Influence of Exercise on Oxygen Consumption, Pulmonary Ventilation, and Blood Gas Analyses in Individuals with Chronic Diseases
Abstract
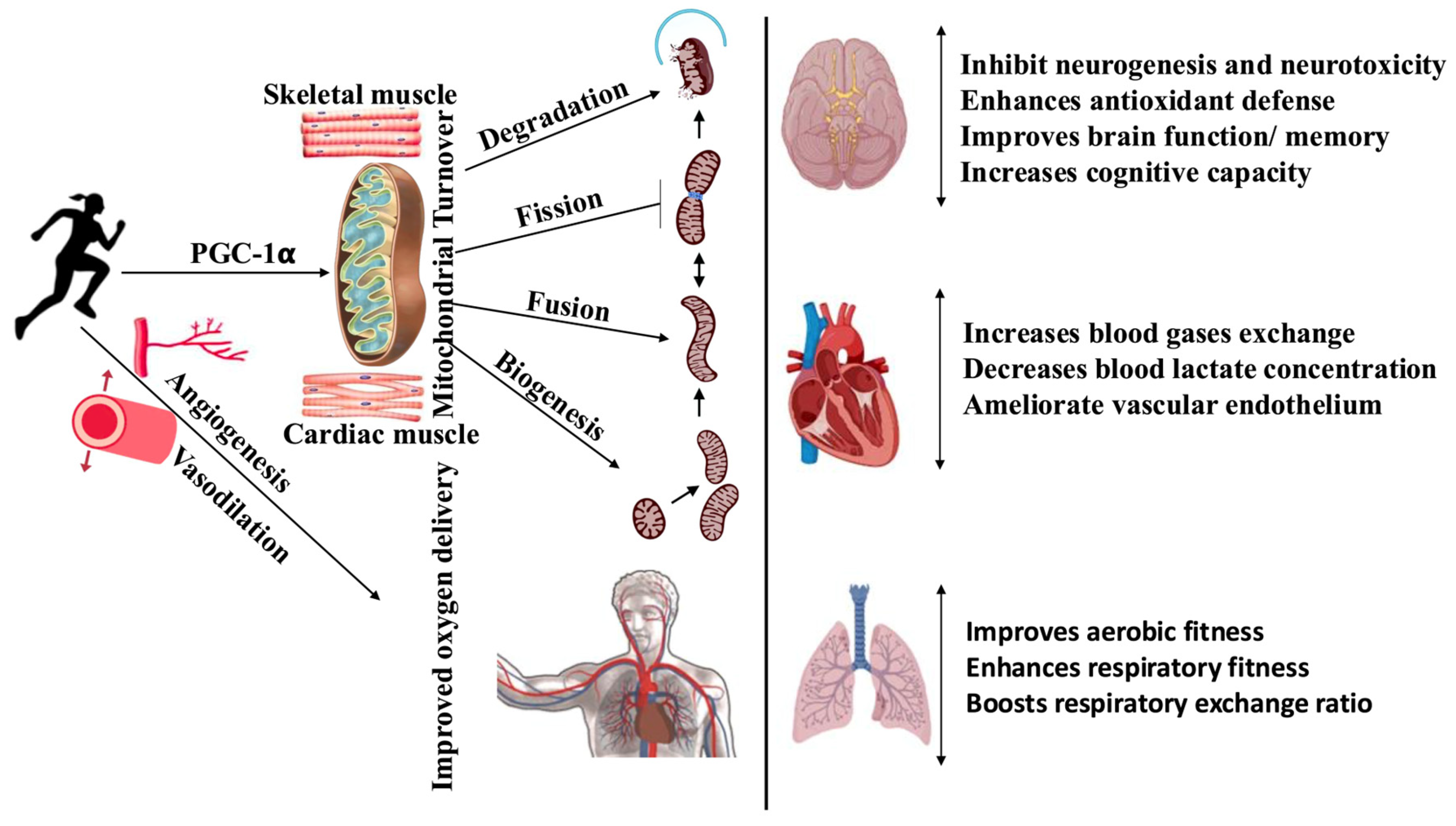
1. Introduction
Exercise and Pathophysiological Conditions
2. Methods
3. Variables Mitigating the Exercise Response
4. Integration of Effects on Oxygen Consumption
4.1. Age Factor
4.2. BMI
4.3. Smokers and Non-Smokers
4.4. Alcoholics and Non-Alcoholics
4.5. Diabetes
4.6. Hypertension
4.7. Parkinson’s Disease (PD)
4.8. COVID-19
5. Integration of Effects on Pulmonary Ventilation and Blood Gases
5.1. Pulmonary Ventilation
5.2. Blood Gas Dynamics
5.3. Limitations of Current Evidence
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- World Health Organization. Recommended population levels of physical activity for health. In Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010; pp. 15–34. [Google Scholar]
- Strain, T.; Flaxman, S.; Guthold, R.; Semenova, E.; Cowan, M.; Riley, L.M.; Bull, F.C.; Stevens, G.A. National, regional, and global trends in insufficient physical activity among adults from 2000 to 2022: A pooled analysis of 507 population-based surveys with 5·7 million participants. Lancet Glob. Health 2024, 12, e1232–e1243. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Morales, J.S.; Valenzuela, P.L.; Martínez-de-Quel, Ó.; Sánchez-Sánchez, J.L.; Muntaner-Mas, A.; Erickson, K.I.; Carbonell-Baeza, A.; Ortega, F.B.; Jiménez-Pavón, D. Exercise Interventions and Intelligence in Children and Adolescents: A Meta-Analysis. Pediatrics 2024, 154, e2023064771. [Google Scholar] [CrossRef]
- Bull, F.; Willumsen, J.; Stevens, G.; Strain, T. Global Levels of Physical Inactivity in Adults: Off Track for 2030; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Korivi, M.; Liu, B.R. Novel and practical approaches to manage diet-induced metabolic disorders: Part-i. Curr. Pharm. Des. 2020, 26, 4953–4954. [Google Scholar] [CrossRef]
- Cui, T.; Sun, Y.; Ye, W.; Liu, Y.; Korivi, M. Efficacy of time restricted eating and resistance training on body composition and mood profiles among young adults with overweight/obesity: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2025, 22, 2481127. [Google Scholar] [CrossRef]
- Unsal, N.; Weaver, G.; Bray, J.W.; Bibeau, D.; Saake, G. Return on Investment of Workplace Wellness: Evidence From a Long-Term Care Company. Workplace Health Saf. 2021, 69, 81–90. [Google Scholar] [CrossRef]
- Burton, W.N.; Chen, C.-Y.; Conti, D.J.; Schultz, A.B.; Edington, D.W. The association between health risk change and presenteeism change. J. Occup. Environ. Med. 2006, 48, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Isham, A.; Mair, S.; Jackson, T. Worker wellbeing and productivity in advanced economies: Re-examining the link. Ecol. Econ. 2021, 184, 106989. [Google Scholar] [CrossRef]
- Bhammar, D.M.; Stickford, J.L.; Bernhardt, V.; Babb, T.G. Effect of weight loss on operational lung volumes and oxygen cost of breathing in obese women. Int. J. Obes. 2016, 40, 998–1004. [Google Scholar] [CrossRef]
- Prieto-González, P.; Yagin, F.H. Energy expenditure, oxygen consumption, and heart rate while exercising on seven different indoor cardio machines at maximum and self-selected submaximal intensity. Front. Sports Act. Living 2024, 6, 1313886. [Google Scholar] [CrossRef]
- Takahashi, T.; Ebihara, S.; Kohzuki, M. Improvement of pulmonary function after comprehensive obesity rehabilitation program in obese patients. Tohoku J. Exp. Med. 2017, 242, 215–221. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization Technical Repport Series; World Health Organization: Geneva, Switzerland, 2000; Volume 894, pp. 1–253. [Google Scholar]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Okamura, M.; Shimizu, M.; Yamamoto, S.; Nishie, K.; Konishi, M. High-intensity interval training versus moderate-intensity continuous training in patients with heart failure: A systematic review and meta-analysis. Heart Fail. Rev. 2023, 28, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Yonas, E.; Siswanto, B.B.; Purwowiyoto, B.S. Exercise Training in Heart Failure: High-intensity Interval Training versus Moderate-intensity Continuous Training. Int. J. Cardiovasc. Acad. 2018, 4, 41–45. [Google Scholar] [CrossRef]
- Gu, S.; Du, X.; Wang, D.; Yu, Y.; Guo, S. Effects of high intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0290362. [Google Scholar] [CrossRef]
- Hurst, C.; Weston, K.L.; Weston, M. The effect of 12 weeks of combined upper-and lower-body high-intensity interval training on muscular and cardiorespiratory fitness in older adults. Aging Clin. Exp. Res. 2019, 31, 661–671. [Google Scholar] [CrossRef]
- Sequeira, S.; Cruz, C.; Pinto, D.; Santos, L.; Marques, A. Prevalence of barriers for physical activity in adults according to gender and socioeconomic status. Br. J. Sports Med. 2011, 45, A18–A19. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Romero-Vera, L.; Ulloa-Díaz, D.; Araya-Sierralta, S.; Guede-Rojas, F.; Andrades-Ramírez, O.; Carvajal-Parodi, C.; Muñoz-Bustos, G.; Matamala-Aguilera, M.; Martínez-García, D. Effects of High-Intensity Interval Training on Blood Pressure Levels in Hypertensive Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Life 2024, 14, 1661. [Google Scholar] [CrossRef]
- Wells, C.M.; Edwards, A.M.; Winter, E.M.; Fysh, M.L.; Drust, B. Sport-specific fitness testing differentiates professional from amateur soccer players where VO2max and VO2 kinetics do not. J. Sports Med. Phys. Fit. 2012, 52, 245–254. [Google Scholar]
- Deliceoğlu, G.; Kabak, B.; Çakır, V.O.; Ceylan, H.İ.; Raul-Ioan, M.; Alexe, D.I.; Stefanica, V. Respiratory Muscle Strength as a Predictor of VO2max and Aerobic Endurance in Competitive Athletes. Appl. Sci. 2024, 14, 8976. [Google Scholar] [CrossRef]
- Phillips, D.B.; Stickland, M.K. Respiratory limitations to exercise in health: A brief review. Curr. Opin. Physiol. 2019, 10, 173–179. [Google Scholar] [CrossRef]
- Tonga, K.O.; Oliver, B.G. Effectiveness of Pulmonary Rehabilitation for Chronic Obstructive Pulmonary Disease Therapy: Focusing on Traditional Medical Practices. J. Clin. Med. 2023, 12, 4815. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.; Criner, G.J.; Papi, A.; Singh, D.; Anzueto, A.; Martinez, F.J.; Agusti, A.A.; Vogelmeier, C.F. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2021, 203, 24–36. [Google Scholar] [CrossRef]
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.-P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef]
- Cardoso, S.R.; Pereira, J.S. Análise da função respiratória na doença de Parkinson. Arq. De Neuro-Psiquiatr. 2002, 60, 91–95. [Google Scholar] [CrossRef]
- Docu Axelerad, A.; Stroe, A.Z.; Arghir, O.C.; Docu Axelerad, D.; Gogu, A.E. Respiratory Dysfunctions in Parkinson’s Disease Patients. Brain Sci. 2021, 11, 595. [Google Scholar] [CrossRef]
- Tamaki, A.; Matsuo, Y.; Yanagihara, T.; Abe, K. Influence of thoracoabdominal movement on pulmonary function in patients with Parkinson’s disease: Comparison with healthy subjects. Neurorehabilit. Neural Repair 2000, 14, 43–47. [Google Scholar] [CrossRef]
- Goldstein, D.S. Dysautonomia in Parkinson’s disease: Neurocardiological abnormalities. Lancet Neurol. 2003, 2, 669–676. [Google Scholar] [CrossRef]
