Mediating Impact of Intranasal Oxytocin on the Interaction Between Irritability and Reactive Aggression in Youth with Severe Irritability
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Screening/Assessment
2.3. Double-Blind, Randomized Intranasal Oxytocin Administration
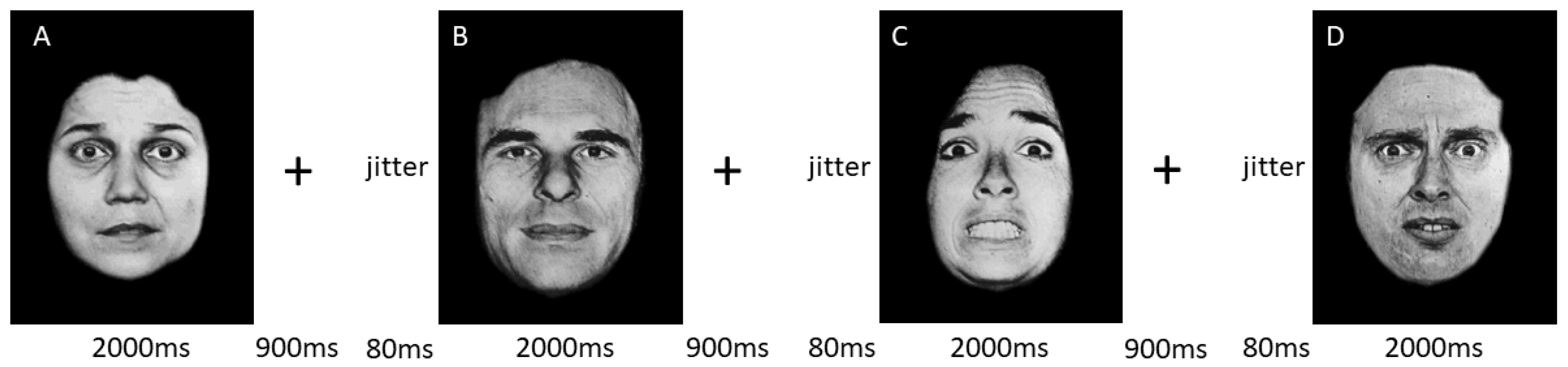
2.4. Functional MRI Experimental Design
2.5. Functional MRI Scans
2.6. Image Acquisition
2.7. Imaging Data Preprocessing
2.8. Statistical Analysis
2.8.1. Statistical Analysis of Clinical Data
2.8.2. Behavioral Data
2.8.3. MRI Data
2.9. Mediation Analysis
3. Results
3.1. Clinical Characteristics
3.2. Symptom Profile Changes
3.3. Behavioral Data
3.4. MRI Data
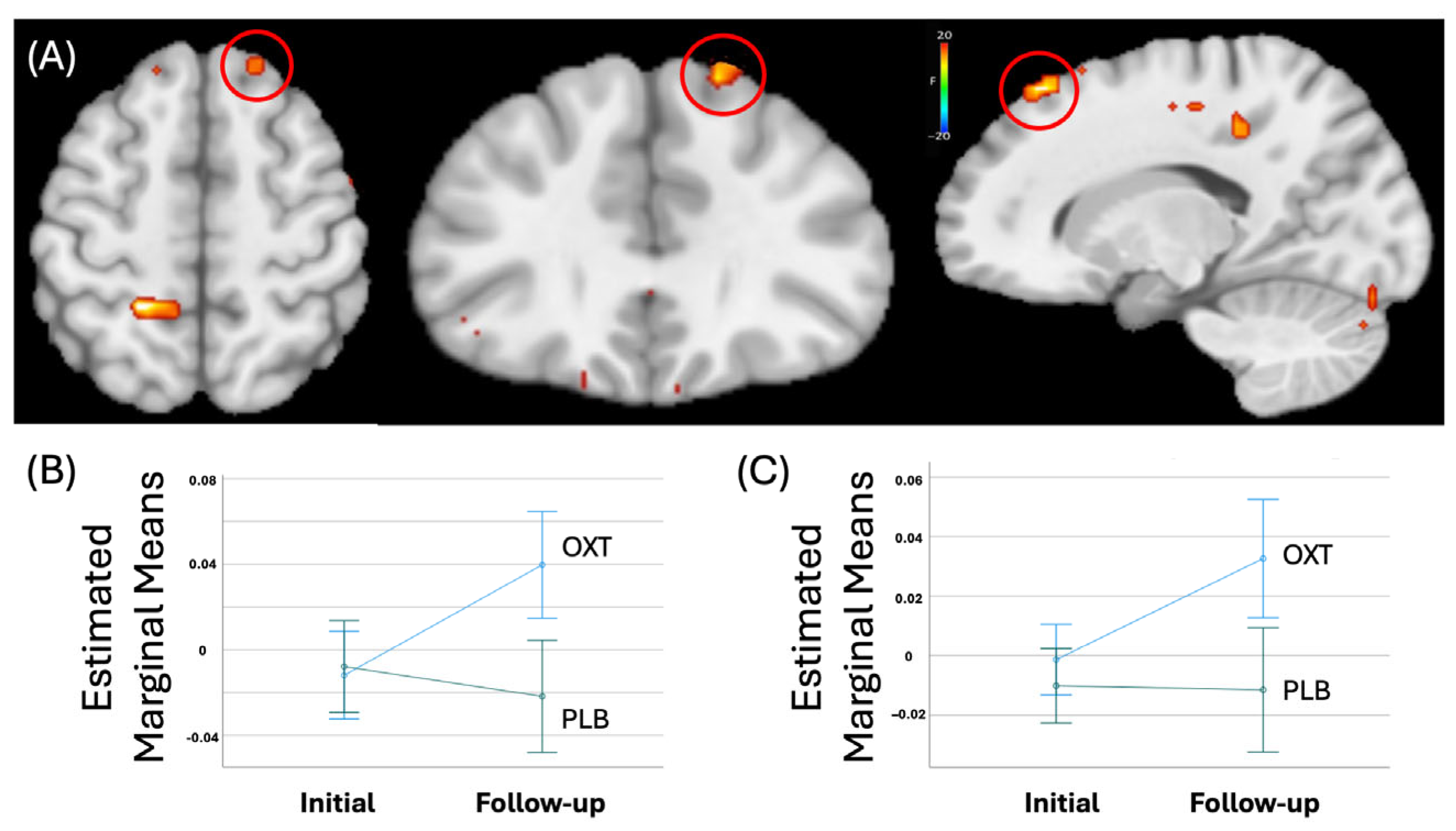
3.4.1. Whole-Brain Analysis
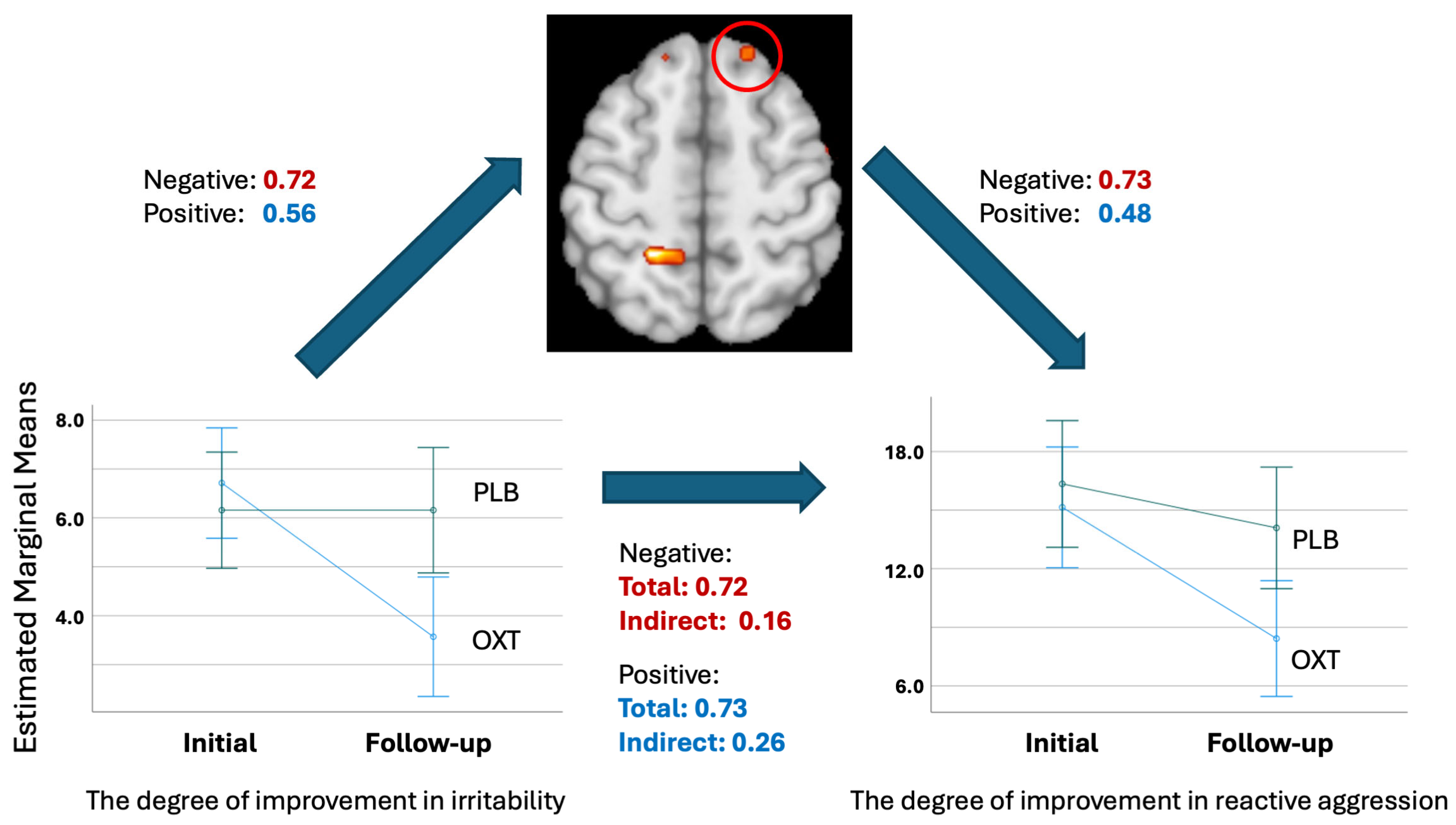
3.4.2. Mediation Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuhrmann, D.; Knoll, L.J.; Blakemore, S.J. Adolescence as a Sensitive Period of Brain Development. Trends Cogn. Sci. 2015, 19, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Lasalvia, A.; Ruggeri, M. Prevention and early intervention in youth mental health: Is it time for a multidisciplinary and trans-diagnostic model for care? Int. J. Ment. Health Syst. 2020, 14, 23. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual 5; Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Barata, P.C.; Holtzman, S.; Cunningham, S.; O’Connor, B.P.; Stewart, D.E. Building a Definition of Irritability from Academic Definitions and Lay Descriptions. Emot. Rev. 2016, 8, 164–172. [Google Scholar] [CrossRef]
- Silver, J.; Hawes, M.T.; Carlson, G.A.; Klein, D.N. Irritability: Associations with real-time affect dynamics, social interactions, and daily substance use in older adolescents. Eur. Child Adolesc. Psychiatry 2025, 34, 623–632. [Google Scholar] [CrossRef]
- Deveney, C.M.; Stoddard, J.; Evans, R.; Chavez, G.; Harney, M.; Wulff, R. On Defining Irritability and its Relationship to Affective Traits and Social Interpretations. Pers. Individ. Differ. 2019, 144, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.S.; Zhang, H.; Santa Lucia, R.; King, R.A.; Lewis, M. Risk factors for presenting problems in child psychiatric emergencies. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1162–1173. [Google Scholar] [CrossRef]
