Traits Across Different Branching Orders
4.2. The Trade-Off Between Structural and Nutrient Investments
4.3. The Coordination of Economic Spectra Across Different Branching Orders
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Lusk, C.H.; Bellingham, P.J.; Burslem, D.F.R.P.; Simpson, A.H.; Kramer-Walter, K.R. Intraspecific Trait Variation Can Weaken Interspecific Trait Correlations When Assessing the Whole-Plant Economic Spectrum. Ecol. Evol. 2017, 7, 8936–8949. [Google Scholar] [CrossRef]
- Osnas, J.L.D.; Katabuchi, M.; Kitajima, K.; Wright, S.J.; Reich, P.B.; Van Bael, S.A.; Kraft, N.J.B.; Samaniego, M.J.; Pacala, S.W.; Lichstein, J.W. Divergent Drivers of Leaf Trait Variation within Species, among Species, and among Functional Groups. Proc. Natl. Acad. Sci. USA 2018, 115, 5480–5485. [Google Scholar] [CrossRef]
- Freschet, G.T.; Bellingham, P.J.; Lyver, P.O.; Bonner, K.I.; Wardle, D.A. Plasticity in Above- and Belowground Resource Acquisition Traits in Response to Single and Multiple Environmental Factors in Three Tree Species. Ecol. Evol. 2013, 3, 1065–1078. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide ‘Fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Lusk, C.H.; Reich, P.B.; Montgomery, R.A.; Ackerly, D.D.; Cavender-Bares, J. Why Are Evergreen Leaves so Contrary about Shade? Trends Ecol. Evol. 2008, 23, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, L.D.L.; Berner, L.T.; Badgley, G.; Sethi, M.L.; Law, B.E.; HilleRisLambers, J. Within-Species Patterns Challenge Our Understanding of the Leaf Economics Spectrum. Ecol. Lett. 2018, 21, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mo, Y.-X.; Cui, H.-L.; Zhang, Y.-J.; Dossa, G.G.O.; Tan, Z.-H.; Song, L. Intraspecific Plasticity and Co-Variation of Leaf Traits Facilitate Ficus Tinctoria to Acclimate Hemiepiphytic and Terrestrial Habitats. Tree Physiol. 2024, 44, tpae007. [Google Scholar] [CrossRef]
- Martin, A.R.; Rapidel, B.; Roupsard, O.; Van den Meersche, K.; de Melo Virginio Filho, E.; Barrios, M.; Isaac, M.E. Intraspecific Trait Variation across Multiple Scales: The Leaf Economics Spectrum in Coffee. Funct. Ecol. 2017, 31, 604–612. [Google Scholar] [CrossRef]
- Rixen, C.; Wipf, S.; Rumpf, S.B.; Giejsztowt, J.; Millen, J.; Morgan, J.W.; Nicotra, A.B.; Venn, S.; Zong, S.; Dickinson, K.J.M.; et al. Intraspecific Trait Variation in Alpine Plants Relates to Their Elevational Distribution. J. Ecol. 2022, 110, 860–875. [Google Scholar] [CrossRef]
- Ren, L.; Guo, X.; Liu, S.; Yu, T.; Guo, W.; Wang, R.; Ye, S.; Lambertini, C.; Brix, H.; Eller, F. Intraspecific Variation in Phragmites Australis: Clinal Adaption of Functional Traits and Phenotypic Plasticity Vary with Latitude of Origin. J. Ecol. 2020, 108, 2531–2543. [Google Scholar] [CrossRef]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Martí, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The Plant Traits That Drive Ecosystems: Evidence from Three Continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Muraoka, H.; Valladares, F. Crown Architecture in Sun and Shade Environments: Assessing Function and Trade-Offs with a Three-Dimensional Simulation Model. New Phytol. 2005, 166, 791–800. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological Limits to Plant Phenotypic Plasticity. New Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. A Review of Light Interception in Plant Stands from Leaf to Canopy in Different Plant Functional Types and in Species with Varying Shade Tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Alternative Height Strategies among 45 Dicot Rain Forest Species from Tropical Queensland, Australia. J. Ecol. 2005, 93, 521–535. [Google Scholar] [CrossRef]
- Valladares, F.; Pearcy, R.W. The Functional Ecology of Shoot Architecture in Sun and Shade Plants of Heteromeles Arbutifolia M. Roem., a Californian Chaparral Shrub. Oecologia 1998, 114, 1–10. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornelissen, J.H.C.; Van Logtestijn, R.S.P.; Aerts, R. Evidence of the ‘Plant Economics Spectrum’ in a Subarctic Flora. J. Ecol. 2010, 98, 362–373. [Google Scholar] [CrossRef]
