The Functional Ingredients of the Combined Extract of Mulberry Leaves and Butterfly Pea Flowers Improve Insomnia, Anxiolytic, Memory-Enhancing, and Antidepressant-like Activities in Stress-Exposed Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Functional Ingredients of the Combined Extract of Mulberry Leaves and Butterfly Pea Flowers
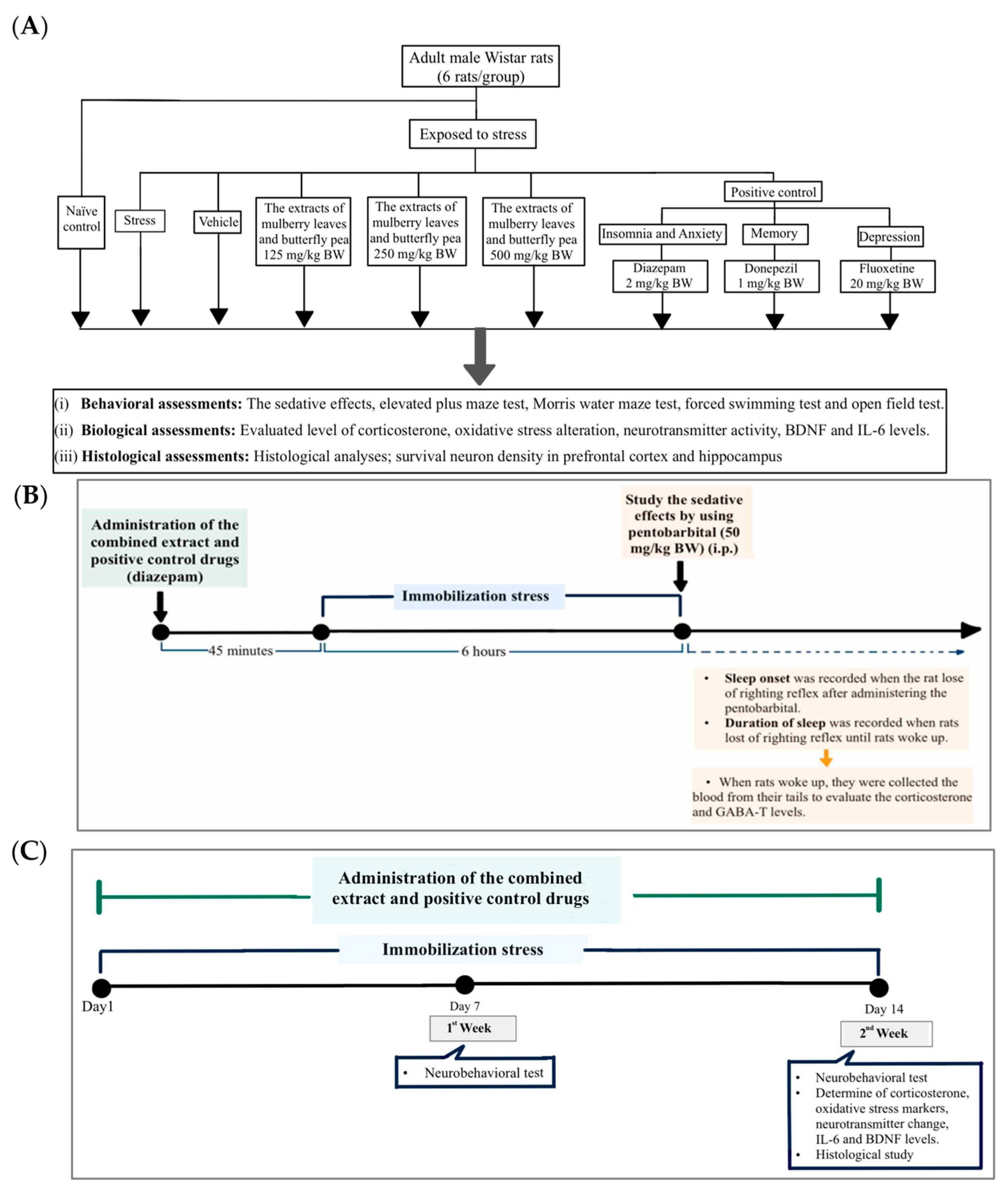
2.2. Experimental Animals and Protocol