- Collishaw, S.; Maughan, B.; Natarajan, L.; Pickles, A. Trends in adolescent emotional problems in England: A comparison of two national cohorts twenty years apart. J. Child Psychol. Psychiatry Allied Discip. 2010, 51, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Bushman, B.J.; Anderson, C.A. Is it time to pull the plug on the hostile versus instrumental aggression dichotomy? Psychol. Rev. 2001, 108, 273–279. [Google Scholar] [CrossRef]
- Rosan, A.M.; Costea-Barlutiu, C. Associations between Callous-Unemotional Traits, Aggression, and Psychopathology in Detailed Adolescent Males. J. Cogn. Behav. Psychother. 2013, 13, 397–407. [Google Scholar]
- Hudley, C.; Friday, J. Attributional bias and reactive aggression. Am. J. Prev. Med. 1996, 12, 75–81. [Google Scholar] [CrossRef]
- Perhamus, G.R.; Ostrov, J.M. Inhibitory Control in Early Childhood Aggression Subtypes: Mediation by Irritability. Child Psychiatry Hum. Dev. 2023, 54, 366–378. [Google Scholar] [CrossRef]
- Sukhodolsky, D.G.; Smith, S.D.; McCauley, S.A.; Ibrahim, K.; Piasecka, J.B. Behavioral Interventions for Anger, Irritability, and Aggression in Children and Adolescents. J. Child Adolesc. Psychopharmacol. 2016, 26, 58–64. [Google Scholar] [CrossRef]
- Kumsta, R.; Heinrichs, M. Oxytocin, stress and social behavior: Neurogenetics of the human oxytocin system. Curr. Opin. Neurobiol. 2013, 23, 11–16. [Google Scholar] [CrossRef]
- Fragkaki, I.; Cima, M.; Granic, I. The role of trauma in the hormonal interplay of cortisol, testosterone, and oxytocin in adolescent aggression. Psychoneuroendocrinology 2018, 88, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010, 65, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Tillman, R.; Gordon, I.; Naples, A.; Rolison, M.; Leckman, J.F.; Feldman, R.; Pelphrey, K.A.; McPartland, J.C. Oxytocin Enhances the Neural Efficiency of Social Perception. Front. Hum. Neurosci. 2019, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, K.M.; Guastella, A.J.; Becker, B. Overview of Human Oxytocin Research. Curr. Top Behav. Neurosci. 2018, 35, 321–348. [Google Scholar]
- Froemke, R.C.; Young, L.J. Oxytocin, Neural Plasticity, and Social Behavior. Annu. Rev. Neurosci. 2021, 44, 359–381. [Google Scholar] [CrossRef]
- Herba, C.; Phillips, M. Annotation: Development of facial expression recognition from childhood to adolescence: Behavioural and neurological perspectives. J. Child Psychol. Psychiatry 2004, 45, 1185–1198. [Google Scholar] [CrossRef]
