The Impact of Blood Flow Restriction Training on Glucose and Lipid Metabolism in Overweight or Obese Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Literature Search
2.3. Literature Selection
2.4. Inclusion and Exclusion Criteria
2.5. Literature Screening and Data Extraction
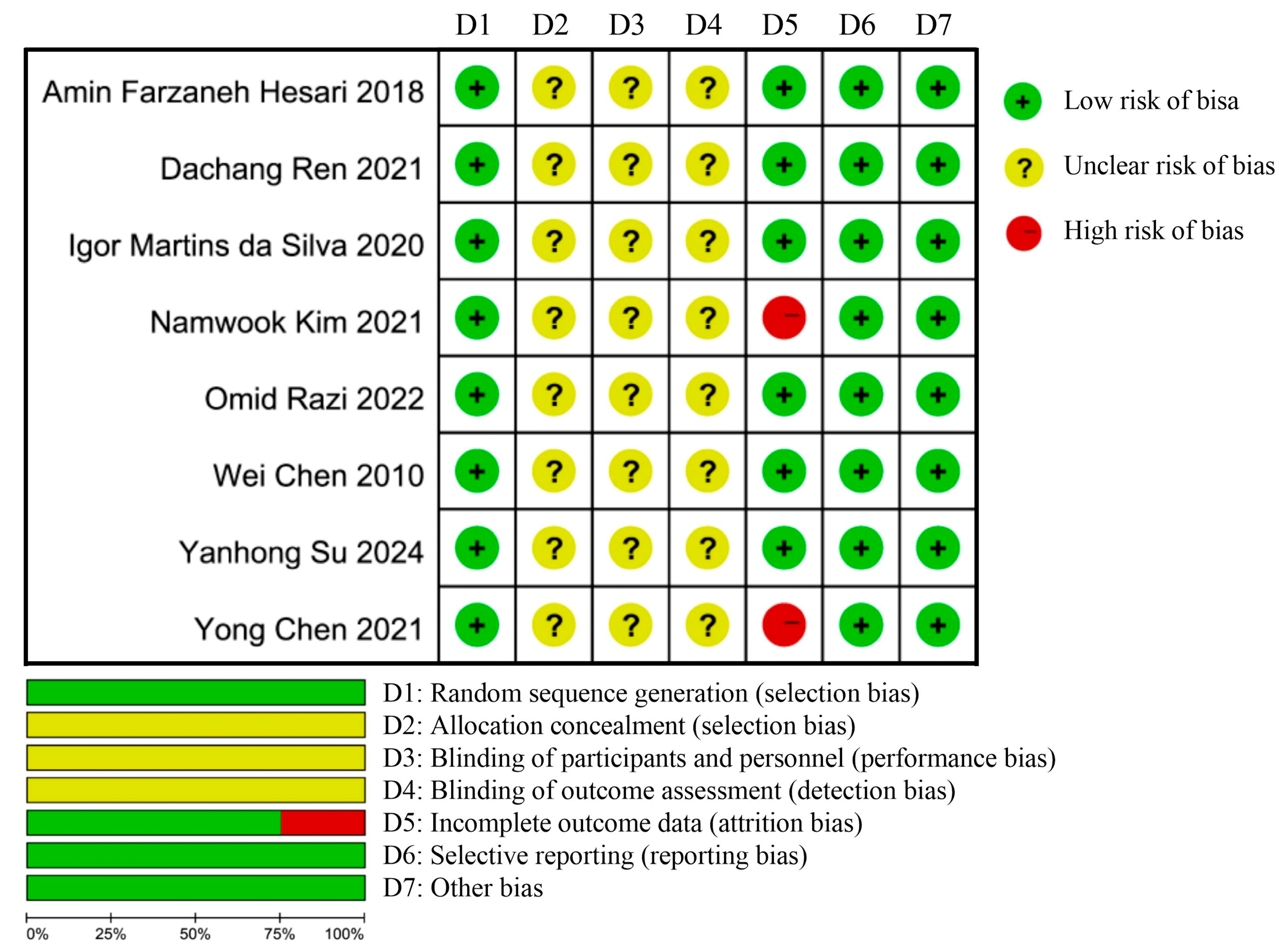
2.6. Risk of Bias Assessment for Included Studies
2.7. Certainty of Evidence
2.8. Statistical Analysis
3. Results
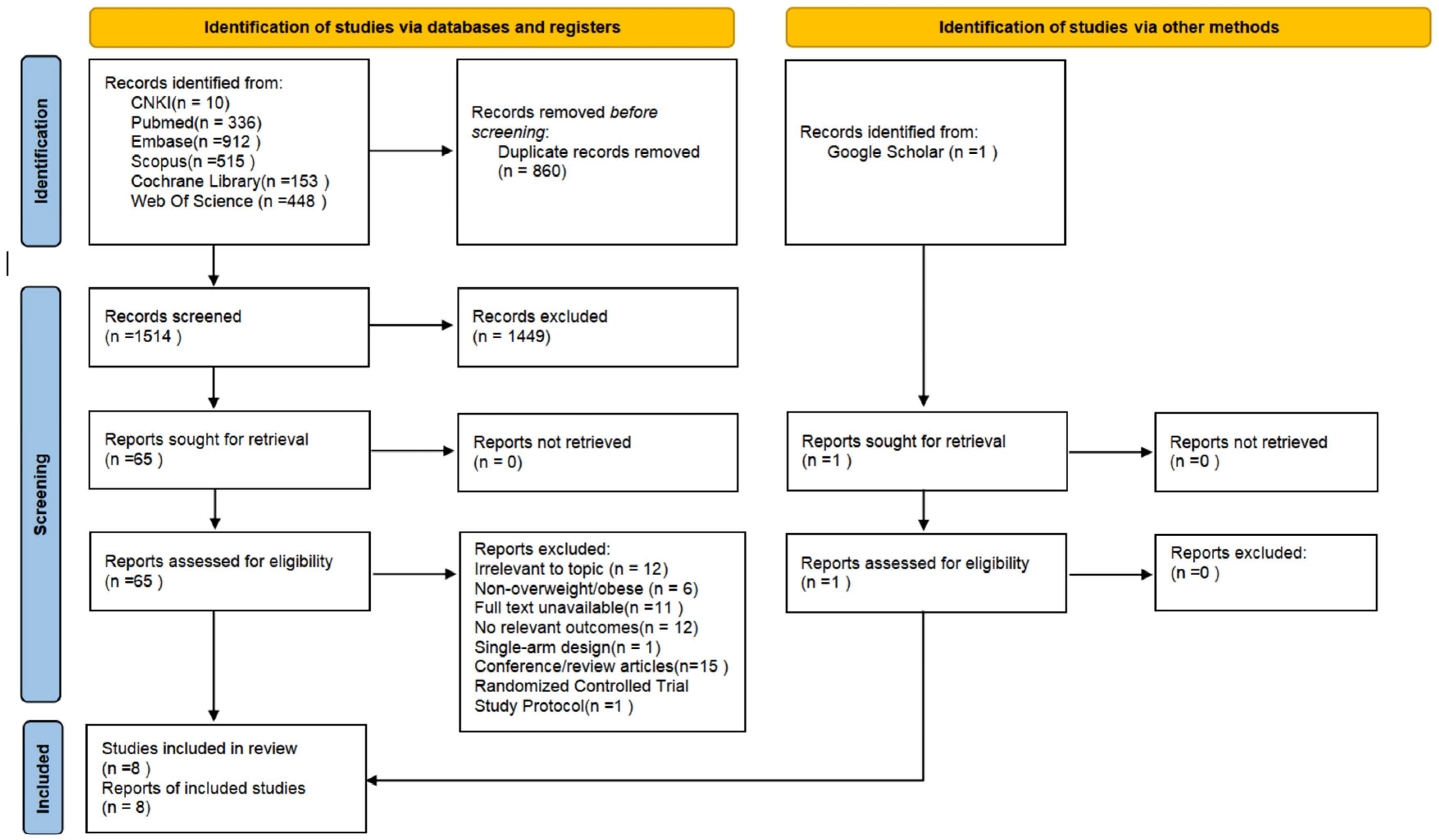
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Risk of Bias Assessment Results
3.4. Meta-Analysis, Certainty of Evidence, and Publication Bias Detection
3.4.1. Glucose Metabolism
- (1)
- Five RCTs were included in the FBG analysis. Compared with GT, BFRT showed significantly greater reductions in FBG (Hedges’ g = −0.77, 95% CI: −1.18 to −0.37; p < 0.01, I2 = 27.09%; GRADE: moderate; Egger’s test: p = 0.5420). Compared with BC, BFR training also demonstrated significant FBG reductions (Hedges’ g = −1.88, 95% CI: −2.47 to −1.29; p < 0.01, I2 = 13.02%; GRADE: moderate; Egger’s test: p = 0.1316).
- (2)
- Three RCTs were included in the HOMA-IR analysis. Compared with conventional training, BFRT significantly improved HOMA-IR (Hedges’ g = −0.69, 95% CI: −1.13 to −0.25; p < 0.01, I2 = 0%; GRADE: moderate; Egger’s test: p = 0.8005). Compared with BC, BFRT showed greater improvements in HOMA−IR (Hedges’ g = −1.33, 95% CI: −1.81 to −0.85; p < 0.01, I2 = 0.00%; GRADE: moderate; Egger’s test: p = 0.2115).