- Poorter, H.; Ryser, P. The Limits to Leaf and Root Plasticity: What Is so Special about Specific Root Length? New Phytol. 2015, 206, 1188–1190. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T.L. A Meta-Analysis of Plant Responses to Light Intensity for 70 Traits Ranging from Molecules to Whole Plant Performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B.J. Ecological and Evolutionary Determinants of a Key Plant Functional Trait: Wood Density and Its Community-Wide Variation across Latitude and Elevation. Am. J. Bot. 2007, 94, 451–459. [Google Scholar] [CrossRef]
- Niklas, K.J.; Spatz, H.-C. Worldwide Correlations of Mechanical Properties and Green Wood Density. Am. J. Bot. 2010, 97, 1587–1594. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Niklas, K.J.; Sun, J.; Wang, Z.; Zhong, Q.; Hu, D.; Cheng, D. A Whole-Plant Economics Spectrum Including Bark Functional Traits for 59 Subtropical Woody Plant Species. J. Ecol. 2022, 110, 248–261. [Google Scholar] [CrossRef]
- Tuo, B.; Yan, E.-R.; Guo, C.; Ci, H.; Berg, M.P.; Cornelissen, J.H.C. Influences of the Bark Economics Spectrum and Positive Termite Feedback on Bark and Xylem Decomposition. Ecology 2021, 102, e03480. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gilbert, G.S.; Li, W.; Fang, M.; Lu, H.; Yu, S. Linking Aboveground Traits to Root Traits and Local Environment: Implications of the Plant Economics Spectrum. Front. Plant Sci. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a Worldwide Wood Economics Spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Méndez-Alonzo, R.; Paz, H.; Zuluaga, R.C.; Rosell, J.A.; Olson, M.E. Coordinated Evolution of Leaf and Stem Economics in Tropical Dry Forest Trees. Ecology 2012, 93, 2397–2406. [Google Scholar] [CrossRef]
- Osada, N. Crown Development in a Pioneer Tree, Rhus Trichocarpa, in Relation to the Structure and Growth of Individual Branches. New Phytol. 2006, 172, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-B.; Slik, J.W.F.; Zhang, J.-L.; Cao, K.-F. Spatial Patterns of Wood Traits in China Are Controlled by Phylogeny and the Environment. Glob. Ecol. Biogeogr. 2011, 20, 241–250. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Jiang, Y.; Wang, B.; Jiao, L.; Wang, M. Seasonal Water Use by Larix Principis-Rupprechtii in an Alpine Habitat. For. Ecol. Manag. 2018, 409, 47–55. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.; Dong, M.; Du, E.; Wu, F.; Zhao, S.; Xu, H. Species-Specific Growth-Climate Responses of Dahurian Larch (Larix Gmelinii) and Mongolian Pine (Pinus Sylvestris Var. Mongolica) in the Greater Khingan Range, Northeast China. Dendrochronologia 2021, 65, 125803. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Peng, D.; Liu, X.; Yang, F.; Li, Z.; Cheng, S. Thinning Drives C:N:P Stoichiometry and Nutrient Resorption in Larix Principis-Rupprechtii Plantations in North China. For. Ecol. Manag. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Ji, X.; Zhang, Z.; Zhang, H.; Zha, T.; Jiang, L. Elevation and Total Nitrogen Are the Critical Factors That Control the Spatial Distribution of Soil Organic Carbon Content in the Shrubland on the Bashang Plateau, China. CATENA 2021, 204, 105415. [Google Scholar] [CrossRef]
- Suzuki, A.A.; Suzuki, M. Why Do Lower Order Branches Show Greater Shoot Growth than Higher Order Branches? Considering Space Availability as a Factor Affecting Shoot Growth. Trees 2009, 23, 69–77. [Google Scholar] [CrossRef]
- Suzuki, A. Resource Allocation to Vegetative Growth and Reproduction at Shoot Level in Eurya Japonica (Theaceae): A Hierarchical Investment? New Phytol. 2001, 152, 307–312. [Google Scholar] [CrossRef]
- Suzuki, A.A. Shoot Growth Patterns in Saplings of Cleyera Japonica in Relation to Light and Architectural Position. Tree Physiol. 2003, 23, 67–71. [Google Scholar] [CrossRef]
- Suzuki, A. Patterns of Vegetative Growth and Reproduction in Relation to Branch Orders: The Plant as a Spatially Structured Population. Trees 2000, 14, 329–333. [Google Scholar] [CrossRef]
- Witzell, J.; Decker, V.H.G.; Agostinelli, M.; Romeralo, C.; Cleary, M.; Albrectsen, B.R. Aspen Leaves as a “Chemical Landscape” for Fungal Endophyte Diversity—Effects of Nitrogen Addition. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass Allocation to Leaves, Stems and Roots: Meta-Analyses of Interspecific Variation and Environmental Control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cabrera, H.I.; Jones, C.S.; Espino, S.; Schenk, H.J. Wood Anatomy and Wood Density in Shrubs: Responses to Varying Aridity along Transcontinental Transects. Am. J. Bot. 2009, 96, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Deane, D.C. The Relationship between Trunk- and Twigwood Density Shifts with Tree Size and Species Stature. For. Ecol. Manag. 2016, 372, 137–142. [Google Scholar] [CrossRef]