- Naim, R.; Haller, S.P.; Linke, J.O.; Jaffe, A.; Stoddard, J.; Jones, M.; Harrewijn, A.; Kircanski, K.; Bar-Haim, Y.; Brotman, M.A. Context-dependent amygdala-prefrontal connectivity during the dot-probe task varies by irritability and attention bias to angry faces. Neuropsychopharmacology 2022, 47, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.A.; Rossell, S.L.; Heinrichs, M.; Kordsachia, C.; Labuschagne, I. Oxytocin and brain activity in humans: A systematic review and coordinate-based meta-analysis of functional MRI studies. Psychoneuroendocrinology 2018, 96, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Sydnor, V.J.; Larsen, B.; Bassett, D.S.; Alexander-Bloch, A.; Fair, D.A.; Liston, C.; Mackey, A.P.; Milham, M.P.; Pines, A.; Roalf, D.R.; et al. Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology. Neuron 2021, 109, 2820–2846. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Bunge, S.A.; Gross, J.J.; Gabrieli, J.D. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002, 14, 1215–1229. [Google Scholar] [CrossRef]
- Crone, E.A.; Dahl, R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012, 13, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Sheskier, M.B.; Farokhnia, M.; Feng, N.; Marenco, S.; Lipska, B.K.; Leggio, L. Oxytocin receptor mRNA expression in dorsolateral prefrontal cortex in major psychiatric disorders: A human post-mortem study. Psychoneuroendocrinology 2018, 96, 143–147. [Google Scholar] [CrossRef]
- Towbin, K.; Vidal-Ribas, P.; Brotman, M.A.; Pickles, A.; Miller, K.V.; Kaiser, A.; Vitale, A.D.; Engel, C.; Overman, G.P.; Davis, M.; et al. A Double-Blind Randomized Placebo-Controlled Trial of Citalopram Adjunctive to Stimulant Medication in Youth with Chronic Severe Irritability. J. Am. Acad. Child Adolesc. Psychiatry 2019, 59, 350–361. [Google Scholar] [CrossRef]
- Hwang, S.; Chung, U.; Chang, Y.; Kim, E.; Suk, J.W.; Meffert, H.; Kratochvil, C.; Leibenluft, E.; Blair, J. Neural Responses to Fluoxetine in Youths with Disruptive Behavior and Trauma Exposure: A Pilot Study. J. Child Adolesc. Psychopharmacol. 2021, 31, 562–571. [Google Scholar] [CrossRef]
- Hwang, S.; Suk, J.W.; Meffert, H.; Lerdahl, A.; Garvey, W.F.; Edwards, R.; Delizza, A.; Soltis-Vaughan, B.; Cordts, K.; Leibenluft, E.; et al. Neural Responses to Intranasal Oxytocin in Youths with Severe Irritability. Am. J. Psychiatry 2024, 181, 291–298. [Google Scholar] [CrossRef]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Stringaris, A.; Goodman, R.; Ferdinando, S.; Razdan, V.; Muhrer, E.; Leibenluft, E.; Brotman, M.A. The Affective Reactivity Index: A concise irritability scale for clinical and research settings. J. Child Psychol. Psychiatry 2012, 53, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence, 2nd ed.; WASI-II; Pearson: San Antonio, TX, USA, 2011. [Google Scholar]