2.3. Behavioral Assessments
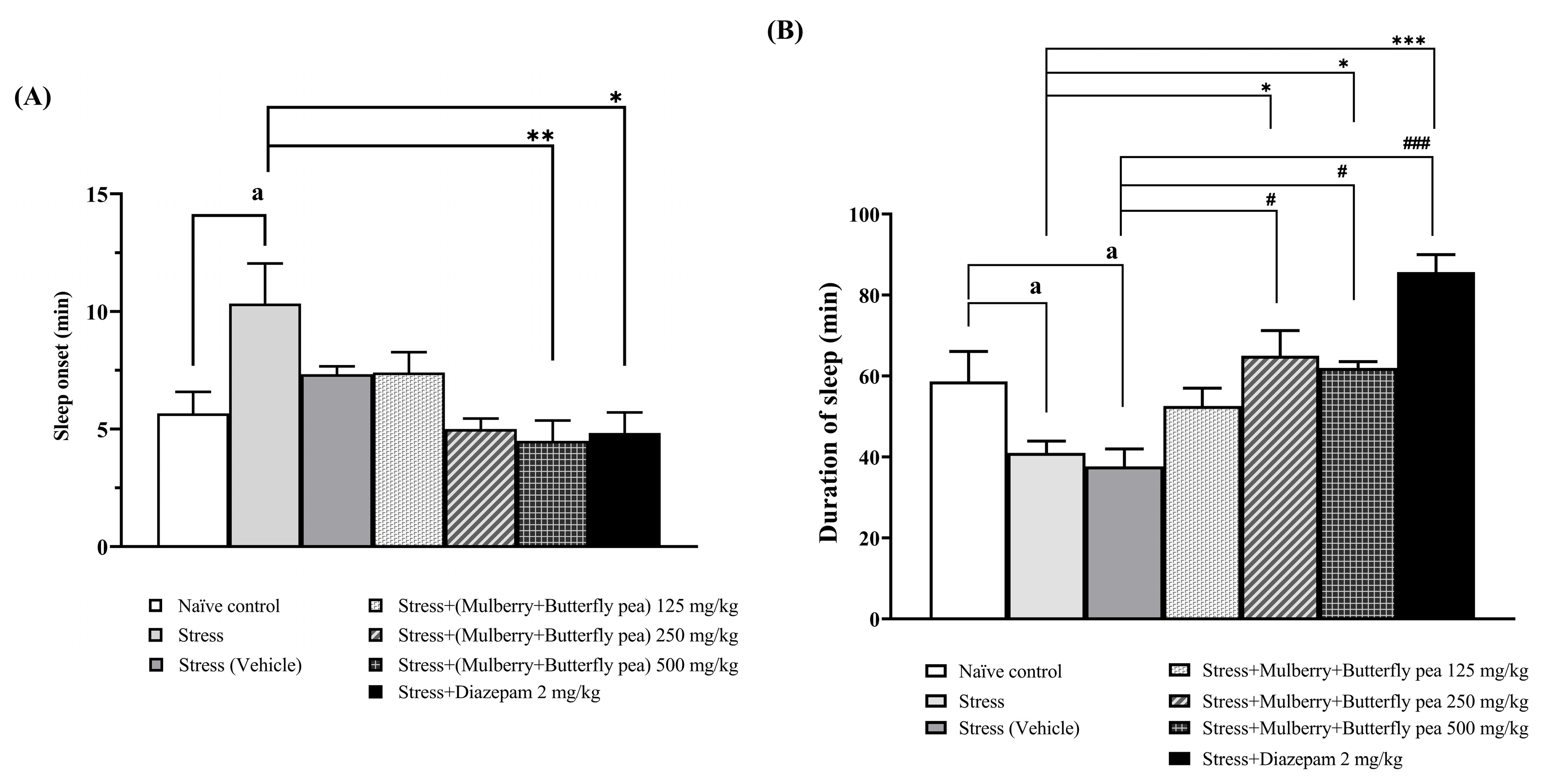
2.3.1. Sleep Assessment
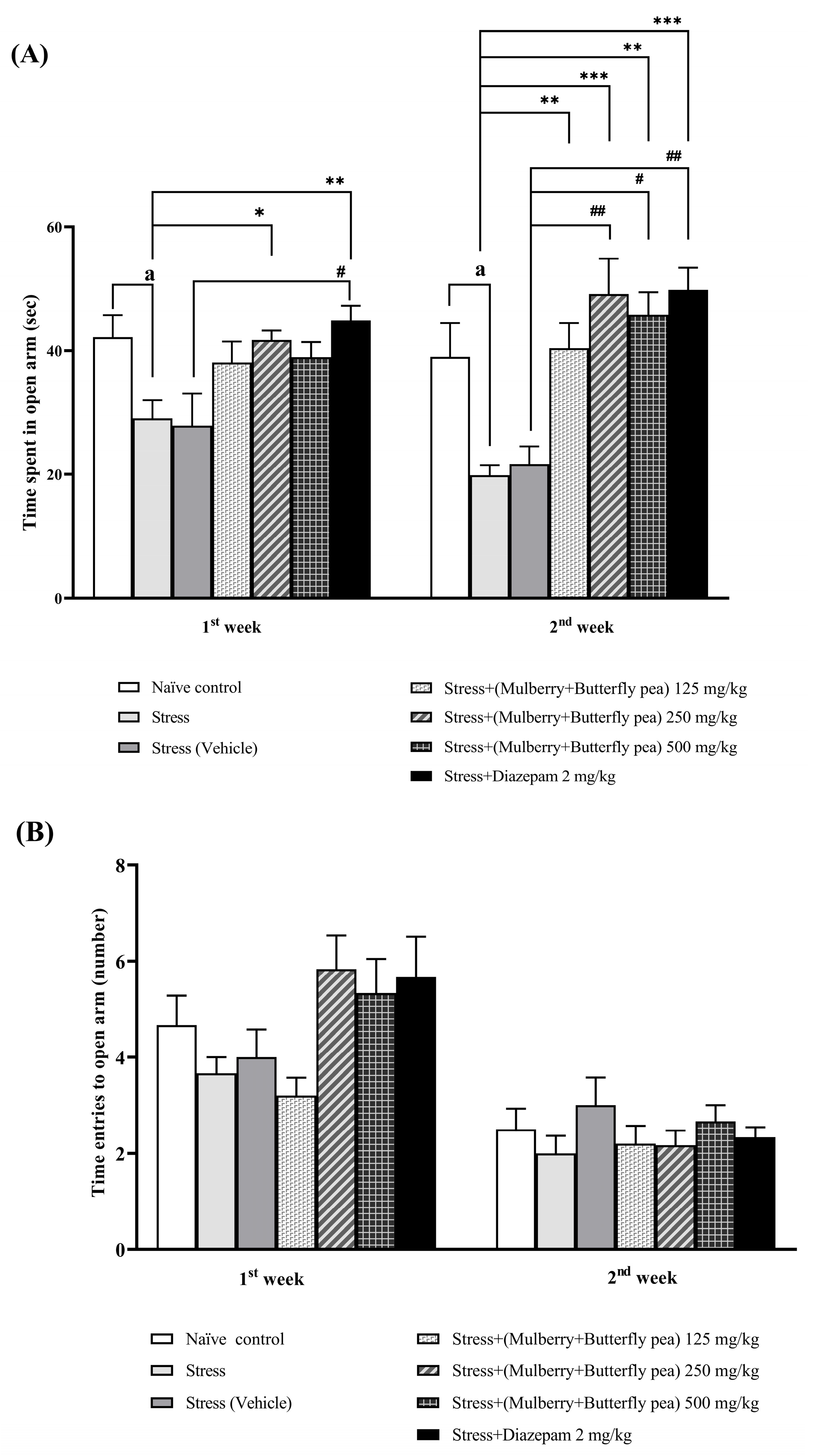
2.3.2. Elevated Plus Maze Test (EPM)
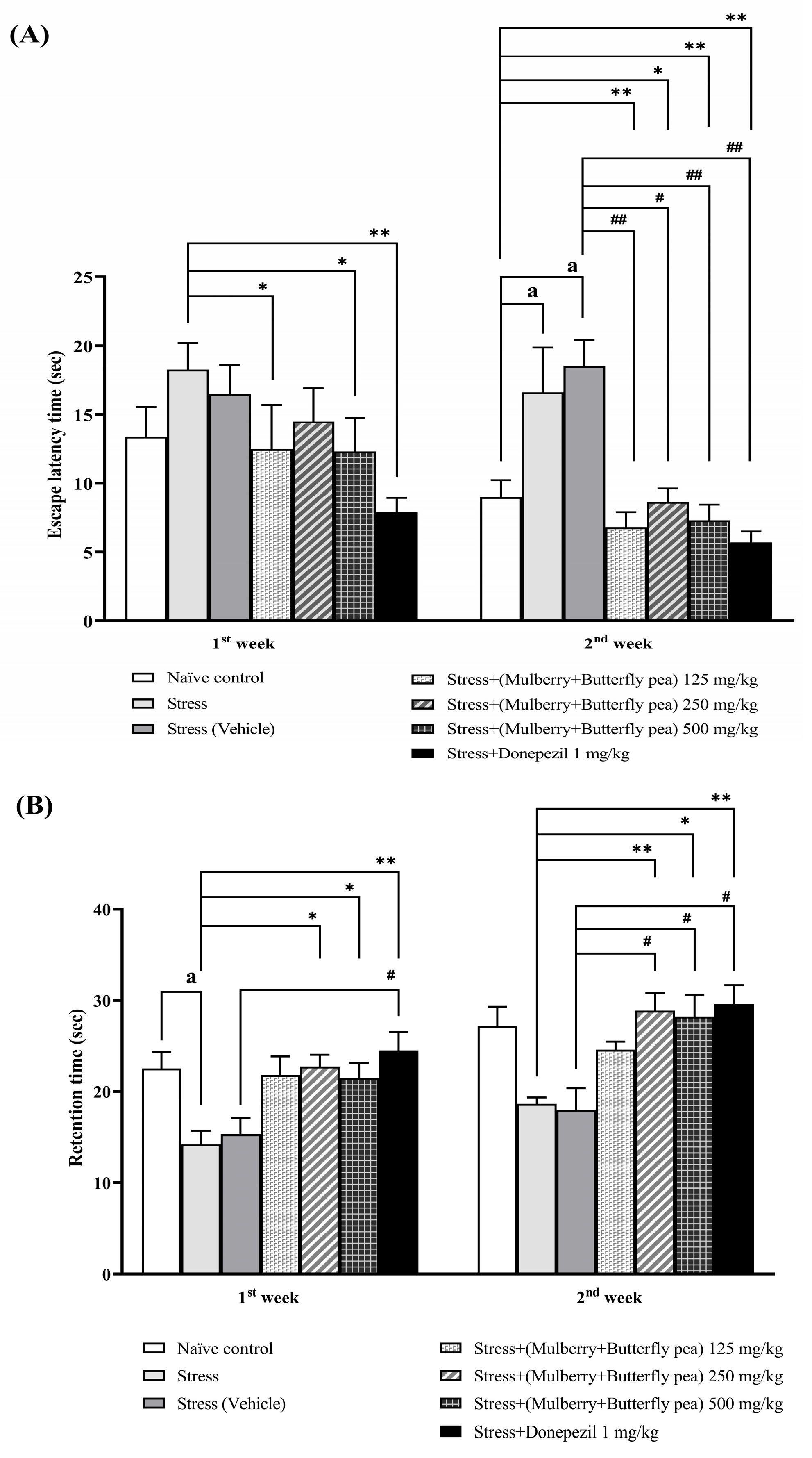
2.3.3. Morris Water Maze Test (MWM)
2.3.4. Forced Swimming Test (FST)
2.3.5. Open Field Test (OFT)
2.4. Biochemical Assessments
2.4.1. Measurement of Oxidative Stress Markers
2.4.2. Measurement of Neurotransmitter Enzyme Changes