- Hackett, P.H.; Roach, R.C. High-altitude illness. N. Engl. J. Med. 2001, 345, 107–114. [Google Scholar] [CrossRef]
- Barberà, J.A.; Riverola, A.; Roca, J.; Ramirez, J.; Wagner, P.D.; Ros, D.; Wiggs, B.R.; Rodriguez-Roisin, R. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1994, 149, 423–429. [Google Scholar] [CrossRef]
- Roach, R.C.; Greene, E.R.; Schoene, R.B.; Hackett, P.H. Arterial oxygen saturation for prediction of acute mountain sickness. Aviat. Space Environ. Med. 1998, 69, 1182–1185. [Google Scholar]
- Ballester, E.; Reyes, A.; Roca, J.; Guitart, R.; Wagner, P.; Rodriguez-Roisin, R. Ventilation-perfusion mismatching in acute severe asthma: Effects of salbutamol and 100% oxygen. Thorax 1989, 44, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Oswald-Mammosser, M.; Weitzenblum, E.; Quoix, E.; Moser, G.; Chaouat, A.; Charpentier, C.; Kessler, R. Prognostic factors in COPD patients receiving long-term oxygen therapy: Importance of pulmonary artery pressure. Chest 1995, 107, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Hobbins, L.; Hunter, S.; Gaoua, N.; Girard, O. Normobaric hypoxic conditioning to maximize weight loss and ameliorate cardio-metabolic health in obese populations: A systematic review. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2017, 313, R251–R264. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Girard, O.; Perez, A.; Rubio-Arias, J.A. Additive stress of normobaric hypoxic conditioning to improve body mass loss and cardiometabolic markers in individuals with overweight or obesity: A systematic review and meta-analysis. Physiol. Behav. 2019, 207, 28–40. [Google Scholar] [CrossRef]
- Netzer, N.C.; Chytra, R.; Küpper, T. Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath. 2008, 12, 129–134. [Google Scholar] [CrossRef]
- Wiesner, S.; Haufe, S.; Engeli, S.; Mutschler, H.; Haas, U.; Luft, F.C.; Jordan, J. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity 2010, 18, 116–120. [Google Scholar] [CrossRef]
- Haufe, S.; Wiesner, S.; Engeli, S.; Luft, F.C.; Jordan, J. Influences of normobaric hypoxia training on metabolic risk markers in human subjects. Med. Sci. Sports Exerc. 2008, 40, 1939–1944. [Google Scholar] [CrossRef]
- De Groote, E.; Britto, F.A.; Bullock, L.; François, M.; De Buck, C.; Nielens, H.; Deldicque, L. Hypoxic training improves normoxic glucose tolerance in adolescents with obesity. Med. Sci. Sports Exerc. 2018, 50, 2200–2208. [Google Scholar] [CrossRef]
- Kong, Z.; Zang, Y.; Hu, Y. Normobaric hypoxia training causes more weight loss than normoxia training after a 4-week residential camp for obese young adults. Sleep Breath. 2014, 18, 591–597. [Google Scholar] [CrossRef]
- Chacaroun, S.; Borowik, A.; Gonzalez, V.-E.Y.; Doutreleau, S.; Wuyam, B.; Belaidi, E.; Tamisier, R.; Pepin, J.-L.; Flore, P.; Verges, S. Hypoxic Exercise Training to Improve Exercise Capacity in Obese Individuals. Med. Sci. Sports Exerc. 2020, 52, 1641–1649. [Google Scholar] [CrossRef]
- Brocherie, F.; Millet, G.P. Hypoxic exercise as an effective nonpharmacological therapeutic intervention. Exp. Mol. Med. 2020, 52, 529–530. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Atakan, M.M.; Kuang, J.; Hu, Y.; Bishop, D.J.; Yan, X. The Molecular Adaptive Responses of Skeletal Muscle to High-Intensity Exercise/Training and Hypoxia. Antioxidants 2020, 9, 656. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Guo, W.; Gao, B.-H. Hypoxic training upregulates mitochondrial turnover and angiogenesis of skeletal muscle in mice. Life Sci. 2022, 291, 119340. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Smith, D.W.; Gardiner, B.S.; Evans, R.G. Stimulation of erythropoietin release by hypoxia and hypoxemia: Similar but different. Kidney Int. 2019, 95, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Walter, M.F.; Korach, K.S.; Noguchi, C.T. Erythropoietin reduces fat mass in female mice lacking estrogen receptor alpha. Mol. Metab. 2021, 45, 101142. [Google Scholar] [CrossRef]
- Shaaban, R.; Kony, S.; Driss, F.; Leynaert, B.; Soussan, D.; Pin, I.; Neukirch, F.; Zureik, M. Change in C-reactive protein levels and FEV1 decline: A longitudinal population-based study. Respir. Med. 2006, 100, 2112–2120. [Google Scholar] [CrossRef]
- Kwon, C.-H.; Rhee, E.-J.; Song, J.-U.; Kim, J.-T.; Kwag, H.J.; Sung, K.-C. Reduced lung function is independently associated with increased risk of type 2 diabetes in Korean men. Cardiovasc. Diabetol. 2012, 11, 38. [Google Scholar] [CrossRef][Green Version]
- Engström, G.; Hedblad, B.; Nilsson, P.; Wollmer, P.; Berglund, G.; Janzon, L. Lung function, insulin resistance and incidence of cardiovascular disease: A longitudinal cohort study. J. Intern. Med. 2003, 253, 574–581. [Google Scholar] [CrossRef]
- Yu, J. Endocrine disorders and the neurologic manifestations. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 184. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Ginis, K.A.M.; Fenuta, A.M.; MacKibbon, K.A.; Motl, R.W. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828.e3. [Google Scholar] [CrossRef] [PubMed]
- Uhrbrand, A.; Stenager, E.; Pedersen, M.S.; Dalgas, U. Parkinson’s disease and intensive exercise therapy–a systematic review and meta-analysis of randomized controlled trials. J. Neurol. Sci. 2015, 353, 9–19. [Google Scholar] [CrossRef] [PubMed]
- LaHue, S.C.; Comella, C.L.; Tanner, C.M. The best medicine? The influence of physical activity and inactivity on Parkinson’s disease. Mov. Disord. 2016, 31, 1444–1454. [Google Scholar] [CrossRef]
- Motl, R.W.; Pilutti, L.A. The benefits of exercise training in multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 487–497. [Google Scholar] [CrossRef]
- Wj, C.-Z.; Proctor, D.; Fiatarone Singh, M. American College of Sports Medicine position stand: Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar]
- Ozemek, C.; Bonikowske, A.; Christle, J.; Gallo, P. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Waltham, MA, USA, 2025. [Google Scholar]
- Hawkins, S.A.; Wiswell, R.A. Rate and mechanism of maximal oxygen consumption decline with aging. Sports Med. 2003, 33, 877–888. [Google Scholar] [CrossRef]
- Cooke, A.B.; Toli, E.; Gomez, Y.-H.; Mutter, A.F.; Eisenberg, M.J.; Mantzoros, C.S.; Daskalopoulou, S.S. From rest to stressed: Endothelin-1 levels in young healthy smokers and non-smokers. Metabolism 2015, 64, 1103–1111. [Google Scholar] [CrossRef]
- Gläser, S.; Ittermann, T.; Koch, B.; Schäper, C.; Felix, S.B.; Völzke, H.; Könemann, R.; Ewert, R.; Hansen, J.E. Influence of smoking and obesity on alveolar-arterial gas pressure differences and dead space ventilation at rest and peak exercise in healthy men and women. Respir. Med. 2013, 107, 919–926. [Google Scholar] [CrossRef]
- Ohuchi, H.; Kato, Y.; Tasato, H.; Arakaki, Y.; Kamiya, T. Ventilatory response and arterial blood gases during exercise in children. Pediatr. Res. 1999, 45, 389–396. [Google Scholar] [CrossRef][Green Version]
- Shur, N.F.; Creedon, L.; Skirrow, S.; Atherton, P.J.; MacDonald, I.A.; Lund, J.; Greenhaff, P.L. Age-related changes in muscle architecture and metabolism in humans: The likely contribution of physical inactivity to age-related functional decline. Ageing Res. Rev. 2021, 68, 101344. [Google Scholar] [CrossRef]