- Raine, A.; Dodge, K.; Loeber, R.; Gatzke-Kopp, L.; Lynam, D.; Reynolds, C.; Stouthamer-Loeber, M.; Liu, J. The Reactive-Proactive Aggression Questionnaire: Differential Correlates of Reactive and Proactive Aggression in Adolescent Boys. Aggress. Behav. 2006, 32, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.A.; Finger, E.C.; Mitchell, D.G.; Reid, M.E.; Sims, C.; Kosson, D.S.; Towbin, K.E.; Leibenluft, E.; Pine, D.S.; Blair, R. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am. J. Psychiatry 2008, 165, 712–720. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W. Pictures of Facial Affect; Consulting Psychologitsts: Palo Alto, CA, USA, 1976. [Google Scholar]
- Phillips, M.L.; Williams, L.M.; Heining, M.; Herba, C.M.; Russell, T.; Andrew, C.; Bullmore, E.T.; Brammer, M.J.; Williams, S.C.; Morgan, M.; et al. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage 2004, 21, 1484–1496. [Google Scholar] [CrossRef]
- Young, A.W.; Rowland, D.; Calder, A.J.; Etcoff, N.L.; Seth, A.; Perrett, D.I. Facial expression megamix: Tests of dimensional and category accounts of emotion recognition. Cognition 1997, 63, 271–313. [Google Scholar] [CrossRef]
- Lange, K.; Williams, L.M.; Young, A.W.; Bullmore, E.T.; Brammer, M.J.; Williams, S.C.; Gray, J.A.; Phillips, M.L. Task instructions modulate neural responses to fearful facial expressions. Biol. Psychiatry 2003, 53, 226–232. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain; Thieme: Stuttgart, NY, USA, 1988. [Google Scholar]
- Cox, R.W.; Chen, G.; Glen, D.R.; Reynolds, R.C.; Taylor, P.A. FMRI clustering in AFNI: False-positive rates redux. Brain Connect. 2017, 7, 152–171. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Cicchetti, D. Emotion dysregulation and emerging psychopathology: A transdiagnostic, transdisciplinary perspective. Dev. Psychopathol. 2019, 31, 799–804. [Google Scholar] [CrossRef] [PubMed]
- McTeague, L.M.; Huemer, J.; Carreon, D.M.; Jiang, Y.; Eickhoff, S.B.; Etkin, A. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. Am. J. Psychiatry 2017, 174, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, J.; Tseng, W.L.; Kim, P.; Chen, G.; Yi, J.; Donahue, L.; Brotman, M.A.; Towbin, K.E.; Pine, D.S.; Leibenluft, E. Association of Irritability and Anxiety with the Neural Mechanisms of Implicit Face Emotion Processing in Youths with Psychopathology. JAMA Psychiatry 2017, 74, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kryza-Lacombe, M.; Iturri, N.; Monk, C.S.; Wiggins, J.L. Face Emotion Processing in Pediatric Irritability: Neural Mechanisms in a Sample Enriched for Irritability with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 1380–1391. [Google Scholar] [CrossRef]
- Aoki, Y.; Watanabe, T.; Abe, O.; Kuwabara, H.; Yahata, N.; Takano, Y.; Iwashiro, N.; Natsubori, T.; Takao, H.; Kawakubo, Y.; et al. Oxytocin’s neurochemical effects in the medial prefrontal cortex underlie recovery of task-specific brain activity in autism: A randomized controlled trial. Mol. Psychiatry 2015, 20, 447–453. [Google Scholar] [CrossRef]
- Domes, G.; Lischke, A.; Berger, C.; Grossmann, A.; Hauenstein, K.; Heinrichs, M.; Herpertz, S.C. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 2010, 35, 83–93. [Google Scholar] [CrossRef]
- Watanabe, T.; Abe, O.; Kuwabara, H.; Yahata, N.; Takano, Y.; Iwashiro, N.; Natsubori, T.; Aoki, Y.; Takao, H.; Kawakubo, Y.; et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: A randomized trial. JAMA Psychiatry 2014, 71, 166–175. [Google Scholar] [CrossRef]
- Lozier, L.M.; Cardinale, E.M.; VanMeter, J.W.; Marsh, A.A. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry 2014, 71, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.W.; Bechara, A.; Damasio, H.; Tranel, D.; Damasio, A.R. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat. Neurosci. 1999, 2, 1032–1037. [Google Scholar] [CrossRef]
- Grafman, J.; Schwab, K.; Warden, D.; Pridgen, A.; Brown, H.R.; Salazar, A.M. Frontal lobe injuries, violence, and aggression: A report of the Vietnam Head Injury Study. Neurology 1996, 46, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Raine, A.; Meloy, J.R.; Bihrle, S.; Stoddard, J.; LaCasse, L.; Buchsbaum, M.S. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behav. Sci. Law. 1998, 16, 319–332. [Google Scholar] [CrossRef]
- Ne’eman, R.; Perach-Barzilay, N.; Fischer-Shofty, M.; Atias, A.; Shamay-Tsoory, S.G. Intranasal administration of oxytocin increases human aggressive behavior. Horm. Behav. 2016, 80, 125–131. [Google Scholar] [CrossRef]