2.4.3. Measurement of Corticosterone
2.4.4. Assessment of Inflammatory Marker and Neuronal Marker
2.5. Histological Procedure and Nissl Staining
2.6. Statistical Analysis
3. Results
3.1. Behavioral Assessment
3.1.1. The Sedative Effect
3.1.2. The Anxiolytic Effect
3.1.3. The Memory Enhancing Effect
3.1.4. The Antidepressant Effect
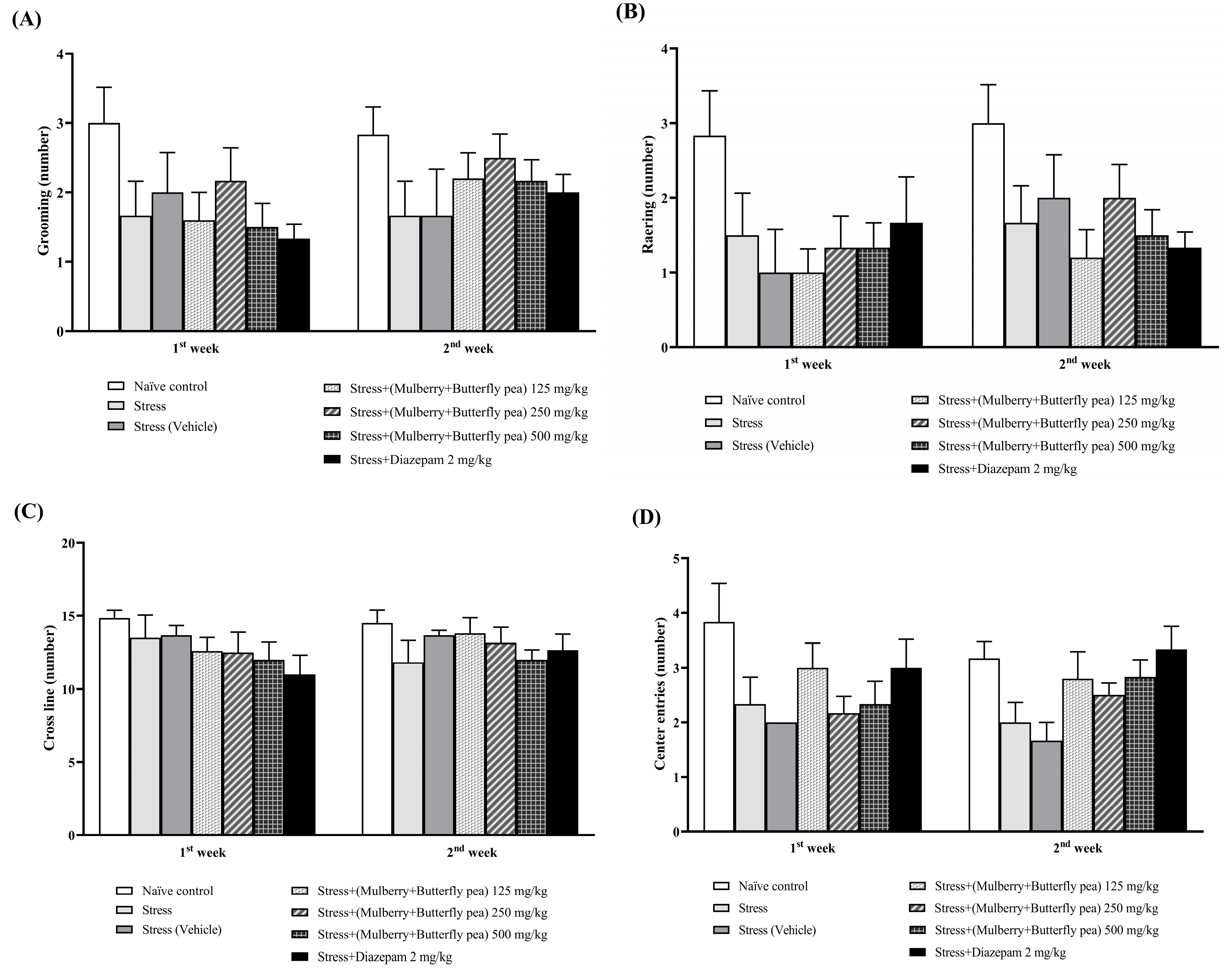
3.1.5. Locomotor Activity
3.2. Changes in Oxidative Stress Markers