- Sengbusch, J.R.; Tiernan, D.L.; Tamulevicius, N.; Martinasek, M.P. The Impact of Smoking on Maximum Oxygen Uptake. Respir. Care 2021, 66, 857–861. [Google Scholar] [CrossRef]
- Degens, H.; Gayan-Ramirez, G.; van Hees, H.W. Smoking-induced skeletal muscle dysfunction: From evidence to mechanisms. Am. J. Respir. Crit. Care Med. 2015, 191, 620–625. [Google Scholar] [CrossRef]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Antó, J.M. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: A population-based cohort study. Am. J. Respir. Crit. Care Med. 2007, 175, 458–463. [Google Scholar] [CrossRef]
- El-Sayed, M.S.; Ali, N.; El-Sayed Ali, Z. Interaction between alcohol and exercise: Physiological and haematological implications. Sports Med. 2005, 35, 257–269. [Google Scholar] [CrossRef]
- Popovic, D.; Damjanovic, S.S.; Plecas-Solarovic, B.; Pešić, V.; Stojiljkovic, S.; Banovic, M.; Ristic, A.; Mantegazza, V.; Agostoni, P. Exercise capacity is not impaired after acute alcohol ingestion: A pilot study. J. Cardiovasc. Med. 2016, 17, 896–901. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. Physical activity and lifestyle modifications in the treatment of neurodegenerative diseases. Front. Aging Neurosci. 2023, 15, 1185671. [Google Scholar] [CrossRef]
- Bull, F.; Goenka, S.; Lambert, V.; Pratt, M. Physical activity for the prevention of cardiometabolic disease. In Disease Control Priorities; World Bank: Washington, DC, USA, 2017; Volume 5. [Google Scholar]
- Haykowsky, M.J.; Brubaker, P.H.; Stewart, K.P.; Morgan, T.M.; Eggebeen, J.; Kitzman, D.W. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2012, 60, 120–128. [Google Scholar] [CrossRef]
- Anderson, L.; Thompson, D.R.; Oldridge, N.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016, 2016, CD001800. [Google Scholar] [CrossRef]
- Aronow, W.S.; Shamliyan, T.A. Exercise for preventing hospitalization and readmission in adults with congestive heart failure. Cardiol. Rev. 2019, 27, 41–48. [Google Scholar] [CrossRef]
- Lundby, C.; Montero, D.; Joyner, M. Biology of VO2max: Looking under the physiology lamp. Acta Physiol. 2017, 220, 218–228. [Google Scholar] [CrossRef]
- Bassett, D.R.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef]
- de Oliveira Bristot, V.J.; de Bem Alves, A.C.; Cardoso, L.R.; da Luz Scheffer, D.; Aguiar Jr, A.S. The role of PGC-1α/UCP2 signaling in the beneficial effects of physical exercise on the brain. Front. Neurosci. 2019, 13, 292. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef]
- Kang, C.; O’Moore, K.M.; Dickman, J.R.; Ji, L.L. Exercise activation of muscle peroxisome proliferator-activated receptor-γ coactivator-1α signaling is redox sensitive. Free Radic. Biol. Med. 2009, 47, 1394–1400. [Google Scholar] [CrossRef]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal. 2014, 21, 154–176. [Google Scholar] [CrossRef]
- Jones, A.M.; Kirby, B.S.; Clark, I.E.; Rice, H.M.; Fulkerson, E.; Wylie, L.J.; Wilkerson, D.P.; Vanhatalo, A.; Wilkins, B.W. Physiological demands of running at 2-hour marathon race pace. J. Appl. Physiol. 2021, 130, 369–379. [Google Scholar] [CrossRef]
- Ansdell, P.; Thomas, K.; Hicks, K.M.; Hunter, S.K.; Howatson, G.; Goodall, S. Physiological sex differences affect the integrative response to exercise: Acute and chronic implications. Exp. Physiol. 2020, 105, 2007–2021. [Google Scholar] [CrossRef]
- Sheel, A.W.; Dominelli, P.B.; Molgat-Seon, Y. Revisiting dysanapsis: Sex-based differences in airways and the mechanics of breathing during exercise. Exp. Physiol. 2016, 101, 213–218. [Google Scholar] [CrossRef]
- Molgat-Seon, Y.; Peters, C.M.; Sheel, A.W. Sex-differences in the human respiratory system and their impact on resting pulmonary function and the integrative response to exercise. Curr. Opin. Physiol. 2018, 6, 21–27. [Google Scholar] [CrossRef]
- Dempsey, J.A.; La Gerche, A.; Hull, J.H. Is the healthy respiratory system built just right, overbuilt, or underbuilt to meet the demands imposed by exercise? J. Appl. Physiol. 2020, 129, 1235–1256. [Google Scholar] [CrossRef]
- Chambault, J.; Grand, G.; Kayser, B. Sex-Specific Effects of Respiratory Muscle Endurance Training on Cycling Time Trial Performance in Normoxia and Hypoxia. Front. Physiol. 2021, 12, 700620. [Google Scholar] [CrossRef] [PubMed]
- Geary, C.M.; Welch, J.F.; McDonald, M.R.; Peters, C.M.; Leahy, M.G.; Reinhard, P.A.; Sheel, A.W. Diaphragm fatigue and inspiratory muscle metaboreflex in men and women matched for absolute diaphragmatic work during pressure-threshold loading. J. Physiol. 2019, 597, 4797–4808. [Google Scholar] [CrossRef]
- Duscha, B.D.; Slentz, C.A.; Johnson, J.L.; Houmard, J.A.; Bensimhon, D.R.; Knetzger, K.J.; Kraus, W.E. Effects of exercise training amount and intensity on peak oxygen consumption in middle-age men and women at risk for cardiovascular disease. Chest 2005, 128, 2788–2793. [Google Scholar] [CrossRef]
- Burn, N.; Weston, M.; Atkinson, G.; Graham, M.; Weston, K. Brief Exercise at Work (BE@ Work): A mixed-methods pilot trial of a workplace high-intensity interval training intervention. Front. Sports Act. Living 2021, 3, 179. [Google Scholar] [CrossRef]
- Torres, G.; Fouche, J.; Redelinghuys, R.; Brussow, B.; Cronson, D.; Zanuso, S.; Constantinou, D. The effectiveness of a corporate exercise intervention programme on cardiovascular risk profile, fitness and productivity: A South African view. S. Afr. Med. J. 2020, 110, 1045–1049. [Google Scholar] [CrossRef]
- Jang, D.-J.; Kim, H.-C.; Kim, J.-K.; Jung, S.-Y.; Kim, D.-Y. Effects of habitual smoking on cardiopulmonary function in taekwondo athletes. J. Exerc. Rehabil. 2017, 13, 711. [Google Scholar] [CrossRef]
- Molina-Hidalgo, C.; De-la, O.A.; Dote-Montero, M.; Amaro-Gahete, F.J.; Castillo, M.J. Influence of daily beer or ethanol consumption on physical fitness in response to a high-intensity interval training program. The BEER-HIIT study. J. Int. Soc. Sports Nutr. 2020, 17, 29. [Google Scholar] [CrossRef]
- Abe, T.; Yokota, T.; Fukushima, A.; Kakutani, N.; Katayama, T.; Shirakawa, R.; Maekawa, S.; Nambu, H.; Obata, Y.; Yamanashi, K. Type 2 diabetes is an independent predictor of lowered peak aerobic capacity in heart failure patients with non-reduced or reduced left ventricular ejection fraction. Cardiovasc. Diabetol. 2020, 19, 142. [Google Scholar] [CrossRef]
- Hoeper, M.; Pletz, M.; Golpon, H.; Welte, T. Prognostic value of blood gas analyses in patients with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2007, 29, 944–950. [Google Scholar] [CrossRef]
- Shulman, L.M.; Katzel, L.I.; Ivey, F.M.; Sorkin, J.D.; Favors, K.; Anderson, K.E.; Smith, B.A.; Reich, S.G.; Weiner, W.J.; Macko, R.F. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol. 2013, 70, 183–190. [Google Scholar] [CrossRef]
- Rinaldo, R.F.; Mondoni, M.; Parazzini, E.M.; Baccelli, A.; Pitari, F.; Brambilla, E.; Luraschi, S.; Balbi, M.; Guazzi, M.; Di Marco, F. Severity does not impact on exercise capacity in COVID-19 survivors. Respir. Med. 2021, 187, 106577. [Google Scholar] [CrossRef]
- Mahindru, A.; Patil, P.; Agrawal, V. Role of Physical Activity on Mental Health and Well-Being: A Review. Cureus 2023, 15, e33475. [Google Scholar] [CrossRef] [PubMed]