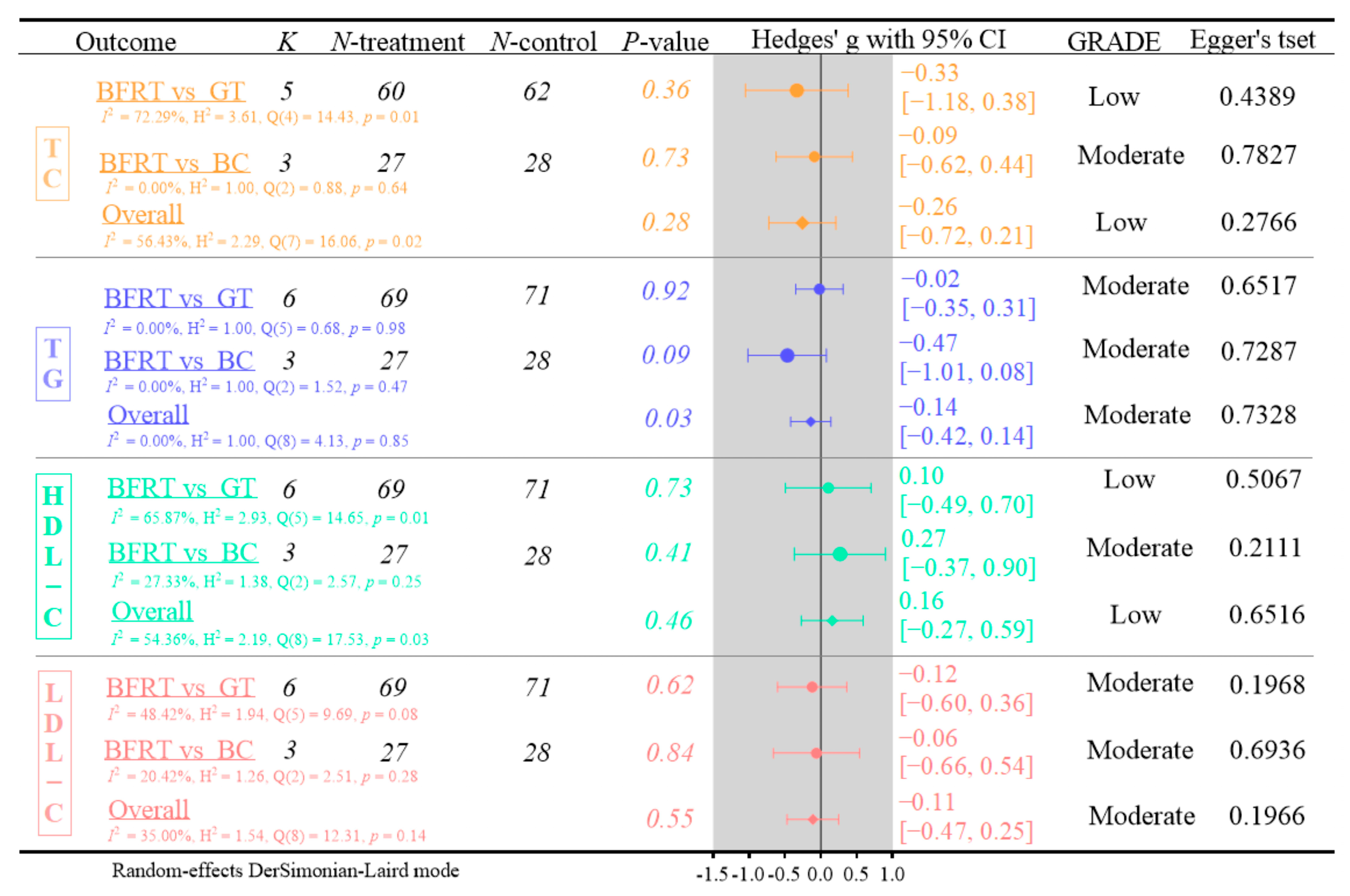
3.4.2. Lipid Metabolism
- (1)
- Five RCTs were included in the TC analysis. BFRT demonstrated no significant effects on TC levels compared to GT or BC (Hedges’ g = −0.26, 95% CI: −0.72 to 0.21; p = 0.28, I2 = 56.43%; GRADE: low; Egger’s test: p = 0.2766).
- (2)
- Six RCTs were included in the TG analysis. Blood flow restriction (BFR) training showed no significant effects on TG levels compared to GT or BC (Hedges’ g = −0.14, 95% CI: −0.42 to 0.14; p = 0.03, I2 = 0.00%; GRADE: moderate; Egger’s test: p = 0.7328).
- (3)
- Six RCTs examined HDL-C levels. This meta-analysis demonstrated no significant effects of BFRT on HDL-C compared to GT or BC (Hedges’ g = 0.16, 95% CI: −0.27 to 0.59; p = 0.46, I2 = 54.36%; GRADE: low; Egger’s test: p = 0.6516).
- (4)
- Six RCTs assessed LDL-C levels. This meta-analysis indicated no significant effects of BFRT on LDL-C concentrations compared to GT or no BC (Hedges’ g = −0.11, 95% CI: −0.47 to 0.25; p = 0.55, I2 = 35%; GRADE: moderate; Egger’s test: p = 0.1966).