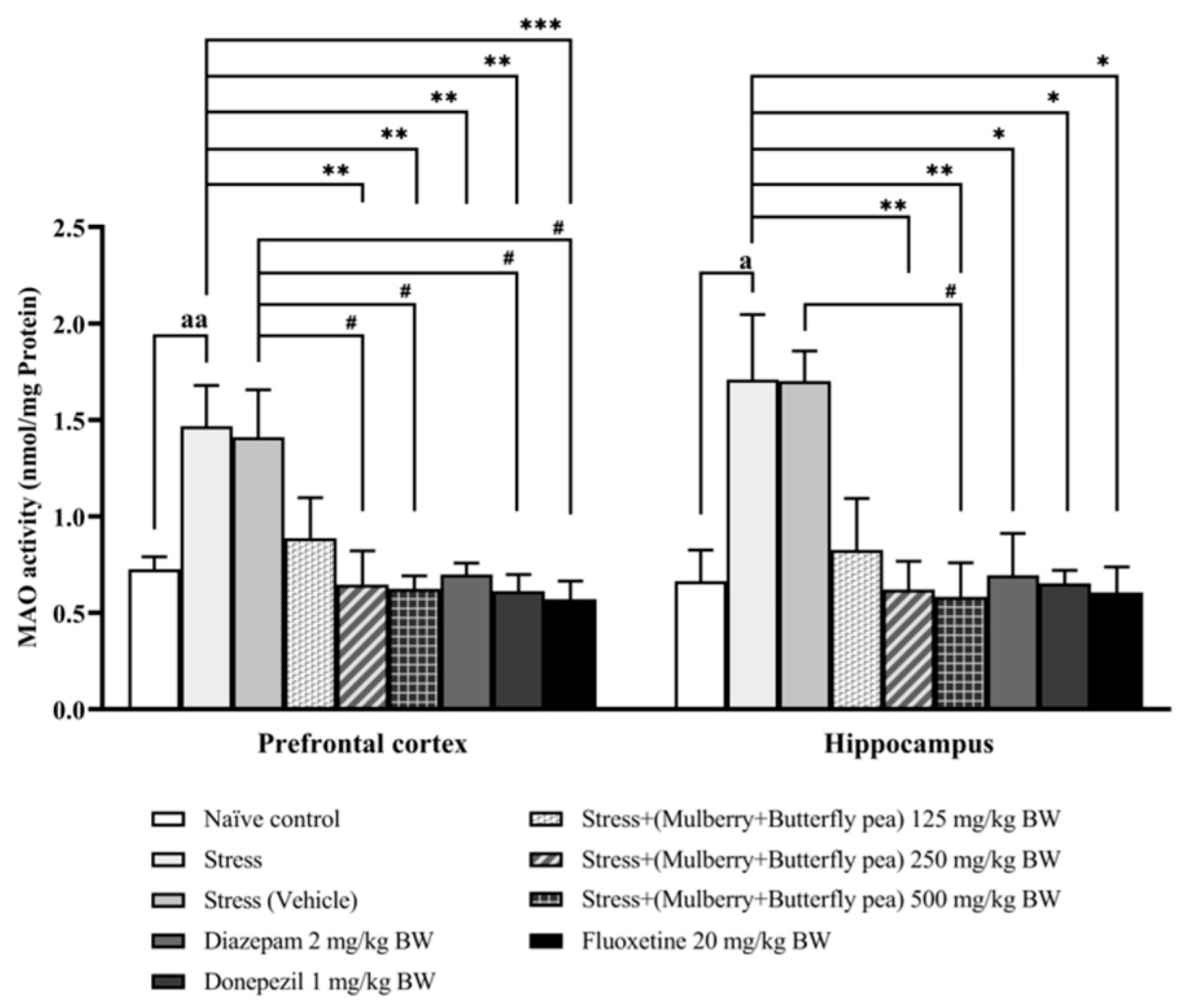
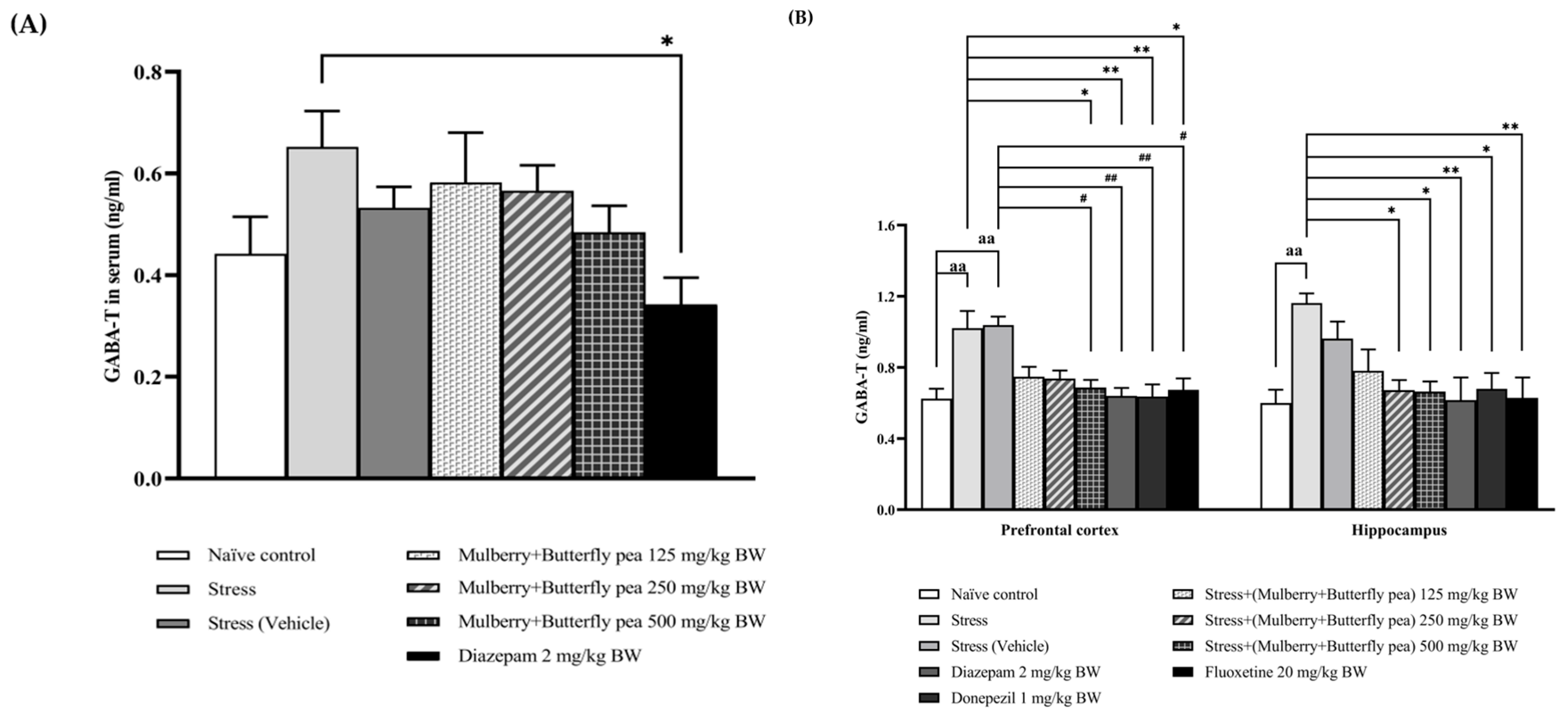
3.3. Neurotransmitters Enzyme Changes
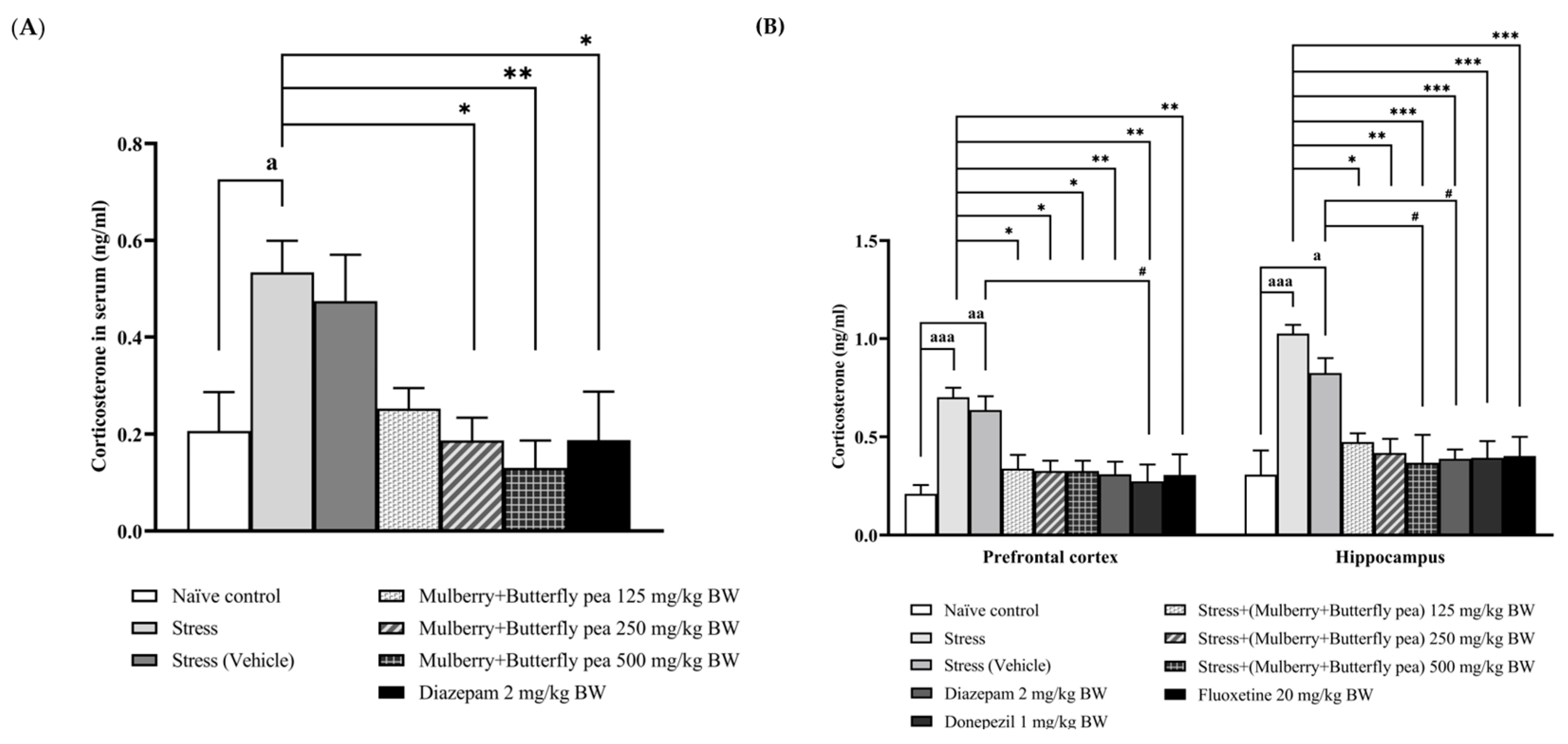
3.4. Hormonal Essay
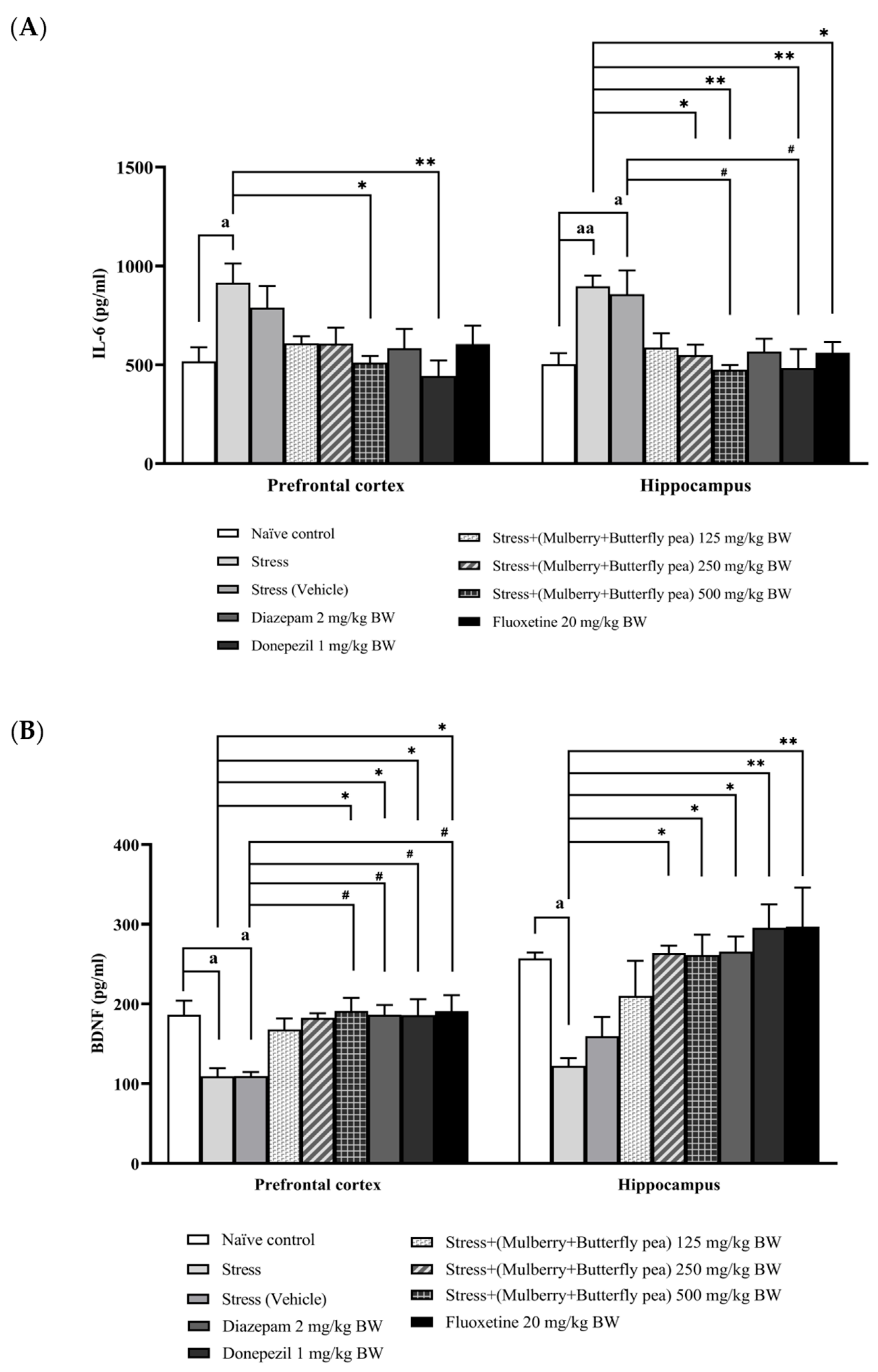
3.5. Assessment of Inflammatory Marker and Neuronal Biomarker
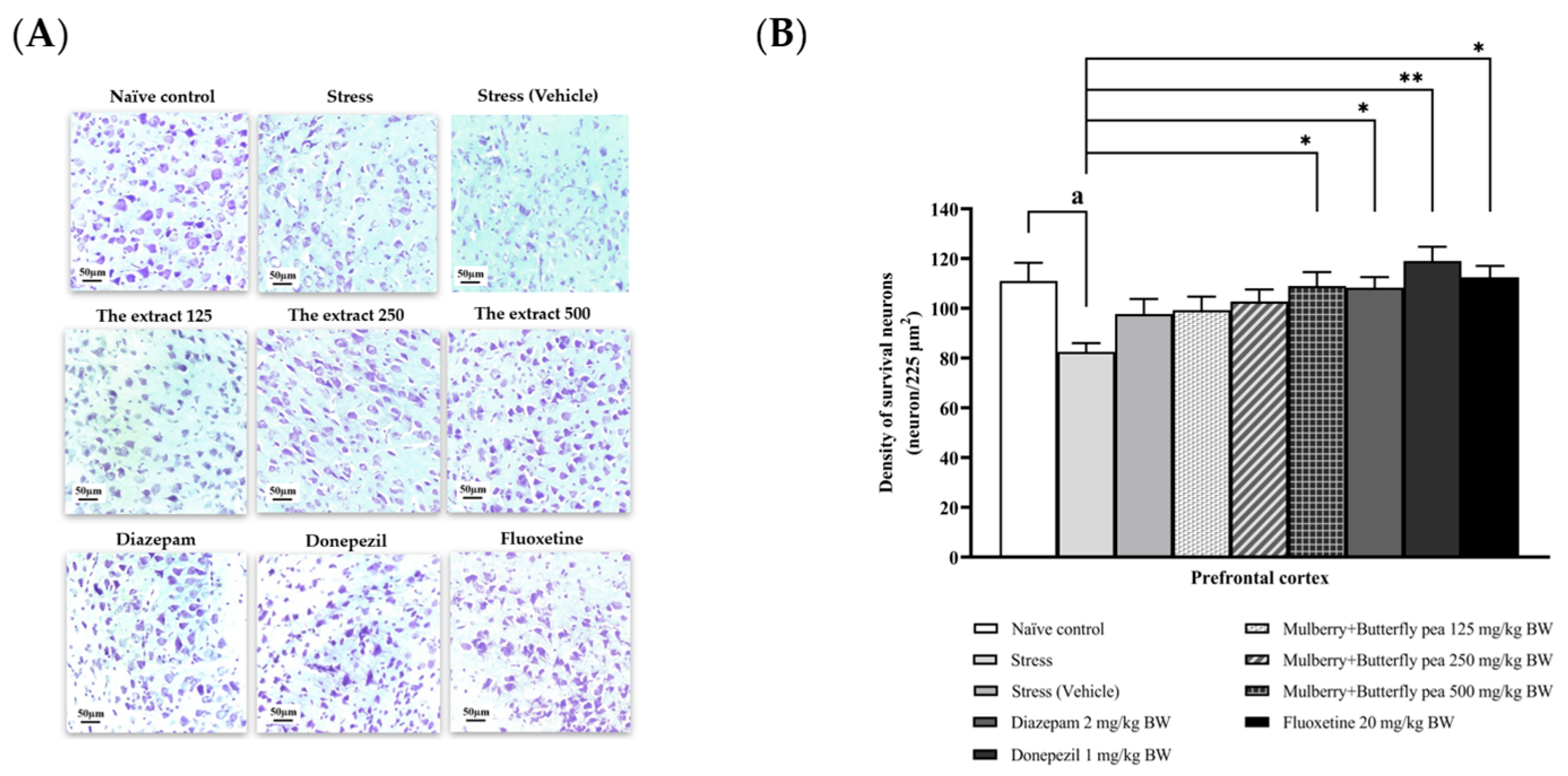
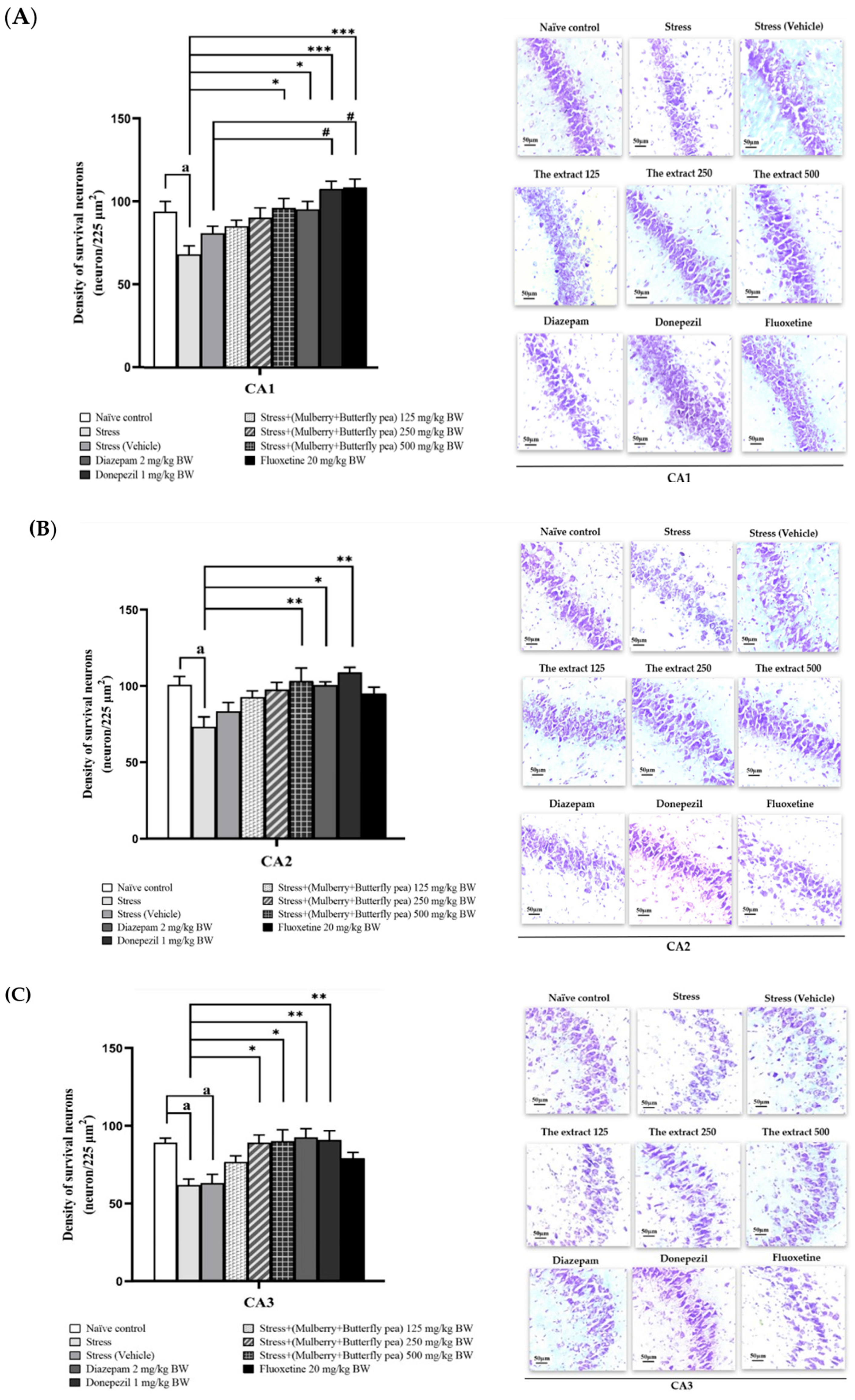
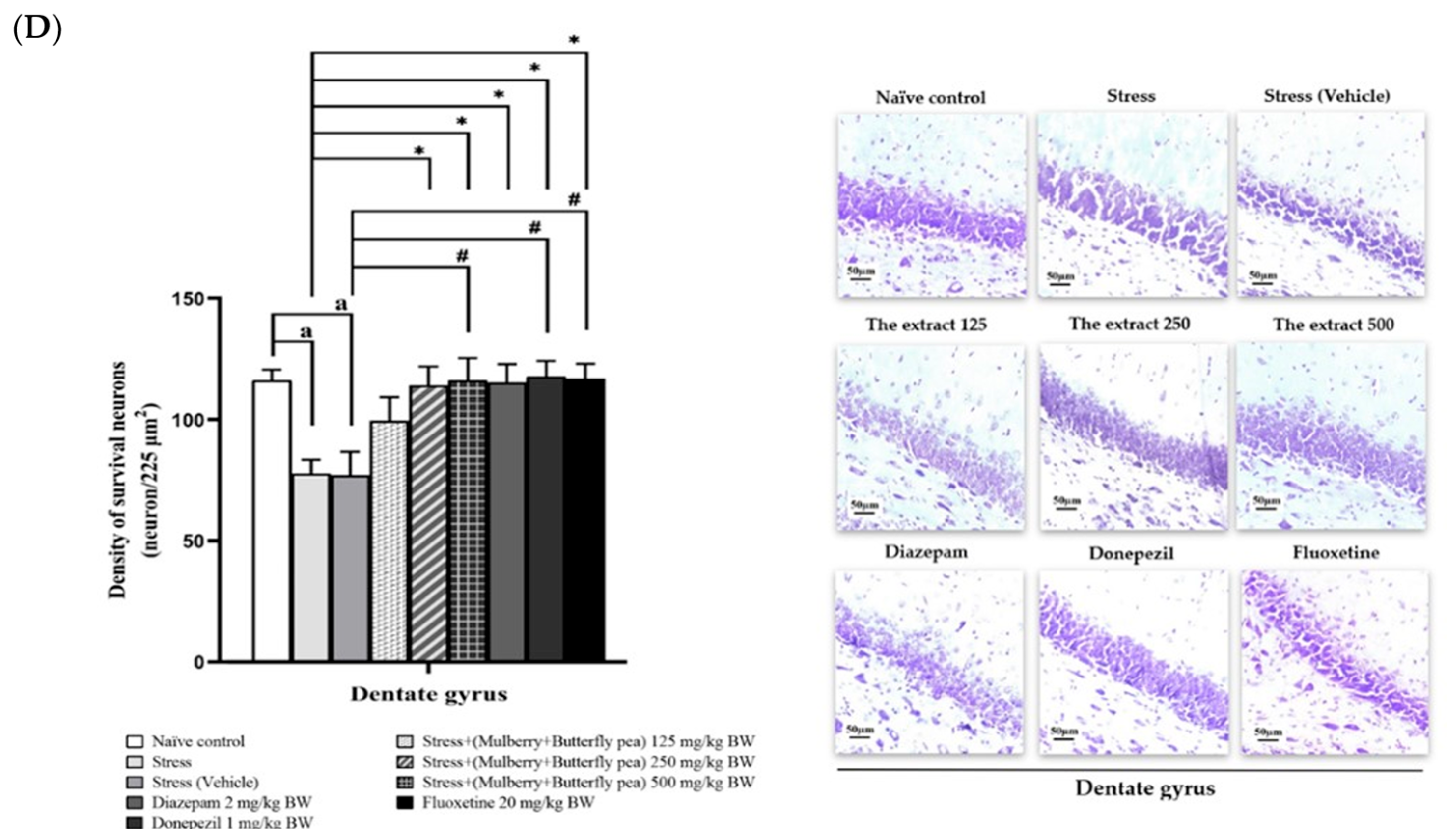
3.6. Histological Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrari, A.J.; Somerville, A.J.; Baxter, A.J.; Norman, R.; Patten, S.B.; Vos, T.; Whiteford, H.A. Global variation in the prevalence and incidence of major depressive disorder: A systematic review of the epidemiological literature. Psychol. Med. 2013, 43, 471–481. [Google Scholar] [CrossRef]
- The Lancet Global Health. Mental health matters. Lancet Glob. Health 2020, 8, e1352. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; 24p, Available online: https://www.who.int/publications/i/item/depression-global-health-estimates (accessed on 25 November 2024).
- World Health Organization. World Failing to Address Dementia Challenge; World Health Organization: Geneva, Switzerland, 2021; 30p, Available online: https://www.who.int/news/item/02-09-2021-world-failing-to-address-dementia-challenge (accessed on 25 November 2024).