- Ayinapudi, K.; Singh, T.; Motwani, A.; Le Jemtel, T.H.; Oparil, S. Obesity and pulmonary hypertension. Curr. Hypertens. Rep. 2018, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, H.; Khayat, A.; Shikora, S.; Unterborn, J.N. Relationship of dyspnea to respiratory drive and pulmonary function tests in obese patients before and after weight loss. Chest 2005, 128, 3870–3874. [Google Scholar] [CrossRef] [PubMed]
- Babb, T.G. Obesity: Challenges to ventilatory control during exercise—A brief review. Respir. Physiol. Neurobiol. 2013, 189, 364–370. [Google Scholar] [CrossRef]
- Zavorsky, G.S.; Murias, J.M.; Kim, D.J.; Gow, J.; Christou, N.V. Poor compensatory hyperventilation in morbidly obese women at peak exercise. Respir. Physiol. Neurobiol. 2007, 159, 187–195. [Google Scholar] [CrossRef]
- Balmain, B.N.; Halverson, Q.M.; Tomlinson, A.R.; Edwards, T.; Ganio, M.S.; Babb, T.G. Obesity Blunts the Ventilatory Response to Exercise in Men and Women. Ann. Am. Thorac. Soc. 2021, 18, 1167–1174. [Google Scholar] [CrossRef]
- Corrêa, P.C.R.P.; Sales, R.K.B.d.; Knorst, M.M.; Pinto, S.R.H.L.; Ragnini, L.F.Q.; Tourinho, C.A.P.; Storrer, K.M.; Scuarcialupi, M.E.C.D.A.; Castellano, M.V.C.D.O.; Albuquerque, A.A.D. The challenge of tobacco and nicotine use among women. Rev. Da Assoc. Médica Bras. 2023, 69, e2023S2124. [Google Scholar] [CrossRef]
- Goodchild, M.; Nargis, N.; d’Espaignet, E.T. Global economic cost of smoking-attributable diseases. Tob. Control 2018, 27, 58–64. [Google Scholar] [CrossRef]
- Reitsma, M.B.; Fullman, N.; Ng, M.; Salama, J.S.; Abajobir, A.; Abate, K.H.; Abbafati, C.; Abera, S.F.; Abraham, B.; Abyu, G.Y. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef]
- David, J.-C.; Fonte, D.; Sutter-Dallay, A.-L.; Auriacombe, M.; Serre, F.; Rascle, N.; Loyal, D. The stigma of smoking among women: A systematic review. Soc. Sci. Med. 2024, 340, 116491. [Google Scholar] [CrossRef] [PubMed]
- Su, F.-Y.; Wang, S.-H.; Lu, H.H.-S.; Lin, G.-M. Association of tobacco smoking with physical fitness of military males in taiwan: The CHIEF study. Can. Respir. J. 2020, 2020, 5968189. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Takeuchi, T.; Hosoi, T.; Loeppky, J.A. Effects of habitual smoking on cardiorespiratory responses to sub-maximal exercise. J. Physiol. Anthropol. Appl. Hum. Sci. 2004, 23, 163–169. [Google Scholar] [CrossRef]
- Holmen, T.; Barrett-Connor, E.; Clausen, J.; Holmen, J.; Bjermer, L. Physical exercise, sports, and lung function in smoking versus nonsmoking adolescents. Eur. Respir. J. 2002, 19, 8–15. [Google Scholar] [CrossRef]
- Tantisuwat, A.; Thaveeratitham, P. Effects of smoking on chest expansion, lung function, and respiratory muscle strength of youths. J. Phys. Ther. Sci. 2014, 26, 167–170. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef]
- Vella, L.D.; Cameron-Smith, D. Alcohol, athletic performance and recovery. Nutrients 2010, 2, 781–789. [Google Scholar] [CrossRef]
- Siggins, R.W.; McTernan, P.M.; Simon, L.; Souza-Smith, F.M.; Molina, P.E. Mitochondrial Dysfunction: At the Nexus between Alcohol-Associated Immunometabolic Dysregulation and Tissue Injury. Int. J. Mol. Sci. 2023, 24, 8650. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Yokus, B.; Paes-Leme, B.; Bátkai, S.; Ungvári, Z.; Haskó, G.; Pacher, P. Chronic alcohol consumption accelerates cardiovascular aging and decreases cardiovascular reserve capacity. GeroScience 2025. online ahead of print. [Google Scholar] [CrossRef]
- Chicco, A.J.; McCarty, H.; Reed, A.H.; Story, R.R.; Westerlind, K.C.; Turner, R.T.; Hayward, R. Resistance exercise training attenuates alcohol-induced cardiac oxidative stress. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 74–79. [Google Scholar] [CrossRef]
- Bonita, R.; Magnusson, R.; Bovet, P.; Zhao, D.; Malta, D.C.; Geneau, R.; Suh, I.; Thankappan, K.R.; McKee, M.; Hospedales, J. Country actions to meet UN commitments on non-communicable diseases: A stepwise approach. Lancet 2013, 381, 575–584. [Google Scholar] [CrossRef]
- Bond, V.; Franks, B.; Howley, E. Alcohol, cardiorespiratory function and work performance. Br. J. Sports Med. 1984, 18, 203–206. [Google Scholar] [CrossRef]
- Roerecke, M.; Rehm, J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: A systematic review and meta-analysis. Addiction 2012, 107, 1246–1260. [Google Scholar] [CrossRef]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Coker, R.H.; Williams, R.H.; Yeo, S.E.; Kortebein, P.M.; Bodenner, D.L.; Kern, P.A.; Evans, W.J. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J. Clin. Endocrinol. Metab. 2009, 94, 4258–4266. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Yoshikawa, T. Role of oxidative stress in impaired insulin signaling associated with exercise-induced muscle damage. Free Radic. Biol. Med. 2013, 65, 1265–1272. [Google Scholar] [CrossRef]
- Syeda, U.S.A.; Battillo, D.; Visaria, A.; Malin, S.K. The importance of exercise for glycemic control in type 2 diabetes. Am. J. Med. Open 2023, 9, 100031. [Google Scholar] [CrossRef]
- Lee, S.R.; Jeong, K.J.; Mukae, M.; Lee, J.; Hong, E.J. Exercise promotes peripheral glycolysis in skeletal muscle through miR-204 induction via the HIF-1α pathway. Sci. Rep. 2025, 15, 1487. [Google Scholar] [CrossRef]
- Nguyen, T.-T.P.; Jacobs, P.G.; Castle, J.R.; Wilson, L.M.; Kuehl, K.; Branigan, D.; Gabo, V.; Guillot, F.; Riddell, M.C.; Haidar, A. Separating insulin-mediated and non-insulin-mediated glucose uptake during and after aerobic exercise in type 1 diabetes. Am. J. Physiol.-Endocrinol. Metab. 2021, 320, E425–E437. [Google Scholar] [CrossRef]
- Romeres, D.; Schiavon, M.; Basu, A.; Cobelli, C.; Basu, R.; Dalla Man, C. Exercise effect on insulin-dependent and insulin-independent glucose utilization in healthy individuals and individuals with type 1 diabetes: A modeling study. Am. J. Physiol.-Endocrinol. Metab. 2021, 321, E122–E129. [Google Scholar] [CrossRef]
- Molina-Sotomayor, E.; Gómez-Campos, R.; Ulloa-Tapia, E.; Arroyo-Jofre, P.; González-Jurado, J.; Celis-Morales, C.; Rodríguez-Rodríguez, F.; Cossio-Bolaños, M. Effects of physical exercise on aerobic fitness and cognition in older women with type 2 diabetes mellitus. Rev. Medica De Chile 2021, 149, 37–44. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fazekas-Pongor, V.; Csiszar, A.; Kunutsor, S.K. The multifaceted benefits of walking for healthy aging: From Blue Zones to molecular mechanisms. Geroscience 2023, 45, 3211–3239. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Nichols, W.W. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 2005, 45, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; Van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Bikos, A.; Angeloudi, E.; Memmos, E.; Loutradis, C.; Karpetas, A.; Ginikopoulou, E.; Panagoutsos, S.; Pasadakis, P.; Liakopoulos, V.; Papagianni, A. A comparative study of short-term blood pressure variability in hemodialysis patients with and without intradialytic hypertension. Am. J. Nephrol. 2018, 48, 295–305. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Persu, A.; Agarwal, R.; Burnier, M.; De Leeuw, P.; Ferro, C.J.; Halimi, J.-M.; Heine, G.H.; Jadoul, M.; Jarraya, F. Hypertension in dialysis patients: A consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) and the Hypertension and the Kidney working group of the European Society of Hypertension (ESH). Nephrol. Dial. Transplant. 2017, 32, 620–640. [Google Scholar]