- Timmermann, M.; Jeung, H.; Schmitt, R.; Boll, S.; Freitag, C.M.; Bertsch, K.; Herpertz, S.C. Oxytocin improves facial emotion recognition in young adults with antisocial personality disorder. Psychoneuroendocrinology 2017, 85, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Curhan, A.L.; Rabinowitz, J.A.; Pas, E.T.; Bradshaw, C.P. Informant Discrepancies in Internalizing and Externalizing Symptoms in an At-Risk Sample: The Role of Parenting and School Engagement. J. Youth Adolesc. 2020, 49, 311–322. [Google Scholar] [CrossRef]
- Auerbach, R.P. RDoC and the developmental origins of psychiatric disorders: How did we get here and where are we going? J. Child Psychol. Psychiatry 2022, 63, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Marquand, A.F.; Rezek, I.; Buitelaar, J.; Beckmann, C.F. Understanding Heterogeneity in Clinical Cohorts Using Normative Models: Beyond Case-Control Studies. Biol. Psychiatry 2016, 80, 552–561. [Google Scholar] [CrossRef]
- Marquand, A.F.; Wolfers, T.; Mennes, M.; Buitelaar, J.; Beckmann, C.F. Beyond Lumping and Splitting: A Review of Computational Approaches for Stratifying Psychiatric Disorders. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.; Aoki, Y.; Watanabe, T.; Endo, N.; Abe, O.; Kuroda, M.; Kuwabara, H.; Kawakubo, Y.; Takao, H.; Kunimatsu, A.; et al. Neurochemical evidence for differential effects of acute and repeated oxytocin administration. Mol. Psychiatry 2021, 26, 710–720. [Google Scholar] [CrossRef]
- DeMayo, M.M.; Song, Y.J.C.; Hickie, I.B.; Guastella, A.J. A Review of the Safety, Efficacy and Mechanisms of Delivery of Nasal Oxytocin in Children: Therapeutic Potential for Autism and Prader-Willi Syndrome, and Recommendations for Future Research. Paediatr. Drugs 2017, 19, 391–410. [Google Scholar] [CrossRef]
- Blair, R.J.; Leibenluft, E.; Pine, D.S. Conduct disorder and callous-unemotional traits in youth. N. Engl. J. Med. 2014, 371, 2207–2216. [Google Scholar] [CrossRef]
- Waschbusch, D.A.; Mayes, S.D.; Waxmonsky, J.G.; Babinski, D.E.; Baweja, R. Reactive, Proactive, Relational, and Slow Dissipation of Aggression in Children. JAACAP Open. 2024, 2, 90–99. [Google Scholar] [CrossRef]
- Engel, S.; Klusmann, H.; Ditzen, B.; Knaevelsrud, C.; Schumacher, S. Menstrual cycle-related fluctuations in oxytocin concentrations: A systematic review and meta-analysis. Front. Neuroendocrinol. 2019, 52, 144–155. [Google Scholar] [CrossRef] [PubMed]
| Youth with Disruptive Behavior Disorders on OXT Treatment (n = 21) | Youth with Disruptive Behavior Disorders on PLB Treatment (n = 19) | p Value | |
|---|---|---|---|
| Age | 14.4 (2.1) | 14.2 (2.2) | 0.85 |
| Sex | 8 girls/13 boys | 3 girls/18 boys | 0.09 |
| IQ | 103.4 (16.2) | 100.7 (14.6) | 0.91 |