- McEwen, B.S.; Gianaros, P.J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Carlson, N.G.; Wieggel, W.A.; Chen, J.; Bacchi, A.; Rogers, S.W.; Gahring, L.C. Inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin through distinct pathways. J. Immunol. 1999, 163, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Abolhassani, N.; Leon, J.; Sheng, Z.; Oka, S.; Hamasaki, H.; Iwaki, T.; Nakabeppu, Y. Molecular pathophysiology of impaired glucose metabolism, mitochondrial dysfunction, and oxidative DNA damage in Alzheimer’s disease brain. Mech. Ageing Dev. 2017, 161, 95–104. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Telean, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; Grosso, G.; et al. Polyphenols and Neuroprotection: Therapeutic Implications for Cognitive Decline. Pharmacol. Ther. 2021, 232, 108013. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G. Dietary Antioxidants and Brain Health: Focus on Cognitive and Affective Disorders. Antioxidants 2021, 10, 1659. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Wesolowski, M.; Viapiana, A. Morus alba L. and Morus nigra L. Leaves as a Promising Food Source of Phenolic Compounds with Antioxidant Activity. Plant. Foods. Hum. Nutr. 2021, 76, 458–465. [Google Scholar] [CrossRef]
- Kaewkaen, P.; Tong-Un, T.; Wattanathorn, J.; Muchimapura, S.; Kaewrueng, W.; Wongcharoenwanakit, S. Mulberry Fruit Extract Protects against Memory Impairment and Hippocampal Damage in Animal Model of Vascular Dementia. Evid.-Based Complement. Altern. Med. 2012, 2012, 263520. [Google Scholar] [CrossRef] [PubMed]
- Wattanathorn, J.; Phunchago, N.; Muchimapura, S.; Thukhum-Mee, W.; Chaisiwamongkol, K.; Kaewrueng, W.; Wongareonwanakij, S. Mulberry fruit mitigates alcohol neurotoxicity and memory impairment induced by chronic alcohol intake. Am. J. Appl. Sci. 2012, 9, 484–491. [Google Scholar] [CrossRef]
- Yadav, A.V.; Kawale, L.A.; Nade, V.S. Effect of Morus alba L. (mulberry) leaves on anxiety in mice. Indian. J. Pharmacol. 2008, 40, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Aditya Rao, S.; Ramesh, C.; Kuppast, I.J.; Mahmood, R.; Prabhakar, B. CNS Depressant activity in two species of Mulberry. J. Pharm. Res. 2012, 5, 4879–4880. [Google Scholar]
- Ahmed, N.; Tabassum, N.; Rashid, P.T.; Deea, B.J.; Richi, F.T.; Chandra, A.; Agarwal, S.; Mollick, S.; Dipto, K.Z.; Mim, S.A.; et al. Clitoria ternatea L. (Butterfly Pea) Flower Against Endometrial Pain: Integrating Preliminary In Vivo and In Vitro Experimentations Supported by Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation Studies. Life 2024, 14, 1473. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Anthocyanins From Clitoria ternatea Flower: Biosynthesis, Extraction, Stability, Antioxidant Activity, and Applications. Front. Plant Sci. 2020, 12, 792303. [Google Scholar] [CrossRef]
- Jain, N.N.; Ohal, C.C.; Shroff, S.K.; Bhutada, R.H.; Somani, R.S.; Kasture, V.S.; Kasture, S.B. Clitoria ternatea and the CNS. Pharmacol. Biochem. Behav. 2003, 75, 529–536. [Google Scholar] [CrossRef]
- Choi, J.J.; Oh, E.H.; Lee, M.K.; Chung, Y.B.; Hong, J.T.; Oh, K.W. Gastrodiae Rhizoma Ethanol Extract Enhances Pentobarbital-Induced Sleeping Behaviors and Rapid Eye Movement Sleep via the Activation of GABA A-ergic Transmission in Rodents. Evid. Based Complement. Alternat. Med. 2014, 2014, 426843. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Nakyam, T.; Wattanathorn, J.; Thukham-Mee, W.; Muchimapura, S. The Polyherbal Functional Ingredient Containing Ginger, Chinese Date, and Wood Ear Mushroom Protects against Dementia following Metabolic Syndrome. Biomed. Res. Int. 2023, 2023, 9911397. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Thongwong, P.; Wattanathorn, J.; Thukhammee, W.; Tiamkao, S. The potential role of the novel orodispersible film from rice polymer loaded with silkworm pupae hydrolysate and the combined extract of holy basil and ginger for the management of stroke with stress. Biomaterials 2023, 299, 122175. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Kirisattayakul, W.; Suriharn, B.; Lertrat, K. Functional Drink Containing the Extracts of Purple Corn Cob and Pandan Leaves, the Novel Cognitive Enhancer, Increases Spatial Memory and Hippocampal Neuron Density Through the Improvement of Extracellular Signal Regulated Protein Kinase Expression, Cholinergic Function, and Oxidative Status in Ovariectomized Rats. Rejuvenation Res. 2018, 21, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.; Sharman, D.F.; Baker, G.B.; Palcic, M.M. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal. Biochem. 1997, 244, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: San Diego, CA, USA, 2008; pp. 443–493. [Google Scholar]
- Joëls, M.; Baram, T.Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W. Antimetabolic Syndrome Effect of Phytosome Containing the Combined Extracts of Mulberry and Ginger in an Animal Model of Metabolic Syndrome. Oxid. Med. Cell. Longev. 2019, 2019, 5972575. [Google Scholar] [CrossRef]
- Mehla, J.; Pahuja, M.; Gupta, P.; Dethe, S.; Agarwal, A.; Gupta, Y.K. Clitoria ternatea ameliorated the intracerebroventricularly injected streptozotocin induced cognitive impairment in rats: Behavioral and biochemical evidence. Psychopharmacology 2013, 230, 589–605. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Schiavone, S.; Jaquet, V.; Trabace, L.; Krause, K.H. Severe life stress and oxidative stress in the brain: From animal models to human pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Harahap, N.S.; Lelo, A.; Purba, A.; Sibuea, A.; Amelia, R.; Zulaini, Z. The effect of red-fleshed pitaya (Hylocereus polyrhizus) on heat shock protein 70 and cortisol expression in strenuous exercise induced rats. F1000Research 2019, 8, 130. [Google Scholar] [CrossRef]
- Ohno, S.; Shinoda, S.; Toyoshima, S.; Nakazawa, H.; Makino, T.; Nakajin, S. Effects of flavonoid phytochemicals on cortisol production and on activities of steroidogenic enzymes in human adrenocortical H295R cells. J. Steroid Biochem. Mol. Biol. 2002, 80, 355–363. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.A.; Murphy, K.M.; Albano, D.L.; Ceballos, R.M. Stress, cortisol, and B lymphocytes: A novel approach to understanding academic stress and immune function. Stress 2016, 19, 185–191. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- Abbas, Z.; Tong, Y.; Wang, J.; Zhang, J.; Wei, X.; Si, D.; Zhang, R. Potential Role and Mechanism of Mulberry Extract in Immune Modulation: Focus on Chemical Compositions, Mechanistic Insights, and Extraction Techniques. Int. J. Mol. Sci. 2024, 25, 5333. [Google Scholar] [CrossRef]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Zhou, G.; Xiong, W.; Zhang, X.; Ge, S. Retrieval of Consolidated Spatial Memory in the Water Maze Is Correlated with Expression of pCREB and Egr1 in the Hippocampus of Aged Mice. Dement. Geriatr. Cogn. Disord. Extra 2013, 3, 39–47. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Nyberg, L.; McIntosh, A.R.; Cabeza, R.; Habib, R.; Houle, S.; Tulving, E. General and specific brain regions involved in encoding and retrieval of events: What, where, and when. Proc. Natl. Acad. Sci. USA 1996, 93, 11280–11285. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Radley, J.J.; Rocher, A.B.; Rodriguez, A.; Ehlenberger, D.B.; Dammann, M.; McEwen, B.S.; Morrison, J.H.; Wearne, S.L.; Hof, P.R. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 2008, 507, 1141–1150. [Google Scholar] [CrossRef]