- Agarwal, R. Epidemiology of interdialytic ambulatory hypertension and the role of volume excess. Am. J. Nephrol. 2011, 34, 381–390. [Google Scholar] [CrossRef]
- Ferrari, L.; Vicenzi, M.; Tarantini, L.; Barretta, F.; Sironi, S.; Baccarelli, A.A.; Guazzi, M.; Bollati, V. Effects of physical exercise on endothelial function and DNA methylation. Int. J. Environ. Res. Public Health 2019, 16, 2530. [Google Scholar] [CrossRef]
- Miranda Furtado, C.L.; Hansen, M.; Kogure, G.S.; Ribeiro, V.B.; Taylor, N.; Racy Soares, M.; Ferriani, R.A.; Aston, K.I.; Jenkins, T.; Dos Reis, R.M. Resistance and aerobic training increases genome-wide DNA methylation in women with polycystic ovary syndrome. Epigenetics 2024, 19, 2305082. [Google Scholar] [CrossRef]
- Murphy, R.M.; Watt, M.J.; Febbraio, M.A. Metabolic communication during exercise. Nat. Metab. 2020, 2, 805–816. [Google Scholar] [CrossRef]
- Secher, N.H.; Seifert, T.; Van Lieshout, J.J. Cerebral blood flow and metabolism during exercise: Implications for fatigue. J. Appl. Physiol. 2008, 104, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.J.; Kramer, A.F.; Erickson, K.I.; Scalf, P.; McAuley, E.; Cohen, N.J.; Webb, A.; Jerome, G.J.; Marquez, D.X.; Elavsky, S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. USA 2004, 101, 3316–3321. [Google Scholar] [CrossRef]
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Malkowski, E.; Alves, H.; Kim, J.S.; Morris, K.S.; White, S.M.; Wójcicki, T.R. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia 2010, 48, 1394–1406. [Google Scholar] [CrossRef]
- Brienesse, L.A.; Emerson, M.N. Effects of resistance training for people with Parkinson’s disease: A systematic review. J. Am. Med. Dir. Assoc. 2013, 14, 236–241. [Google Scholar] [CrossRef]
- David, F.J.; Rafferty, M.R.; Robichaud, J.A.; Prodoehl, J.; Kohrt, W.M.; Vaillancourt, D.E.; Corcos, D.M. Progressive resistance exercise and Parkinson’s disease: A review of potential mechanisms. Park. Dis. 2012, 2012, 124527. [Google Scholar] [CrossRef]
- Lima, L.O.; Scianni, A.; Rodrigues-de-Paula, F. Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson’s disease: A systematic review. J. Physiother. 2013, 59, 7–13. [Google Scholar] [CrossRef]
- Saltychev, M.; Bärlund, E.; Paltamaa, J.; Katajapuu, N.; Laimi, K. Progressive resistance training in Parkinson’s disease: A systematic review and meta-analysis. BMJ Open 2016, 6, e008756. [Google Scholar] [CrossRef]
- Almikhlafi, M.A. The role of exercise in Parkinson’s Disease. Neurosciences (Riyadh) 2023, 28, 4–12. [Google Scholar] [CrossRef]
- Katzel, L.I.; Sorkin, J.D.; Macko, R.F.; Smith, B.; Ivey, F.M.; Shulman, L.M. Repeatability of aerobic capacity measurements in Parkinson disease. Med. Sci. Sports Exerc. 2011, 43, 2381. [Google Scholar] [CrossRef]
- Clavario, P.; De Marzo, V.; Lotti, R.; Barbara, C.; Porcile, A.; Russo, C.; Beccaria, F.; Bonavia, M.; Bottaro, L.C.; Caltabellotta, M. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int. J. Cardiol. 2021, 340, 113–118. [Google Scholar] [CrossRef]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Millis, R.M.; Bond, V., Jr.; Asadi, M.S.; Haddad, G.E.; Adams, R.G. Oxygen Consumption at 30 W of Exercise Is Surrogate for Peak Oxygen Consumption in Evaluation of Cardiorespiratory Fitness in Young-Adult African-American Females. ISRN Physiol. 2013, 2013, 756276. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.G.; Kim, G.; Jeong, H.S.; So, W.Y. Association between Cigarette Smoking and Physical Fitness Level of Korean Adults and the Elderly. Healthcare 2021, 9, 185. [Google Scholar] [CrossRef]
- Lu, M.C.; Fang, W.C.; Li, W.C.; Yeh, W.C.; Shieh, Y.H.; Chen, J.Y. The Association between Insulin Resistance and Cardiovascular Disease Risk: A Community-Based Cross-Sectional Study among Taiwanese People Aged over 50 Years. Int. J. Environ. Res. Public Health 2020, 17, 7195. [Google Scholar] [CrossRef]
- Alosco, M.L.; Spitznagel, M.B.; Cohen, R.; Sweet, L.H.; Josephson, R.; Hughes, J.; Rosneck, J.; Gunstad, J. Obesity and cognitive dysfunction in heart failure: The role of hypertension, type 2 diabetes, and physical fitness. Eur. J. Cardiovasc. Nurs. 2015, 14, 334–341. [Google Scholar] [CrossRef]
- Gil Obando, L.M.; López López, A.; Avila, C.L. Normal values of the maximal respiratory pressures in healthy people older than 20 years old in the City of Manizales—Colombia. Colomb. Med. (Cali) 2012, 43, 119–125. [Google Scholar] [CrossRef]
- Aliverti, A.; Quaranta, M.; Chakrabarti, B.; Albuquerque, A.L.P.; Calverley, P.M. Paradoxical movement of the lower ribcage at rest and during exercise in COPD patients. Eur. Respir. J. 2009, 33, 49–60. [Google Scholar] [CrossRef]
- Cala, S.; Kenyon, C.; Ferrigno, G.; Carnevali, P.; Aliverti, A.; Pedotti, A.; Macklem, P.; Rochester, D. Chest wall and lung volume estimation by optical reflectance motion analysis. J. Appl. Physiol. 1996, 81, 2680–2689. [Google Scholar] [CrossRef]
- Ribeiro, R.; Brandão, D.; Noronha, J.; Lima, C.; Fregonezi, G.; Resqueti, V.; de Andrade, A.D. Breath-stacking and incentive spirometry in Parkinson’s disease: Randomized crossover clinical trial. Respir. Physiol. Neurobiol. 2018, 255, 11–16. [Google Scholar] [CrossRef]
- Bijl, R.C.; Cornette, J.M.; van der Ham, K.; de Zwart, M.L.; Dos Reis Miranda, D.; Steegers-Theunissen, R.P.; Franx, A.; Molinger, J.; Koster, M. The physiological effect of early pregnancy on a woman’s response to a submaximal cardiopulmonary exercise test. Physiol. Rep. 2020, 8, e14624. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.-N.; Zong, H.-Y.; Ou, Y.; Yu, X.; Cheng, H.; Du, C.-P.; He, H.-C. Exoskeleton-assisted walking improves pulmonary function and walking parameters among individuals with spinal cord injury: A randomized controlled pilot study. J. Neuroeng. Rehabil. 2021, 18, 86. [Google Scholar] [CrossRef] [PubMed]
- Mehr, S.A.; Singh, M.; Knox, D.; Ketter, D.M.; Pickens-Jones, D.; Atwood, S.; Lucas, C.; Jacoby, N.; Egner, A.A.; Hopkins, E.J. Universality and diversity in human song. Science 2019, 366, eaax0868. [Google Scholar] [CrossRef]
- Philip, K.E.; Lewis, A.; Buttery, S.C.; McCabe, C.; Manivannan, B.; Fancourt, D.; Orton, C.M.; Polkey, M.I.; Hopkinson, N.S. Physiological demands of singing for lung health compared with treadmill walking. BMJ Open Respir. Res. 2021, 8, e000959. [Google Scholar] [CrossRef]
- Marume, K.; Takashio, S.; Nakanishi, M.; Kumasaka, L.; Fukui, S.; Nakao, K.; Arakawa, T.; Yanase, M.; Noguchi, T.; Yasuda, S. Efficacy of cardiac rehabilitation in heart failure patients with low body mass index. Circ. J. 2019, 83, 334–341. [Google Scholar] [CrossRef]
- Richman, P.S.; Yeung, P.; Bilfinger, T.V.; Yang, J.; Stringer, W.W. Exercise Capacity in Unilateral Diaphragm Paralysis: The Effect of Obesity. Pulm. Med. 2019, 2019, 1090982. [Google Scholar] [CrossRef]
- Miki, K.; Maekura, R.; Kitada, S.; Miki, M.; Yoshimura, K.; Yamamoto, H.; Kawabe, T.; Kagawa, H.; Oshitani, Y.; Satomi, A. Pulmonary rehabilitation for COPD improves exercise time rather than exercise tolerance: Effects and mechanisms. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1061. [Google Scholar] [CrossRef]