| Diagnosis (Primary) | |||
| ADHD | 12 (57.1%) | 10 (52.6%) | |
| DMDD | 4 (19.0%) | 4 (21.1%) | |
| CD | 2 (9.5%) | 0 (0%) | |
| Other | 2 (9.5%) | 5 (26.3%) |
| Youth with DBDs on OXT Treatment (n = 21) | Youth with DBDs on PLB Treatment (n = 19) | ||||
|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | ||
| ARI-Y | 6.71 (2.15) | 3.57 (2.36) | 6.16 (2.95) | 6.16 (3.15) | <0.001 */0.363 |
| ARI-P | 7.76 (2.81) | 4.14 (2.82) | 8.53 (2.09) | 6.31 (3.04) | 0.20/0.043 |
| RPAQ-Y | 15.14 (6.09) | 8.43 (6.70) | 16.35 (7.89) | 14.09 (6.72 | 0.07/0.177 |
| RA-Y | 12.33 (3.85) | 6.95 (4.76) | 12.37 (5.20) | 10.95 (4.65) | 0.005 */0.190 |
| PA-Y | 2.80 (2.84) | 1.55 (2.66) | 3.96 (2.95) | 3.13 (3.78) | 0.54/0.010 |
| RPAQ-P | 19.71 (7.27) | 10.86 (6.24) | 22.42 (6.53) | 16.82 (10.22) | 0.21/0.041 |
| RA-P | 14.86 (3.92) | 8.81 (4.26) | 16.05 (3.87) | 12.40 (6.28) | 0.11/0.066 |
| PA-P | 2.81 (2.84) | 1.55 (2.66) | 3.96 (2.95) | 3.13 (3.78) | 0.54/0.010 |
| Coordinates of Peak Activation b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region a | Left/Right | BA | x | y | z | F | Voxels | η2 |
| Group by time | ||||||||
| Superior Frontal Cortex | Right | 8 | 16.5 | 28.5 | 50.5 | 22.63 | 40 | 1.305 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.J.; Suk, J.-W.; Garvey, W.F.; Edwards, R.T.; Leibenluft, E.; Blair, R.J.R.; Hwang, S. Mediating Impact of Intranasal Oxytocin on the Interaction Between Irritability and Reactive Aggression in Youth with Severe Irritability. Life 2025, 15, 1253. https://doi.org/10.3390/life15081253
Son JJ, Suk J-W, Garvey WF, Edwards RT, Leibenluft E, Blair RJR, Hwang S. Mediating Impact of Intranasal Oxytocin on the Interaction Between Irritability and Reactive Aggression in Youth with Severe Irritability. Life. 2025; 15(8):1253. https://doi.org/10.3390/life15081253
Chicago/Turabian StyleSon, Jake J., Ji-Woo Suk, William F. Garvey, Ryan T. Edwards, Ellen Leibenluft, R. J. R. Blair, and Soonjo Hwang. 2025. "Mediating Impact of Intranasal Oxytocin on the Interaction Between Irritability and Reactive Aggression in Youth with Severe Irritability" Life 15, no. 8: 1253. https://doi.org/10.3390/life15081253
APA StyleSon, J. J., Suk, J.-W., Garvey, W. F., Edwards, R. T., Leibenluft, E., Blair, R. J. R., & Hwang, S. (2025). Mediating Impact of Intranasal Oxytocin on the Interaction Between Irritability and Reactive Aggression in Youth with Severe Irritability. Life, 15(8), 1253. https://doi.org/10.3390/life15081253