- McEwen, B.S.; Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- Varinthra, P.; Anwar, S.N.M.N.; Shih, S.C.; Liu, I.Y. The role of the GABAergic system on insomnia. Tzu Chi Med. J. 2024, 36, 103–109. [Google Scholar] [CrossRef]
- Kondziella, D. The Top 5 Neurotransmitters from a Clinical Neurologist’s Perspective. Neurochem. Res. 2017, 42, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hille, B.; Koh, D.S. Serotonin modulates melatonin synthesis as an autocrine neurotransmitter in the pineal gland. Proc. Natl. Acad. Sci. USA 2021, 118, e2113852118. [Google Scholar] [CrossRef] [PubMed]
- Rios, R.L.; Kafashan, M.; Hyche, O.; Lenard, E.; Lucey, B.P.; Lenze, E.J.; Palanca, B.J.A. Targeting Slow Wave Sleep Deficiency in Late-Life Depression: A Case Series with Propofol. Am. J. Geriatr. Psychiatry 2023, 31, 643–652. [Google Scholar] [CrossRef]
- Goldschmied, J.R.; Cheng, P.; Hoffmann, R.; Boland, E.M.; Deldin, P.J.; Armitage, R. Effects of slow-wave activity on mood disturbance in major depressive disorder. Psychol. Med. 2019, 49, 639–645. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Muskała, M.; Merecz-Sadowska, A.; Sikora, J.; Picot, L.; Sitarek, P. Anti-Inflammatory and Anticancer Effects of Anthocyanins in In Vitro and In Vivo Studies. Antioxidants 2024, 13, 1143. [Google Scholar] [CrossRef]
- Shan, X.; Chen, J.; Dai, S.; Wang, J.; Huang, Z.; Lv, Z.; Wang, Q.; Wu, Q. Cyanidin-related antidepressant-like efficacy requires PI3K/AKT/FoxG1/FGF-2 pathway modulated enhancement of neuronal differentiation and dendritic maturation. Phytomedicine 2020, 76, 153269. [Google Scholar] [CrossRef]
- Inan, S.; Meissler, J.J.; Shekarabi, A.; Foss, J.; Wiah, S.; Eisenstein, T.K.; Rawls, S.M. Cyanidin prevents MDPV withdrawal-induced anxiety-like effects and dysregulation of cytokine systems in rats. Brain Res. 2023, 1806, 148310. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef] [PubMed]


| Treatment Group | Experimental Details |
|---|---|
| Group I Naïve control | Rats not receiving any treatment |
| Group II Stress | Rats were immobilized for 6 h to stress, and they were not given any treatment. |
| Group III Stress + Vehicle | Rats were immobilized for 6 h to stress after they were given distilled water. |
| Group IV Stress + the combined extract of mulberry leaves and butterfly pea flowers 125 mg/kg BW | Rats were immobilized for 6 h to stress after they were given the combined extract at a dose of 125 mg/kg BW. |
| Group V Stress + the combined extract of mulberry leaves and butterfly pea flowers 250 mg/kg BW | Rats were immobilized for 6 h to stress after they were given the combined extract at a dose of 250 mg/kg BW. |
| Group VI Stress + combined extract of mulberry leaves and butterfly pea flowers 500 mg/kg BW | Rats were immobilized for 6 h to stress after they were given the combined extract at a dose of 500 mg/kg BW. |
| Group VII-IX Stress+ Positive control | Rats were immobilized for 6 h to stress after they were given diazepam (2 mg/kg BW) (GPO®, Bangkok, Thailand) to assess its ability to reduce sedative effect and anxiety, while donepezil (1 kg/kg BW) (Tonizep®, Bangkok, Thailand) and fluoxetine (20 mg/kg BW) (FULOX®, Bangkok, Thailand) were also tested for their effects. |
| Groups | Prefrontal Cortex | ||
|---|---|---|---|
| Malondialdehyde Level (nmol/mg Protein) | Superoxide Dismutase Activity (U/mg Protein) | Catalase Activity (U/mg Protein) | |
| Naïve control | 0.072 ± 0.02 | 36.07 ± 1.66 | 9.22 ± 0.72 |
| Stress | 0.158 ± 0.01 aaa | 25.56 ± 1.18 aa | 3.61 ± 0.93 a |
| Stress + Vehicle | 0.149 ± 0.01 a | 28.59 ± 1.84 | 4.07 ± 0.41 |
| Stress + The combined extract of mulberry leaves and butterfly pea flowers 125 mg/kg BW | 0.010 ± 0.01 | 31.73 ± 1.87 | 7.25 ± 1.83 |
| Stress + The combined extract of mulberry leaves and butterfly pea flowers 250 mg/kg BW | 0.079 ± 0.02 ** | 34.19 ± 0.97 * | 9.02 ± 0.79 * |
| Stress + The combined extract of mulberry leaves and butterfly pea flowers 500 mg/kg BW | 0.077 ± 0.01 **, # | 36.08 ± 1.96 ** | 10.39 ± 1.48 ** |
| Stress + Diazepam | 0.095 ± 0.01 * | 32.89 ± 1.17 * | 8.93 ± 1.06 |
| Stress + Donepezil | 0.090 ± 0.01 * | 34.00 ± 1.89 * | 10.53 ± 1.49 ** |
| Stress + Fluoxetine | 0.083 ± 0.01 ** | 34.37 ± 2.27 | 9.74 ± 1.09 * |
| Groups | Hippocampus | ||
|---|---|---|---|
| Malondialdehyde Level (nmol/mg Protein) | Superoxide Dismutase Activity (U/mg Protein) | Catalase Activity (U/mg Protein) | |
| Naïve control | 0.081 ± 0.01 | 36.53 ± 0.85 | 11.54 ± 1.48 |
| Stress | 0.154 ± 0.01 aa | 25.45 ± 3.73 aa | 4.95 ± 0.56 a |
| Stress + Vehicle | 0.146 ± 0.03 a | 26.30 ± 1.21a | 6.16 ± 1.45 |
| Stress + The combined extract of mulberry leaves and butterfly pea flowers 125 mg/kg BW | 0.091 ± 0.02 ** | 36.00 ± 1.15 ** | 9.77 ± 1.19 |
| Stress + The combined extract of mulberry leaves and butterfly pea flowers 250 mg/kg BW | 0.083 ± 0.01 ** | 37.08 ± 2.13 ***, # | 10.2 ± 1.97 |
| Stress + The combined extract of mulberry leaves and butterfly pea flowers 500 mg/kg BW | 0.083 ± 0.01 **, # | 38.00 ± 0.90 ***, # | 11.30 ± 1.57 * |
| Stress + Diazepam | 0.088 ± 0.01 ** | 34.24 ± 1.08 * | 8.23 ± 0.90 |
| Stress + Donepezil | 0.086 ± 0.01 ** | 35.57 ± 1.28 ** | 11.42 ± 1.08 * |
| Stress + Fluoxetine | 0.089 ± 0.01 ** | 35.64 ± 1.15 ** | 8.63 ± 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suna, O.; Wattanathorn, J.; Muchimapura, S.; Thukham-mee, W.; Iamsaard, S.; Uabundit, N. The Functional Ingredients of the Combined Extract of Mulberry Leaves and Butterfly Pea Flowers Improve Insomnia, Anxiolytic, Memory-Enhancing, and Antidepressant-like Activities in Stress-Exposed Rats. Life 2025, 15, 1308. https://doi.org/10.3390/life15081308
Suna O, Wattanathorn J, Muchimapura S, Thukham-mee W, Iamsaard S, Uabundit N. The Functional Ingredients of the Combined Extract of Mulberry Leaves and Butterfly Pea Flowers Improve Insomnia, Anxiolytic, Memory-Enhancing, and Antidepressant-like Activities in Stress-Exposed Rats. Life. 2025; 15(8):1308. https://doi.org/10.3390/life15081308
Chicago/Turabian StyleSuna, Orraya, Jintanaporn Wattanathorn, Supaporn Muchimapura, Wipawee Thukham-mee, Sitthichai Iamsaard, and Nongnut Uabundit. 2025. "The Functional Ingredients of the Combined Extract of Mulberry Leaves and Butterfly Pea Flowers Improve Insomnia, Anxiolytic, Memory-Enhancing, and Antidepressant-like Activities in Stress-Exposed Rats" Life 15, no. 8: 1308. https://doi.org/10.3390/life15081308
APA StyleSuna, O., Wattanathorn, J., Muchimapura, S., Thukham-mee, W., Iamsaard, S., & Uabundit, N. (2025). The Functional Ingredients of the Combined Extract of Mulberry Leaves and Butterfly Pea Flowers Improve Insomnia, Anxiolytic, Memory-Enhancing, and Antidepressant-like Activities in Stress-Exposed Rats. Life, 15(8), 1308. https://doi.org/10.3390/life15081308