- Fuertes, E.; Markevych, I.; Jarvis, D.; Vienneau, D.; de Hoogh, K.; Antó, J.M.; Bowatte, G.; Bono, R.; Corsico, A.G.; Emtner, M. Residential air pollution does not modify the positive association between physical activity and lung function in current smokers in the ECRHS study. Environ. Int. 2018, 120, 364–372. [Google Scholar] [CrossRef]
- Inomoto, A.; Yamato, H.; Michishita, R.; Jiang, Y.; Nishiyama, S.; Fukuda, R.; Deguchi, J. Frequency of exposure to secondhand smoke outside the home is associated with a lower FEV1/FVC in male workers regardless of smoking status. J. UOEH 2019, 41, 15–24. [Google Scholar] [CrossRef]
- Abid, N.; Rao, A.R.; Babar, M.N.; Ansari, M.; Awan, W.A. Effect of deep breathing exercises in healthy smokers: A pilot study. JPMA. J. Pak. Med. Assoc. 2020, 70, 1209–1213. [Google Scholar]
- Fuertes, E.; Carsin, A.-E.; Antó, J.M.; Bono, R.; Corsico, A.G.; Demoly, P.; Gislason, T.; Gullón, J.-A.; Janson, C.; Jarvis, D. Leisure-time vigorous physical activity is associated with better lung function: The prospective ECRHS study. Thorax 2018, 73, 376–384. [Google Scholar] [CrossRef]
- Kervern, M.; Dubois, C.; Naassila, M.; Daoust, M.; Pierrefiche, O. Perinatal alcohol exposure in rat induces long-term depression of respiration after episodic hypoxia. Am. J. Respir. Crit. Care Med. 2009, 179, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Mirrakhimov, A.E. Chronic obstructive pulmonary disease and glucose metabolism: A bitter sweet symphony. Cardiovasc. Diabetol. 2012, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.L.; Meltzer, D.; Carnethon, M.; Krishnan, J.A. Type II diabetes mellitus is associated with decreased measures of lung function in a clinical setting. Respir. Med. 2011, 105, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- van den Borst, B.; Gosker, H.R.; Zeegers, M.P.; Schols, A.M. Pulmonary function in diabetes: A metaanalysis. Chest 2010, 138, 393–406. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Punjabi, N.M.; Wang, N.-Y.; Pankow, J.S.; Duncan, B.B.; Cox, C.E.; Selvin, E.; Brancati, F.L. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: The Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2008, 31, 741–746. [Google Scholar] [CrossRef]
- Lange, P.; Parner, J.; Schnohr, P.; Jensen, G. Copenhagen City Heart Study: Longitudinal analysis of ventilatory capacity in diabetic and nondiabetic adults. Eur. Respir. J. 2002, 20, 1406–1412. [Google Scholar] [CrossRef]
- Lazarus, R.; Sparrow, D.; Weiss, S. Baseline ventilatory function predicts the development of higher levels of fasting insulin and fasting insulin resistance index: The Normative Aging Study. Eur. Respir. J. 1998, 12, 641–645. [Google Scholar] [CrossRef]
- Dieterle, C.D.; Schmauss, S.; Arbogast, H.; Domsch, C.; Huber, R.M.; Landgraf, R. Pulmonary function in patients with type 1 diabetes before and after simultaneous pancreas and kidney transplantation. Transplantation 2007, 83, 566–569. [Google Scholar] [CrossRef]
- Kinney, G.L.; Black-Shinn, J.L.; Wan, E.S.; Make, B.; Regan, E.; Lutz, S.; Soler, X.; Silverman, E.K.; Crapo, J.; Hokanson, J.E. Pulmonary function reduction in diabetes with and without chronic obstructive pulmonary disease. Diabetes Care 2014, 37, 389–395. [Google Scholar] [CrossRef]
- Billings, C.G.; Hurdman, J.A.; Condliffe, R.; Elliot, C.A.; Smith, I.A.; Austin, M.; Armstrong, I.J.; Hamilton, N.; Charalampopoulos, A.; Sabroe, I. Incremental shuttle walk test distance and autonomic dysfunction predict survival in pulmonary arterial hypertension. J. Heart Lung Transplant. 2017, 36, 871–879. [Google Scholar] [CrossRef]
- Jaijee, S.; Quinlan, M.; Tokarczuk, P.; Clemence, M.; Howard, L.S.; Gibbs, J.S.R.; O’Regan, D.P. Exercise cardiac MRI unmasks right ventricular dysfunction in acute hypoxia and chronic pulmonary arterial hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H950–H957. [Google Scholar] [CrossRef] [PubMed]
- Gouzi, F.; Maury, J.; Bughin, F.; Blaquière, M.; Ayoub, B.; Mercier, J.; Perez-Martin, A.; Pomiès, P.; Hayot, M. Impaired training-induced adaptation of blood pressure in COPD patients: Implication of the muscle capillary bed. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2349. [Google Scholar] [CrossRef]
- Sathyaprabha, T.; Kapavarapu, P.; Pal, P.; Thennarasu, K.; Raju, T. Pulmonary functions in Parkinson’s disease. Indian J. Chest Dis. Allied Sci. 2005, 47, 251. [Google Scholar]
- Bosnak-Guclu, M.; Guclu-Gunduz, A.; Nazliel, B.; Irkec, C. Comparison of functional exercise capacity, pulmonary function and respiratory muscle strength in patients with multiple sclerosis with different disability levels and healthy controls. J. Rehabil. Med. 2012, 44, 80–86. [Google Scholar] [CrossRef]
- Reyes, A.; Ziman, M.; Nosaka, K. Respiratory muscle training for respiratory deficits in neurodegenerative disorders: A systematic review. Chest 2013, 143, 1386–1394. [Google Scholar] [CrossRef]
- Pereira, C.; Neder, J.; Tisiologia, S.B.d.P.e. SBPT. Diretrizes para testes de função pulmonar. J. Pneumol. 2002, 29, 207–221. [Google Scholar]
- Sheers, N.L.; O’Sullivan, R.; Howard, M.E.; Berlowitz, D.J. The role of lung volume recruitment therapy in neuromuscular disease: A narrative review. Front. Rehabil. Sci. 2023, 4, 1164628. [Google Scholar] [CrossRef]
- Fumagalli, A.; Misuraca, C.; Bianchi, A.; Borsa, N.; Limonta, S.; Maggiolini, S.; Bonardi, D.R.; Corsonello, A.; Di Rosa, M.; Soraci, L. Pulmonary function in patients surviving to COVID-19 pneumonia. Infection 2021, 49, 153–157. [Google Scholar] [CrossRef]
- Li, X.; Yu, R.; Wang, P.; Wang, A.; Huang, H. Effects of Exercise Training on Cardiopulmonary Function and Quality of Life in Elderly Patients with Pulmonary Fibrosis: A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 7643. [Google Scholar] [CrossRef]
- Dufu, K.; Yalcin, O.; Ao-Ieong, E.S.; Hutchaleelala, A.; Xu, Q.; Li, Z.; Vlahakis, N.; Oksenberg, D.; Lehrer-Graiwer, J.; Cabrales, P. GBT1118, a potent allosteric modifier of hemoglobin O2 affinity, increases tolerance to severe hypoxia in mice. Am. J. Physiol.-Heart Circ. Physiol. 2017, 313, H381–H391. [Google Scholar] [CrossRef]
- Winslow, R.M.; Monge, C.C.; Statham, N.J.; Gibson, C.G.; Charache, S.; Whittembury, J.; Moran, O.; Berger, R.L. Variability of oxygen affinity of blood: Human subjects native to high altitude. J. Appl. Physiol. 1981, 51, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Gersonde, K.; Nicolau, C. Modification of the oxygen affinity of intracellular haemoglobin by incorporation of polyphosphates into intact red blood cells and enhanced O2 release in the capillary system. Surg. Hemotherapy 1980, 46, 81–95. [Google Scholar]
- Perutz, M.F.; Fermi, G.; Abraham, D.J.; Poyart, C.; Bursaux, E. Hemoglobin as a receptor of drugs and peptides: X-ray studies of the stereochemistry of binding. J. Am. Chem. Soc. 1986, 108, 1064–1078. [Google Scholar] [CrossRef]
- Kunert, M.; Liard, J.; Abraham, D.; Lombard, J. Low-Affinity Hemoglobin Increases Tissue PO2 and Decreases Arteriolar Diameter and Flow in the Rat Cremaster Muscle. Microvasc. Res. 1996, 52, 58–68. [Google Scholar] [CrossRef]
- Fuller, A.; Okwose, N.; Scragg, J.; Eggett, C.; Luke, P.; Bandali, A.; Velicki, R.; Greaves, L.; MacGowan, G.A.; Jakovljevic, D.G. The effect of age on mechanisms of exercise tolerance: Reduced arteriovenous oxygen difference causes lower oxygen consumption in older people. Exp. Gerontol. 2021, 149, 111340. [Google Scholar] [CrossRef]