3.5. Sensitivity Analysis
3.6. Subgroup Analysis
4. Discussion
4.1. Effects on Glucose Metabolism
4.2. Effects on Lipid Metabolism
4.3. Future Research Directions
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanem, N.; Murray, C.J.L.; Horton, R. The Lancet Commission on 21st-Century Global Health Threats. Lancet 2023, 401, 10–11. [Google Scholar] [CrossRef]
- Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Breen, C.; O’Connell, J.; Geoghegan, J.; O’Shea, D.; Birney, S.; Tully, L.; Gaynor, K.; O’Kelly, M.; O’Malley, G.; O’Donovan, C.; et al. Obesity in Adults: A 2022 Adapted Clinical Practice Guideline for Ireland. Obes. Facts 2022, 15, 736–752. [Google Scholar] [CrossRef] [PubMed]
- Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Powis, J.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for 161 countries. BMJ Glob. Health 2022, 7, e009773. [Google Scholar] [CrossRef] [PubMed]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Clifton, P.M. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 1062–1073. [Google Scholar] [CrossRef]
- Lacy, M.; Atzler, D.; Liu, R.; de Winther, M.; Weber, C.; Lutgens, E. Interactions between dyslipidemia and the immune system and their relevance as putative therapeutic targets in atherosclerosis. Pharmacol. Ther. 2019, 193, 50–62. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Terada, T.; Reed, J.L.; Vidal-Almela, S.; Mistura, M.; Kamiya, K.; Way, K.L. Sex-specific associations of fat mass and muscle mass with cardiovascular disease risk factors in adults with type 2 diabetes living with overweight and obesity: Secondary analysis of the Look AHEAD trial. Cardiovasc. Diabetol. 2022, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Lawman, H.G.; Troiano, R.P.; Perna, F.M.; Wang, C.Y.; Fryar, C.D.; Ogden, C.L. Associations of Relative Handgrip Strength and Cardiovascular Disease Biomarkers in U.S. Adults, 2011–2012. Am. J. Prev. Med. 2016, 50, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Al-Mhanna, S.; Leão, C.; Ghazali, W.; Mohamed, M.; Batrakoulis, A.; Afolabi, H.; Abubakar, B.; Aldhahi, M.; Gülü, M.; Yagin, F.H.; et al. Impact of Exercise on High-Density Lipoprotein Cholesterol in Adults with Overweight and Obesity: A Narrative Review. Ann. Appl. Sport Sci. 2024, 12, e1300. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Jomard, A.; Osto, E. High Density Lipoproteins: Metabolism, Function, and Therapeutic Potential. Front. Cardiovasc. Med. 2020, 7, 39. [Google Scholar] [CrossRef]
- Bressan, J.; de Carvalho Vidigal, F.; Hermsdorff, H.H. Social Components of the Obesity Epidemic. Curr. Obes. Rep. 2012, 2, 32–41. [Google Scholar] [CrossRef][Green Version]
- Wiesner, S.; Haufe, S.; Engeli, S.; Mutschler, H.; Haas, U.; Luft, F.C.; Jordan, J. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity 2010, 18, 116–120. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, L.; Duolikun, D.; Yao, Q. Aerobic Exercise Training Under Normobaric Hypoxic Conditions to Improve Glucose and Lipid Metabolism in Overweight and Obese Individuals: A Systematic Review and Meta-Analysis. High Alt. Med. Biol. 2023, 24, 312–320. [Google Scholar] [CrossRef]
- Oppert, J.M.; Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Carraça, E.V.; Encantado, J.; Ermolao, A.; Pramono, A.; et al. Exercise training in the management of overweight and obesity in adults: Synthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obes. Rev. 2021, 22 (Suppl. S4), e13273. [Google Scholar] [CrossRef]
- Atakan, M.M.; Türkel, İ.; Özerkliğ, B.; Koşar, Ş.N.; Taylor, D.F.; Yan, X.; Bishop, D.J. Small peptides: Could they have a big role in metabolism and the response to exercise? J. Physiol. 2024, 602, 545–568. [Google Scholar] [CrossRef]
- Al-Mhanna, S.B.; Rocha-Rodriguesc, S.; Mohamed, M.; Batrakoulis, A.; Aldhahi, M.I.; Afolabi, H.A.; Yagin, F.H.; Alhussain, M.H.; Gülü, M.; Abubakar, B.D.; et al. Effects of combined aerobic exercise and diet on cardiometabolic health in patients with obesity and type 2 diabetes: A systematic review and meta-analysis. BMC Sports Sci. Med. Rehabil. 2023, 15, 165. [Google Scholar] [CrossRef]
- Wu, T.; Gao, X.; Chen, M.; van Dam, R.M. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: A meta-analysis. Obes. Rev. 2009, 10, 313–323. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Q.; Chen, B.; Chen, J.; Wang, W.; Hu, Y.; Yu, J.; Huang, H. Effects of high-intensity intermittent exercise on glucose and lipid metabolism in type 2 diabetes patients: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1360998. [Google Scholar] [CrossRef]