- Poorter, L.; McNeil, A.; Hurtado, V.-H.; Prins, H.H.T.; Putz, F.E. Bark Traits and Life-History Strategies of Tropical Dry- and Moist Forest Trees. Funct. Ecol. 2014, 28, 232–242. [Google Scholar] [CrossRef]
- Baraloto, C.; Timothy Paine, C.E.; Poorter, L.; Beauchene, J.; Bonal, D.; Domenach, A.-M.; Hérault, B.; Patiño, S.; Roggy, J.-C.; Chave, J. Decoupled Leaf and Stem Economics in Rain Forest Trees. Ecol. Lett. 2010, 13, 1338–1347. [Google Scholar] [CrossRef]
- Olson, M.; Rosell, J.A.; Martínez-Pérez, C.; León-Gómez, C.; Fajardo, A.; Isnard, S.; Cervantes-Alcayde, M.A.; Echeverría, A.; Figueroa-Abundiz, V.A.; Segovia-Rivas, A.; et al. Xylem Vessel-Diameter–Shoot-Length Scaling: Ecological Significance of Porosity Types and Other Traits. Ecol. Monogr. 2020, 90, e01410. [Google Scholar] [CrossRef]
- Hacke, U.G.; Stiller, V.; Sperry, J.S.; Pittermann, J.; McCulloh, K.A. Cavitation Fatigue. Embolism and Refilling Cycles Can Weaken the Cavitation Resistance of Xylem1. Plant Physiol. 2001, 125, 779–786. [Google Scholar] [CrossRef]
- Fortunel, C.; Fine, P.V.A.; Baraloto, C. Leaf, Stem and Root Tissue Strategies across 758 Neotropical Tree Species. Funct. Ecol. 2012, 26, 1153–1161. [Google Scholar] [CrossRef]
- Cardou, F.; Munson, A.D.; Boisvert-Marsh, L.; Anand, M.; Arsenault, A.; Bell, F.W.; Bergeron, Y.; Boulangeat, I.; Delagrange, S.; Fenton, N.J.; et al. Above- and Belowground Drivers of Intraspecific Trait Variability across Subcontinental Gradients for Five Ubiquitous Forest Plants in North America. J. Ecol. 2022, 110, 1590–1605. [Google Scholar] [CrossRef]
- Pierick, K.; Leuschner, C.; Link, R.M.; Báez, S.; Velescu, A.; Wilcke, W.; Homeier, J. Above- and Belowground Strategies of Tropical Montane Tree Species Are Coordinated and Driven by Small-Scale Nitrogen Availability. Funct. Ecol. 2024, 38, 1364–1377. [Google Scholar] [CrossRef]
- Hummel, I.; Vile, D.; Violle, C.; Devaux, J.; Ricci, B.; Blanchard, A.; Garnier, É.; Roumet, C. Relating Root Structure and Anatomy to Whole-Plant Functioning in 14 Herbaceous Mediterranean Species. New Phytol. 2007, 173, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading Dimensions in Absorptive Root Trait Variation across 96 Subtropical Forest Species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of Root Trait Relationships and the Root Economics Spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef]
- Goto, T.; Osada, N. Geographic Variation in Shoot Structure in Association with Fruit Size in an Evergreen Woody Species. AoB PLANTS 2021, 13, plab023. [Google Scholar] [CrossRef]
- Yao, F.; Chen, Y.; Yan, Z.; Li, P.; Han, W.; Fang, J. Biogeographic Patterns of Structural Traits and C:N:P Stoichiometry of Tree Twigs in China’s Forests. PLoS ONE 2015, 10, e0116391. [Google Scholar] [CrossRef]
- Osada, N. Height-Dependent Changes in Shoot Structure and Tree Allometry in Relation to Maximum Height in Four Deciduous Tree Species. Funct. Ecol. 2011, 25, 777–786. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Zhang, H.; Wang, Z.; Liu, Z. The Cortical Chlorenchyma Collaboration Gradient Dominates the Shoot Economics Space in Larix principis-rupprechtii. Life 2025, 15, 1310. https://doi.org/10.3390/life15081310
Yu Y, Zhang H, Wang Z, Liu Z. The Cortical Chlorenchyma Collaboration Gradient Dominates the Shoot Economics Space in Larix principis-rupprechtii. Life. 2025; 15(8):1310. https://doi.org/10.3390/life15081310
Chicago/Turabian StyleYu, Yang, Huayong Zhang, Zhongyu Wang, and Zhao Liu. 2025. "The Cortical Chlorenchyma Collaboration Gradient Dominates the Shoot Economics Space in Larix principis-rupprechtii" Life 15, no. 8: 1310. https://doi.org/10.3390/life15081310
APA StyleYu, Y., Zhang, H., Wang, Z., & Liu, Z. (2025). The Cortical Chlorenchyma Collaboration Gradient Dominates the Shoot Economics Space in Larix principis-rupprechtii. Life, 15(8), 1310. https://doi.org/10.3390/life15081310