- Houghton, D.; Jones, T.W.; Cassidy, S.; Siervo, M.; MacGowan, G.A.; Trenell, M.I.; Jakovljevic, D.G. The effect of age on the relationship between cardiac and vascular function. Mech. Ageing Dev. 2016, 153, 1–6. [Google Scholar] [CrossRef]
- Carrick-Ranson, G.; Hastings, J.L.; Bhella, P.S.; Shibata, S.; Fujimoto, N.; Palmer, D.; Boyd, K.; Levine, B.D. The effect of age-related differences in body size and composition on cardiovascular determinants of VO2max. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 608–616. [Google Scholar] [CrossRef]
- Ogawa, T.; Spina, R.J.; Martin, W., 3rd; Kohrt, W.M.; Schechtman, K.B.; Holloszy, J.O.; Ehsani, A.A. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 1992, 86, 494–503. [Google Scholar] [CrossRef]
- Milanović, Z.; Pantelić, S.; Trajković, N.; Sporiš, G.; Kostić, R.; James, N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging 2013, 8, 549. [Google Scholar] [CrossRef]
- Rivera, A.M.; Pels, A., 3rd; Sady, S.P.; Sady, M.A.; Cullinane, E.M.; Thompson, P.D. Physiological factors associated with the lower maximal oxygen consumption of master runners. J. Appl. Physiol. 1989, 66, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Kraus, W.E.; Brubaker, P.H.; Kitzman, D.W. Healthy aging and cardiovascular function: Invasive hemodynamics during rest and exercise in 104 healthy volunteers. JACC Heart Fail. 2020, 8, 111–121. [Google Scholar] [CrossRef]
- McGuire, D.K.; Levine, B.D.; Williamson, J.W.; Snell, P.G.; Blomqvist, C.G.; Saltin, B.; Mitchell, J.H. A 30-year follow-up of the Dallas Bed Rest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation 2001, 104, 1350–1357. [Google Scholar] [CrossRef]
- Stratton, J.R.; Levy, W.C.; Cerqueira, M.D.; Schwartz, R.S.; Abrass, I.B. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation 1994, 89, 1648–1655. [Google Scholar] [CrossRef]
- Mai, K.; Klug, L.; Rakova, N.; Piper, S.K.; Mähler, A.; Bobbert, T.; Schulz-Menger, J.; Spranger, J.; Boschmann, M.; Luft, F.C. Hypoxia and exercise interactions on skeletal muscle insulin sensitivity in obese subjects with metabolic syndrome: Results of a randomized controlled trial. Int. J. Obes. 2020, 44, 1119–1128. [Google Scholar] [CrossRef]
- Stewart, G.M.; Chase, S.; Cross, T.J.; Wheatley-Guy, C.M.; Joyner, M.J.; Curry, T.; Lehrer-Graiwer, J.; Dufu, K.; Vlahakis, N.E.; Johnson, B.D. Effects of an allosteric hemoglobin affinity modulator on arterial blood gases and cardiopulmonary responses during normoxic and hypoxic low-intensity exercise. J. Appl. Physiol. 2020, 128, 1467–1476. [Google Scholar] [CrossRef]
- Hobbins, L.; Hunter, S.; Gaoua, N.; Girard, O. Short-Term Perceptually Regulated Interval-Walk Training in Hypoxia and Normoxia in Overweight-to-Obese Adults. J. Sports Sci. Med. 2021, 20, 45. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Marasciulo, F.L.; Montagnani, M.; Potenza, M.A. Endothelin-1: The yin and yang on vascular function. Curr. Med. Chem. 2006, 13, 1655–1665. [Google Scholar] [CrossRef]
- Rizzi, M.; Sergi, M.; Andreoli, A.; Pecis, M.; Bruschi, C.; Fanfulla, F. Environmental tobacco smoke may induce early lung damage in healthy male adolescents. Chest 2004, 125, 1387–1393. [Google Scholar] [CrossRef]
- Rizzi, M.; Tarsia, P.; La Spina, T.; Cristiano, A.; Frassanito, F.; Macaluso, C.; Airoldi, A.; Vanni, S.; Legnani, D. A new approach to detect early lung functional impairment in very light smokers. Respir. Physiol. Neurobiol. 2016, 231, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Lee, H.; Kim, J.H.; Jung, K.; Lim, Y.-H.; Hong, Y.-C. Physical activity-and alcohol-dependent association between air pollution exposure and elevated liver enzyme levels: An elderly panel study. J. Prev. Med. Public Health 2015, 48, 151. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Coast, J.R.; Hempleman, S.C.; Baldi, J.C. Type 1 diabetes duration decreases pulmonary diffusing capacity during exercise. Respiration 2016, 91, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Weynand, B.; Jonckheere, A.; Frans, A.; Rahier, J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration 1999, 66, 14–19. [Google Scholar] [CrossRef]
- Niranjan, V.; McBrayer, D.G.; Ramirez, L.C.; Raskin, P.; Hsia, C.C. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am. J. Med. 1997, 103, 504–513. [Google Scholar] [CrossRef]
- Schneider, S.R.; Mayer, L.C.; Lichtblau, M.; Berlier, C.; Schwarz, E.I.; Saxer, S.; Furian, M.; Bloch, K.E.; Ulrich, S. Effect of normobaric hypoxia on exercise performance in pulmonary hypertension: Randomized trial. Chest 2021, 159, 757–771. [Google Scholar] [CrossRef]
- Afzal, S.; Burge, A.T.; Lee, A.L.; Bondarenko, J.; Holland, A.E. Should the 6-minute walk test be stopped if oxyhemoglobin saturation falls below 80%? Arch. Phys. Med. Rehabil. 2018, 99, 2370–2372. [Google Scholar] [CrossRef]
- Penko, A.L.; Zimmerman, N.M.; Crawford, M.; Linder, S.M.; Alberts, J.L. Effect of Aerobic Exercise on Cardiopulmonary Responses and Predictors of Change in Individuals With Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2021, 102, 925–931. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’Arcangelo, G.; Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. [Google Scholar] [CrossRef]
- Cabral, D.A.; da Costa, K.G.; Okano, A.H.; Elsangedy, H.M.; Rachetti, V.P.; Fontes, E.B. Improving cerebral oxygenation, cognition and autonomic nervous system control of a chronic alcohol abuser through a three-month running program. Addict. Behav. Rep. 2017, 6, 83–89. [Google Scholar] [CrossRef]
- Komatsu, W.R.; Gabbay, M.A.; Castro, M.L.; Saraiva, G.L.; Chacra, A.R.; de Barros Neto, T.L.; Dib, S.A. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr. Diabetes 2005, 6, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, R.; Mankowski, R.T.; van Loon, L.J.; Langendonk, J.G.; Sijbrands, E.J.; van den Meiracker, A.H.; Stam, H.J.; Praet, S.F. Hyperoxia increases arterial oxygen pressure during exercise in type 2 diabetes patients: A feasibility study. Eur. J. Med. Res. 2016, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Skjørten, I.; Hilde, J.M.; Melsom, M.N.; Hansteen, V.; Steine, K.; Humerfelt, S. Pulmonary artery pressure and PaO2 in chronic obstructive pulmonary disease. Respir. Med. 2013, 107, 1271–1279. [Google Scholar] [CrossRef]
- Vitacca, M.; Olivares, A.; Comini, L.; Vezzadini, G.; Langella, A.; Luisa, A.; Petrolati, A.; Frigo, G.; Paneroni, M. Exercise Intolerance and Oxygen Desaturation in Patients with Parkinson’s Disease: Triggers for Respiratory Rehabilitation? Int. J. Environ. Res. Public Health 2021, 18, 12298. [Google Scholar] [CrossRef]
- Lai, P.; Xue, J.H.; Xie, M.J.; Ye, J.H.; Yang, N.; Zhong, Y.M.; Liao, Y.L. High-intensity and moderate-intensity interval training in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Medicine 2023, 102, e33010. [Google Scholar] [CrossRef]
- Costache, A.D.; Maștaleru, A.; Leon, M.M.; Roca, M.; Gavril, R.S.; Cosău, D.E.; Rotundu, A.; Amagdalinei, A.I.; Mitu, O.; Costache Enache, I.I.; et al. High-Intensity Interval Training vs. Medium-Intensity Continuous Training in Cardiac Rehabilitation Programs: A Narrative Review. Medicina 2024, 60, 1875. [Google Scholar] [CrossRef]