- Marandi, S.M.; Abadi, N.G.; Esfarjani, F.; Mojtahedi, H.; Ghasemi, G. Effects of intensity of aerobics on body composition and blood lipid profile in obese/overweight females. Int. J. Prev. Med. 2013, 4, S118–S125. [Google Scholar]
- Ahmadi, M.N.; Clare, P.J.; Katzmarzyk, P.T.; Del Pozo Cruz, B.; Lee, I.M.; Stamatakis, E. Vigorous physical activity, incident heart disease, and cancer: How little is enough? Eur. Heart J. 2022, 43, 4801–4814. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, J.H. Does Obesity Affect the Severity of Exercise-Induced Muscle Injury? J. Obes. Metab. Syndr. 2021, 30, 132–140. [Google Scholar] [CrossRef]
- Lorenz, D.S.; Bailey, L.; Wilk, K.E.; Mangine, R.E.; Head, P.; Grindstaff, T.L.; Morrison, S. Blood Flow Restriction Training. J. Athl. Train. 2021, 56, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Clark, B.C. Blood flow restricted exercise and skeletal muscle health. Exerc. Sport Sci. Rev. 2009, 37, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kearns, C.F.; Sato, Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J. Appl. Physiol. 2006, 100, 1460–1466. [Google Scholar] [CrossRef]
- Abe, T.; Loenneke, J.P.; Fahs, C.A.; Rossow, L.M.; Thiebaud, R.S.; Bemben, M.G. Exercise intensity and muscle hypertrophy in blood flow-restricted limbs and non-restricted muscles: A brief review. Clin. Physiol. Funct. Imaging 2012, 32, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Okita, K.; Morita, N.; Yokota, T.; Hirabayashi, K.; Horiuchi, M.; Takada, S.; Takahashi, T.; Omokawa, M.; Kinugawa, S.; et al. Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. J. Appl. Physiol. 2009, 106, 1119–1124. [Google Scholar] [CrossRef]
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with blood flow restriction: An updated evidence-based approach for enhanced muscular development. Sports Med. 2015, 45, 313–325. [Google Scholar] [CrossRef]
- Curty, V.M.; Melo, A.B.; Caldas, L.C.; Guimarães-Ferreira, L.; de Sousa, N.F.; Vassallo, P.F.; Vasquez, E.C.; Barauna, V.G. Blood flow restriction attenuates eccentric exercise-induced muscle damage without perceptual and cardiovascular overload. Clin. Physiol. Funct. Imaging 2018, 38, 468–476. [Google Scholar] [CrossRef]
- de Oliveira, M.F.; Caputo, F.; Corvino, R.B.; Denadai, B.S. Short-term low-intensity blood flow restricted interval training improves both aerobic fitness and muscle strength. Scand. J. Med. Sci. Sports 2016, 26, 1017–1025. [Google Scholar] [CrossRef]
- Ferraz, R.B.; Gualano, B.; Rodrigues, R.; Kurimori, C.O.; Fuller, R.; Lima, F.R.; AL, D.E.S.-P.; Roschel, H. Benefits of Resistance Training with Blood Flow Restriction in Knee Osteoarthritis. Med. Sci. Sports Exerc. 2018, 50, 897–905. [Google Scholar] [CrossRef]
- Picón, M.M.; Chulvi, I.M.; Cortell, J.T.; Tortosa, J.; Alkhadar, Y.; Sanchís, J.; Laurentino, G. Acute Cardiovascular Responses after a Single Bout of Blood Flow Restriction Training. Int. J. Exerc. Sci. 2018, 11, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, C.; Wang, J.; Gu, Y.; Gao, Y. Effects of 40% of Maximum Oxygen Uptake Intensity Cycling Combined with Blood Flow Restriction Training on Body Composition and Serum Biomarkers of Chinese College Students with Obesity. Int. J. Environ. Res. Public Health 2021, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Wernbom, M.; Järrebring, R.; Andreasson, M.A.; Augustsson, J. Acute effects of blood flow restriction on muscle activity and endurance during fatiguing dynamic knee extensions at low load. J. Strength Cond. Res. 2009, 23, 2389–2395. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Abe, T.; Wilson, J.M.; Thiebaud, R.S.; Fahs, C.A.; Rossow, L.M.; Bemben, M.G. Blood flow restriction: An evidence based progressive model (Review). Acta Physiol. Hung. 2012, 99, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Drevon, D.; Fursa, S.R.; Malcolm, A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017, 41, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H. Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group); Cochrane Statistical Methods Group. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In Cochrane Handbook for Systematic Reviews of Interventio; Wiley: Hoboken, NJ, USA, 2019; pp. 375–402. [Google Scholar]