- Milani, J.; Milani, M.; Verboven, K.; Cipriano, G., Jr.; Hansen, D. Exercise intensity prescription in cardiovascular rehabilitation: Bridging the gap between best evidence and clinical practice. Front. Cardiovasc. Med. 2024, 11, 1380639. [Google Scholar] [CrossRef]
| Variables | Sample Size (n) | Age in Years | Intervention | VO2max (mL/kg/min) | Study Reference |
|---|---|---|---|---|---|
| Age | 37 | 52.2 ± 7.1 | Control | 27.4 ± 5.7 | [90] |
| 25 | 53.7 ± 5.2 | Low amount/moderate intensity | 29.6 ± 6.9 | ||
| 36 | 52.0 ± 6.9 | Low amount/high intensity | 32.4 ± 6.4 | ||
| 35 | 50.9 ± 5.4 | High amount/high intensity | 34.6 ± 6.1 | ||
| Workplace | 24 | 46 ± 12 | Control | 36.6 ± 9.0 | [91] |
| 30 | 46 ± 9 | Brief exercise intervention program | 37.7 ± 7.5 | ||
| Body Mass Index | 251 | >18 | Corporate exercise intervention program | 43.1 ± 16.5 * | [92] |
| Smokers | 6 | 23.0 ± 1.4 | Taekwondo athletes | 58.4 ± 10.0 | [93] |
| Non-smokers | 9 | 22.4 ± 1.3 | Taekwondo athletes | 62.23 ± 6.1 | |
| Alcoholic | 6 | 25.2 ± 5.5 | High-intensity interval training | 44 ± 10 | [94] |
| Non-alcoholic | 8 | 19.9 ± 2.3 | High-intensity interval training | 41 ± 8 | |
| Diabetes | 34 | 61 ± 12 | Cardiopulmonary Exercise Testing | 15.16 ± 3.82 | [95] |
| Non-diabetes | 97 | 54 ± 17 | Cardiopulmonary exercise testing | 17.46 ± 5.22 | |
| Hypertensive | 53 | 47 ±14 | Cardiopulmonary exercise testing | 13.4 ± 3.6 | [96] |
| Parkinson’s Disease | 23 | 66.1 ± 9.73 | Higher-intensity treadmill exercise | 22.39 ± 0.9 | [97] |
| 22 | 65.8 ± 11.5 | Lower-intensity treadmill exercise | 25.11 ± 1.4 | ||
| 22 | 65.3 ± 11.3 | Stretching and resistance exercises | 22.89 ± 1 | ||
| COVID-19 | 18 | 50 ± 9 | Mild–moderate disease | 22.1 ± 6.3 | [98] |
| 18 | 58 ± 13 | Severe disease | 18.4 ± 5.0 | ||
| 39 | 59 ± 11 | Critical disease | 19.8 ± 5.1 | ||
| Kawasaki disease (Children) | 7 | 9.7 ± 0.5 | At rest | 6.5 ± 0.4 | [65] |
| Treadmill exercise progressive test | 46.1 ± 1.7 | ||||
| Kawasaki disease (Young adults) | 6 | 18.0 ± 1 | At rest | 4.5 ± 0.3 | |
| Treadmill exercise progressive test | 45.5 ± 1.3 |
| Variables | Pulmonary Ventilations | Blood Gas Dynamics |
|---|---|---|
| Age Factor | During exercise testing in pregnant women, minute ventilation increased from 12 L/min to 28 L/min during 1 min of rest [158]. | Maximal arterial–venous oxygen difference (A-VO2 diff) was higher in exercise-trained individuals (19.8 ± 4.0 vs. 17.3 ± 3.7 mL/dL; p = 0.03) [74]. |
| The A-VO2 difference at rest was 5.4 ± 1.7 in young people and 4.3 ± 1.6 in older people; during maximal exercise, it increased to 15.4 ± 2.6 in young people and 10.1 ± 1.8 in older people, indicating that the A-VO2 difference was greater in young people [194]. | ||
| Body Mass Index | After cardiac rehabilitation, the %∆peak VO2 per 1mL/min increase was significantly higher in patients with lower BMI (17.1 ± 2.8% vs. 7.8 ± 1.5%; p < 0.001) [162]. | SaO2 did not change at rest in normoxia, but increased during exercise on day 15 (96.6 ± 0.3% vs. 95.2 ± 0.4%, p < 0.05). In hypoxia, SaO2 increased at rest (90.9 ± 1.8% vs. 82.9 ± 3.4%, p < 0.05) and during exercise (84.8 ± 2.7% vs. 73.6 ± 2.5%, p < 0.01) on day 15. PaO2 increased and PaCO2 decreased after treatment [203]. |
| SaO2 levels during interval walking in individuals with obesity were 83 ± 1% in hypoxia and 96 ± 1% in normoxia, but this difference was not statistically significant (p > 0.05) [204]. | ||
| Smokers | In healthy smokers, deep breathing exercises caused a significant change in FVC, inspiratory capacity, tidal volume, expiratory reserve volume, and FEV1 (p < 0.05) [167]. | Smoking significantly increases the difference in O2 pressure differences (p(A–a′)O2) and arterial ((a′)–end-tidal (et)) carbon dioxide (CO2) pressure differences (p(a′–et)CO2) during rest and peak exercise, while dead space/tidal volume ratios (VD/VT) increase significantly only during exercise [64]. |
| Non-Smokers | After maximal treadmill exercise, endothelin-1 levels were significantly reduced in non-smokers (p < 0.001). Chronic smokers showed fewer exercise-related changes in tidal volume (p = 0.050), fraction of expired CO2 (p = 0.021), oxygen consumption (p = 0.005), CO2 elimination (p = 0.004), and peak expiratory flow (p = 0.003) [63]. | |
| Alcoholic | A 90-day running program in a patient with alcohol addiction increased his running time from 6 to 45 min, with a VO2max increase from 24.2 to 30.1 mL/kg/min) [217]. | Exercise attenuated the increased liver enzyme levels in older people exposed to air pollutants [209]. |
| Diabetes | In an incremental exercise test, patients with type 1 diabetes showed lower aerobic capacity than healthy controls, with reduced VO2 (41.57 ± 7.68 vs. 51.12 ± 9.94 mL/kg/min), lower VE (76.39 ± 19.93 vs. 96.90 ± 25.72 mL/kg/min), and shorter time to exhaustion (8.75 ± 1.60 vs. 10.82 ± 1.44 min) [218]. | During hyperoxic exercise, the oxygen-binding pressure (pO2) in the blood of patients with type 2 diabetes increased significantly (p < 0.05), but there was no change in arterial pCO2 [219]. |
| Hypertensive | In individuals with hypertension, the distance covered in the incremental shuttle walk test was significantly associated with hemodynamic parameters at baseline (p < 0.001). Further, both at baseline (AUC = 0.655; p = 0.004) and at 1 year after initiation of treatment, distance (AUC = 0.737; p < 0.001) could able to predict mortality [178]. | In COPD outpatients, at peak exercise, PaO2 (<8.5 kPa) better predicted mean pulmonary artery pressure and pulmonary hypertension than PaO2 at rest (<9.5 kPa) [220]. |
| Parkinson’s Disease | Respiratory muscle training improved respiratory volumes and lung capacities in patients with Parkinson’s disease and multiple sclerosis [183]. | Patients with Parkinson’s disease show poor exercise tolerance and respiratory muscle weakness and pulmonary function; exercise training improved FVC [221]. |
| COVID-19 | Exercise training in elderly patients with pulmonary fibrosis significantly improved 6 min walk distance by 34.04 m, peak VO2 by 1.13 mL/kg/min, and predicted FVC by 3.94% (d = 0.42, p = 0.01) [187]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korivi, M.; Ghanta, M.K.; Nuthalapati, P.; Natesh, N.S.; Tang, J.; Bhaskar, L. Influence of Exercise on Oxygen Consumption, Pulmonary Ventilation, and Blood Gas Analyses in Individuals with Chronic Diseases. Life 2025, 15, 1255. https://doi.org/10.3390/life15081255
Korivi M, Ghanta MK, Nuthalapati P, Natesh NS, Tang J, Bhaskar L. Influence of Exercise on Oxygen Consumption, Pulmonary Ventilation, and Blood Gas Analyses in Individuals with Chronic Diseases. Life. 2025; 15(8):1255. https://doi.org/10.3390/life15081255
Chicago/Turabian StyleKorivi, Mallikarjuna, Mohan Krishna Ghanta, Poojith Nuthalapati, Nagabhishek Sirpu Natesh, Jingwei Tang, and LVKS Bhaskar. 2025. "Influence of Exercise on Oxygen Consumption, Pulmonary Ventilation, and Blood Gas Analyses in Individuals with Chronic Diseases" Life 15, no. 8: 1255. https://doi.org/10.3390/life15081255
APA StyleKorivi, M., Ghanta, M. K., Nuthalapati, P., Natesh, N. S., Tang, J., & Bhaskar, L. (2025). Influence of Exercise on Oxygen Consumption, Pulmonary Ventilation, and Blood Gas Analyses in Individuals with Chronic Diseases. Life, 15(8), 1255. https://doi.org/10.3390/life15081255