- Chen, W.; Li, J.; Chen, Q.; Shang, N.; Yang, H.; Gao, L. The Effect of Resistance Exercise Combined with Restriction of Blood Flow on the Body Composition and Insulin Sensitivity in Obese Subjects. Chin. J. Sports Med. 2010, 29, 646–649. [Google Scholar] [CrossRef]
- Farzaneh Hesari, A.; Ebrahimi, A.; Zanjani, M.; Mahdavi, S. Effects of Resistance Training with and without Blood Flow Restriction on Cardiovascular Risk Factors in Overweight Females. Med. Lab. J. 2018, 12, 31–36. [Google Scholar] [CrossRef]
- da Silva, I.M.; Santos, M.A.; Galvão, S.L.; Dorneles, G.P.; Lira, F.S.; Romão, P.R.T.; Peres, A. Blood flow restriction impairs the inflammatory adaptations of strength training in overweight men: A clinical randomized trial. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2020, 45, 659–666. [Google Scholar] [CrossRef]
- Razi, O.; Mohammadi, M.; Zamani, N.; Hackney, A.C.; Tourny, C.; Zouita, S.; Laher, I.; Zouhal, H. Walking exercise and lower-body blood flow restriction: Effects on systemic inflammation, lipid profiles and hematological indices in overweight middle-aged males. Res. Sports Med. 2022, 30, 41–49. [Google Scholar] [CrossRef]
- Ren, D.; LI, A.; Xia, S.; Zhang, F.; Li, G. Impacts of blood flow restriction of motor muscles on body composition and insulin sensitivity of obese people in low-load resistance training. China Med. Pharm. 2021, 11, 151–154. [Google Scholar]
- Kim, N.; Lee, D.; Lee, S. Effects of 5 Week Low-Intensity Blood Flow Restriction Resistance Exercise and Moderate-Intensity Resistance Exercise on Body Composition and Blood Lipids in Normal Weight Obese Women. Exerc. Sci. 2021, 30, 70–79. [Google Scholar] [CrossRef]
- Su, Y.; Wang, F.; Wang, M.; He, S.; Yang, X.; Luan, Z. Effects of blood flow restriction training on muscle fitness and cardiovascular risk of obese college students. Front. Physiol. 2023, 14, 1252052. [Google Scholar] [CrossRef]
- Li, X.; Chen, H. Characteristics of glucolipid metabolism and complications in novel cluster-based diabetes subgroups: A retrospective study. Lipids Health Dis. 2023, 22, 200. [Google Scholar] [CrossRef]
- Gordon, B.A.; Taylor, C.J.; Church, J.E.; Cousins, S.D. A Comparison of the Gluco-Regulatory Responses to High-Intensity Interval Exercise and Resistance Exercise. Int. J. Environ. Res. Public Health 2021, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Şahin, E.; Ayaz, T.; Saglam, M. Acute effects of blood flow restricted aerobic exercise in type 2 diabetes mellitus. Medicine 2024, 103, e39031. [Google Scholar] [CrossRef]
- Wormgoor, S.G.; Dalleck, L.C.; Zinn, C.; Harris, N.K. Effects of High-Intensity Interval Training on People Living with Type 2 Diabetes: A Narrative Review. Can. J. Diabetes 2017, 41, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Brellenthin, A.G.; Lanningham-Foster, L.M.; Kohut, M.L.; Li, Y. Aerobic, resistance, or combined exercise training and cardiovascular risk profile in overweight or obese adults: The CardioRACE trial. Eur. Heart J. 2024, 45, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Sigal, R.J.; Kenny, G.P.; Boulé, N.G.; Wells, G.A.; Prud’homme, D.; Fortier, M.; Reid, R.D.; Tulloch, H.; Coyle, D.; Phillips, P.; et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 357–369. [Google Scholar] [CrossRef]
- McGuigan, M.R.; Egan, A.D.; Foster, C. Salivary Cortisol Responses and Perceived Exertion during High Intensity and Low Intensity Bouts of Resistance Exercise. J. Sports Sci. Med. 2004, 3, 8–15. [Google Scholar]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Balaa, N.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Resistance versus aerobic exercise: Acute effects on glycemia in type 1 diabetes. Diabetes Care 2013, 36, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, S.M.; Golmohammadi, M.; Eslami, R.; Shakiba, N.; Costa, P.B. The Effects of Eight Weeks of Circuit Resistance Training on Serum METRNL Levels and Insulin Resistance in Individuals with Type 2 Diabetes. J. Diabetes Metab. Disord. 2023, 22, 1151–1158. [Google Scholar] [CrossRef]
- Al-Mhanna, S.; Syaheedah, W.; Ghazali, W.; Batrakoulis, A.; Alkhamees, N.; Drenowatz, C.; Mohamed, M.; Gülü, M.; Afolabi, H.; Badicu, G. Impact of Various Types of Exercise on Lipid Metabolism in Patients with Type 2 Diabetes and Concurrent Overweight/Obesity: A Narrative Review. Ann. Appl. Sport Sci. 2024, 12, e1324. [Google Scholar] [CrossRef]
- Ostman, C.; Smart, N.A.; Morcos, D.; Duller, A.; Ridley, W.; Jewiss, D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 110. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Hua, G.; Guo, C.; Gong, S.; Li, M.; Yang, Y. Exercise training modalities in prediabetes: A systematic review and network meta-analysis. Front. Endocrinol. 2024, 15, 1308959. [Google Scholar] [CrossRef]
- Kambič, T.; Novaković, M.; Tomažin, K.; Strojnik, V.; Jug, B. Blood Flow Restriction Resistance Exercise Improves Muscle Strength and Hemodynamics, but Not Vascular Function in Coronary Artery Disease Patients: A Pilot Randomized Controlled Trial. Front. Physiol. 2019, 10, 656. [Google Scholar] [CrossRef]
- Williams, M.A.; Haskell, W.L.; Ades, P.A.; Amsterdam, E.A.; Bittner, V.; Franklin, B.A.; Gulanick, M.; Laing, S.T.; Stewart, K.J. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: A scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007, 116, 572–584. [Google Scholar] [CrossRef]
- Jorge, M.L.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S.; et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2011, 60, 1244–1252. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Fotiadis, G.; Kapelouzou, A.; Kostakis, A.; Liapis, C.D.; Vrabas, I.S. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet. Med. A J. Br. Diabet. Assoc. 2013, 30, e41–e50. [Google Scholar] [CrossRef]
- Cauza, E.; Hanusch-Enserer, U.; Strasser, B.; Ludvik, B.; Metz-Schimmerl, S.; Pacini, G.; Wagner, O.; Georg, P.; Prager, R.; Kostner, K.; et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch. Phys. Med. Rehabil. 2005, 86, 1527–1533. [Google Scholar] [CrossRef]
- Ruschke, K.; Fishbein, L.; Dietrich, A.; Klöting, N.; Tönjes, A.; Oberbach, A.; Fasshauer, M.; Jenkner, J.; Schön, M.R.; Stumvoll, M.; et al. Gene expression of PPARgamma and PGC-1alpha in human omental and subcutaneous adipose tissues is related to insulin resistance markers and mediates beneficial effects of physical training. Eur. J. Endocrinol. 2010, 162, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Durstine, J.L.; Grandjean, P.W.; Davis, P.G.; Ferguson, M.A.; Alderson, N.L.; DuBose, K.D. Blood lipid and lipoprotein adaptations to exercise: A quantitative analysis. Sports Med. 2001, 31, 1033–1062. [Google Scholar] [CrossRef] [PubMed]
- Halverstadt, A.; Phares, D.A.; Wilund, K.R.; Goldberg, A.P.; Hagberg, J.M. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metab. Clin. Exp. 2007, 56, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Xie, Y.; Zhang, Y.; Zhang, R.; Zeng, D.; Yue, X. Effect of different training modalities on lipid metabolism in patients with type ii diabetes mellitus: A network meta-analysis. Ann. Med. 2024, 56, 2428432. [Google Scholar] [CrossRef]
- Thomas, D.E.; Elliott, E.J.; Naughton, G.A. Exercise for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2006, 2006, Cd002968. [Google Scholar] [CrossRef]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.; Chen, S.; Durstine, J.L. The effects of exercise training on the traditional lipid profile and beyond. Curr. Sports Med. Rep. 2014, 13, 253–259. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Vu Tran, Z. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: A meta-analysis of randomized controlled trials. Int. J. Obes. 2005, 29, 881–893. [Google Scholar] [CrossRef]
- Hansen, D.; Dendale, P.; Jonkers, R.A.; Beelen, M.; Manders, R.J.; Corluy, L.; Mullens, A.; Berger, J.; Meeusen, R.; van Loon, L.J. Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA(1c) in obese type 2 diabetes patients. Diabetologia 2009, 52, 1789–1797. [Google Scholar] [CrossRef]
- Racil, G.; Ben Ounis, O.; Hammouda, O.; Kallel, A.; Zouhal, H.; Chamari, K.; Amri, M. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur. J. Appl. Physiol. 2013, 113, 2531–2540. [Google Scholar] [CrossRef]
- Drew, B.G.; Rye, K.A.; Duffy, S.J.; Barter, P.; Kingwell, B.A. The emerging role of HDL in glucose metabolism. Nat. Rev. Endocrinol. 2012, 8, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Pollock, M.L.; Franklin, B.A.; Balady, G.J.; Chaitman, B.L.; Fleg, J.L.; Fletcher, B.; Limacher, M.; Piña, I.L.; Stein, R.A.; Williams, M.; et al. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: Benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation 2000, 101, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Yurgalevitch, S.M.; Flynn, M.M.; Zmuda, J.M.; Spannaus-Martin, D.; Saritelli, A.; Bausserman, L.; Herbert, P.N. Effect of prolonged exercise training without weight loss on high-density lipoprotein metabolism in overweight men. Metab. Clin. Exp. 1997, 46, 217–223. [Google Scholar] [CrossRef]
- Bemben, D.; Bemben, M. Effects of Resistance Exercise and Body Mass Index on Lipoprotein-Lipid Patterns of Postmenopausal Women. J. Strength Cond. Res. 2000, 14, 80–85. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S. Impact of progressive resistance training on lipids and lipoproteins in adults: A meta-analysis of randomized controlled trials. Prev. Med. 2009, 48, 9–19. [Google Scholar] [CrossRef]
- Tambalis, K.; Panagiotakos, D.B.; Kavouras, S.A.; Sidossis, L.S. Responses of blood lipids to aerobic, resistance, and combined aerobic with resistance exercise training: A systematic review of current evidence. Angiology 2009, 60, 614–632. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Durstine, J.L.; Grandjean, P.W.; Cox, C.A.; Thompson, P.D. Lipids, lipoproteins, and exercise. J. Cardiopulm. Rehabil. 2002, 22, 385–398. [Google Scholar] [CrossRef]
| Study | Participant Characteristics | BFR Details | Protocols | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (M ± SD)/year | BMI (M ± SD)/kg/m2 | M/F | N | Position | Pressure | Intervention | Intensity | Sets × Reps or Span | Interval | Frequency | Duration (weeks) | ||
| 1 Chen (2010a) [43] | BFR: 21.9 ± 2.07 GT: 21.6 ± 1.71 | 29.45 ± 1.61 29.57 ± 1.42 | 13/0 | 7 | PT, PUL | 120 mmHg | LI-RT | 20% 1 RM | 4 × 12 | 2 min | 3 t/wk | 18 | FBG, HOMA-IR |
| 1 Chen (2010b) [43] | BFR: 21.9 ± 2.07 BC: 21.1 ± 1.66 | 29.45 ± 1.61 29.83 ± 1.83 | 13/0 | 7 | |||||||||
| 2 Farzaneh Hesari et al. (2018a) [44] | 18–24 | BFR: 27.8 ± 3 GT: 26.4 ± 4 | 0/18 | 9 | PT, PUL | <70% AOP | LI-RT | 30% 1 RM | 1 × 30 + 3 × 15 | 1–4 min | 3 t/wk | 8 | TG, TC, HDL-C, LDL-C |
| 2 Farzaneh Hesari et al. (2018b) [44] | BFR: 27.8 ± 3 BC: 26.7 ± 2 | 0/19 | 9 | ||||||||||
| 3 da Silva IM et al. (2020) [45] | BFR: 25.52 ± 2.19 GT: 24.46 ± 2.56 | 28.45 ± 2.35 28.68 ± 1.76 | 15/0 15/0 | 15 | PT, PUL | 20–40 mmHg | LI-RT | 30% 1 RM | 4 × 23 | 2 min | 3 t/wk | 8 | TG, TC, HDL-C, LDL-C, FBG |
| 4 Razi O et al. (2022) [46] | BFR: 44.77 ± 5.09 GT: 42.77 ± 6.35 | 27.63 ± 1.2 28.36 ± 1.37 | 9/0 9/0 | 9 | PT, PUL | 140–200 mmHg | LI-AT | 50 m/min | 5 × 2 min | 1 min | 3 t/wk | 8 | TG, HDL-C, LDL-C |
| 5 Chen Y et al. (2021) [37] | BFR: 20.3 ± 1.07 GT: 20.1 ± 1.08 | 30.1 ± 0.95 30.3 ± 1.08 | 18/0 19/0 | 18 | PT | 160–200 mmHg | LI-AT | 40% VO2max | 3 × 15 min | 1 min | 2 t/wk | 12 | FBG, TC, TG, HDL-C, LDL-C |
| 6 Ren (2021a) [47] | BFR: 23.77 ± 2.14 CON: 23.73 ± 2.17 | 30.88 ± 0.26 30.75 ± 0.23 | 17/11 16/10 | 28 | PT | 120–220 mmHg | LI-RT | 20–30% 1 RM | 10 × (3–4) | 2 min | 2 t/wk | 12 | FBG, HOMA-1 R |
| 6 Ren (2021b) [47] | BFR: 23.77 ± 2.14 CON: 23.75 ± 2.16 | 30.88 ± 0.26 30.89 ± 0.24 | 17/11 15/11 | 28 | PT | ||||||||
| 7 Kim Namwook et al. (2021a) [48] | BFR: 22.3 ± 1.0 GT: 21.5 ± 0.8 | met the criteria forormal weight obesity (NWO) | 0/9 0/10 | 9 | PT, PUL | n/a | LI-RT | 40%1 RM | 3 × (14–18) | n/a | 2 t/wk | 5 | TC, TG, HDL-C, LDL-C |
| 7 Kim Namwook et al. (2021b) [48] | BFR: 22.3 ± 1.0 BC: 21.9 ± 0.5 | 0/9 0/10 | 9 | ||||||||||
| 8 Su Y et al. (2023a) [49] | 20–24 | met the criteria forormal weight obesity (NWO) | BFR: 9 GT: 9 | 9 | PT | 140–200 mmHg | LI-RT | 30%1 RM | 4 × (15–30) | 30–60 s | 5 t/wk | 12 | FBG, HOMA-IR TC, TG, HDL-C, LDL-C |
| 8 Su Y (2023b) [49] | BFR: 9 BC: 8 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Liu, P.; Deng, Y.; Cai, H.; Liang, P.; Jiang, X. The Impact of Blood Flow Restriction Training on Glucose and Lipid Metabolism in Overweight or Obese Adults: A Systematic Review and Meta-Analysis. Life 2025, 15, 1245. https://doi.org/10.3390/life15081245
Chen H, Liu P, Deng Y, Cai H, Liang P, Jiang X. The Impact of Blood Flow Restriction Training on Glucose and Lipid Metabolism in Overweight or Obese Adults: A Systematic Review and Meta-Analysis. Life. 2025; 15(8):1245. https://doi.org/10.3390/life15081245
Chicago/Turabian StyleChen, Hao, Peng Liu, Yidi Deng, Haibo Cai, Pu Liang, and Xin Jiang. 2025. "The Impact of Blood Flow Restriction Training on Glucose and Lipid Metabolism in Overweight or Obese Adults: A Systematic Review and Meta-Analysis" Life 15, no. 8: 1245. https://doi.org/10.3390/life15081245
APA StyleChen, H., Liu, P., Deng, Y., Cai, H., Liang, P., & Jiang, X. (2025). The Impact of Blood Flow Restriction Training on Glucose and Lipid Metabolism in Overweight or Obese Adults: A Systematic Review and Meta-Analysis. Life, 15(8), 1245. https://doi.org/10.3390/life15081245