Immune Dysregulation Connecting Type 2 Diabetes and Cardiovascular Complications
Abstract
1. Introduction
1.1. Dysregulated Insulin/InsR Signaling and Its Implications in the Development of T2D and CVDs
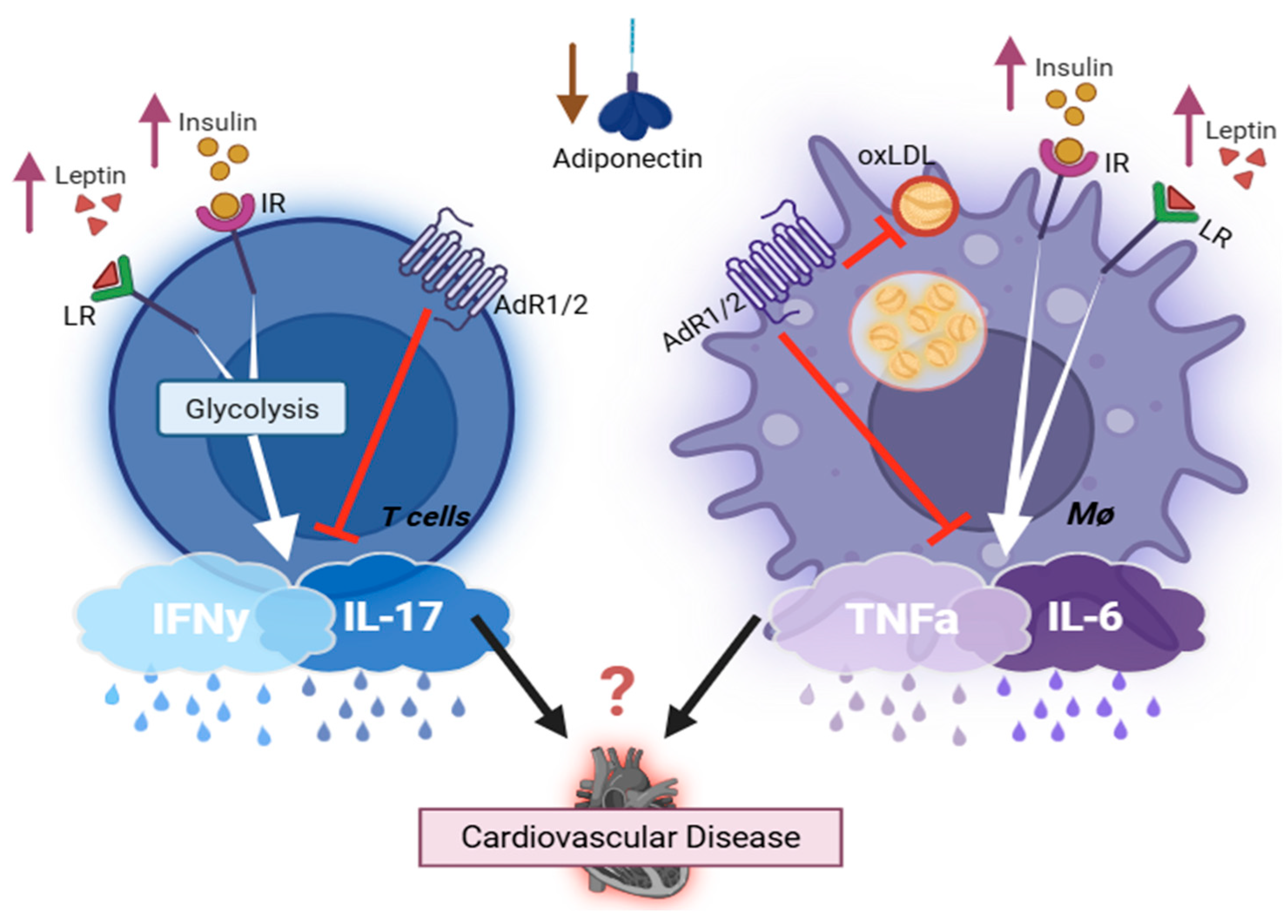
1.2. Association of Adipocyte-Derived Hormone, the Immune System, and T2D-CVDs
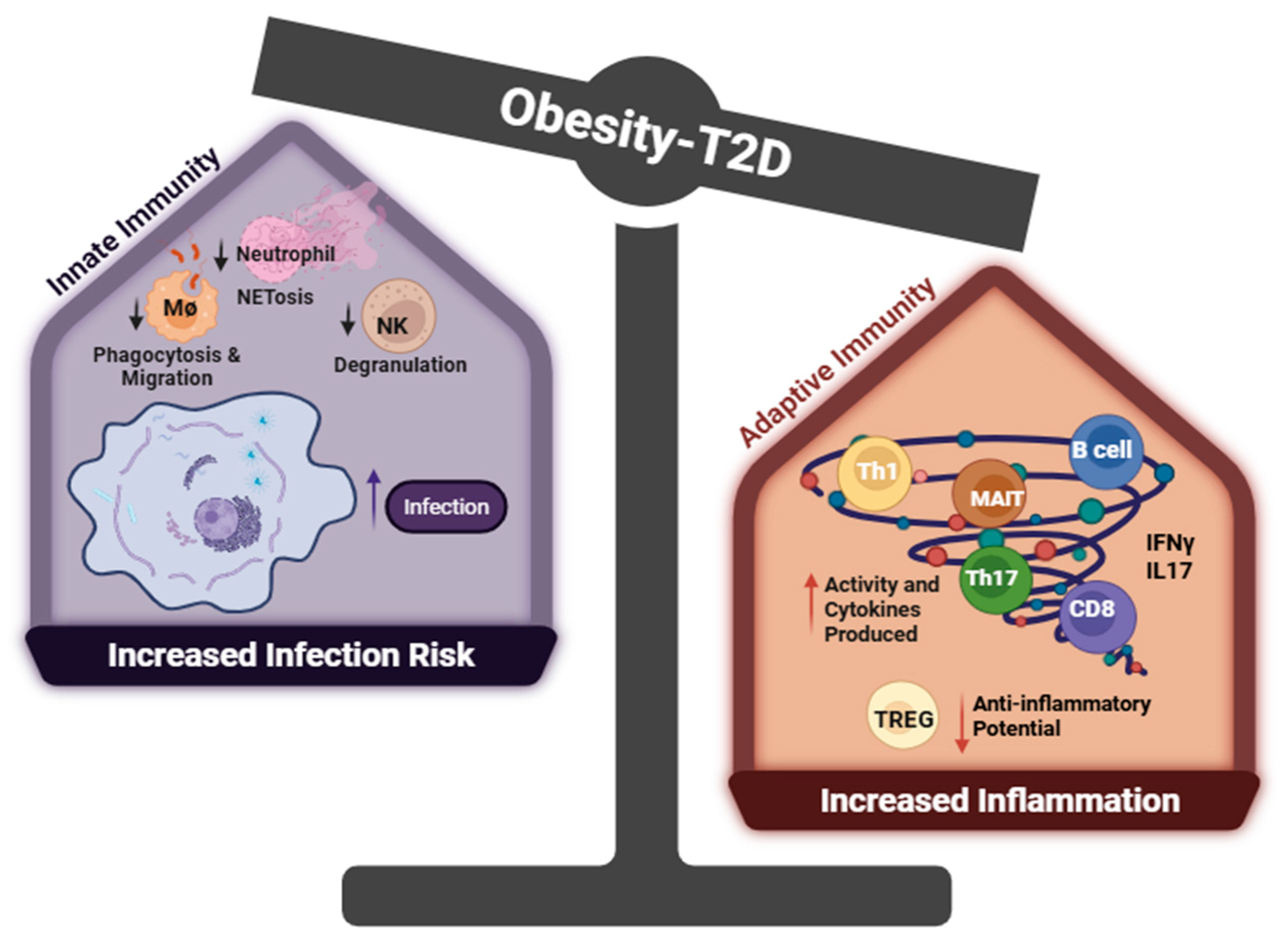
1.3. The Dysregulation of Immune Cells in T2D
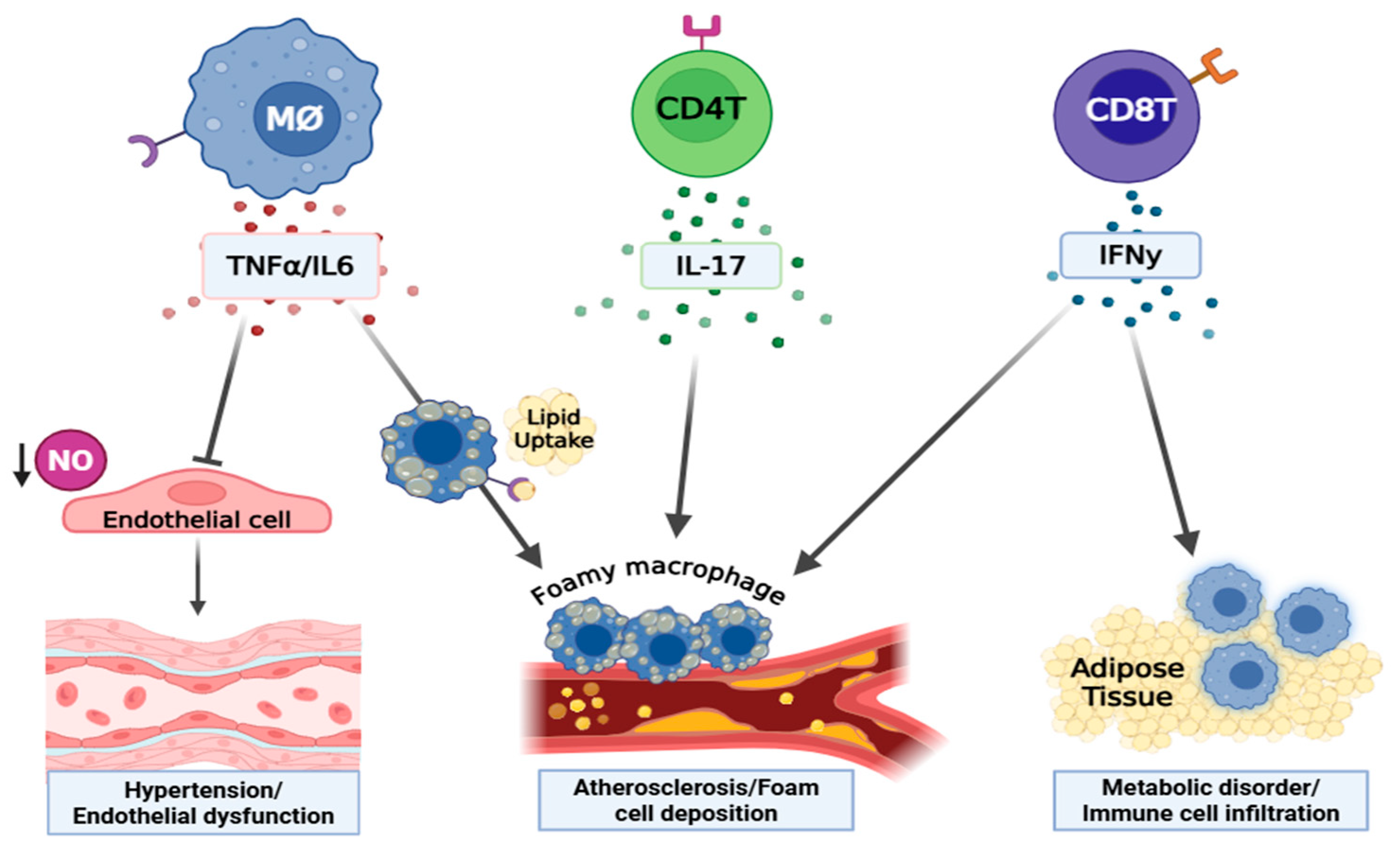
1.4. Effects of Cytokines (IFNγ, TNFα, IL17, IL6) on the Development of T2D and Its Related Cardiovascular Complications
2. Summary/Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Regufe, V.M.G.; Pinto, C.; Perez, P. Metabolic syndrome in type 2 diabetic patients: A review of current evidence. Porto Biomed. J. 2020, 5, e101. [Google Scholar] [CrossRef]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Type 2 diabetes—An autoinflammatory disease driven by metabolic stress. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3805–3823. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzam, N.; Al-Azzam, S.; Elsalem, L.; Karasneh, R. Hypertension prevalence and associated factors among patients with diabetes: A retrospective cross-sectional study from Jordan. Ann. Med. Surg. 2021, 61, 126–131. [Google Scholar] [CrossRef]
- Almalki, Z.S.; Albassam, A.A.; Alhejji, N.S.; Alotaibi, B.S.; Al-Oqayli, L.A.; Ahmed, N.J. Prevalence, risk factors, and management of uncontrolled hypertension among patients with diabetes: A hospital-based cross-sectional study. Prim. Care Diabetes 2020, 14, 610–615. [Google Scholar] [CrossRef]
- Alsaadon, H.; Afroz, A.; Karim, A.; Habib, S.H.; Alramadan, M.J.; Billah, B.; Shetty, A.N. Hypertension and its related factors among patients with type 2 diabetes mellitus—A multi-hospital study in Bangladesh. BMC Public Health 2022, 22, 198. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhou, T.; Heianza, Y.; Li, X.; Fan, M.; Fonseca, V.A.; Qi, L. Type 2 Diabetes and Hypertension. Circ. Res. 2019, 124, 930–937. [Google Scholar] [CrossRef]
- Schernthaner, G. Cardiovascular mortality and morbidity in type-2 diabetes mellitus. Diabetes Res. Clin. Pract. 1996, 31, S3–S13. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates: Deaths by Cause, Age, Sex and Country, 2000–2012; WHO: Geneva, Switzerland, 2014; Volume 9, p. c2014. [Google Scholar]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef]
- Ismail, L.; Materwala, H.; Al Kaabi, J. Association of risk factors with type 2 diabetes: A systematic review. Comput. Struct. Biotechnol. J. 2021, 19, 1759–1785. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Mechanisms of Disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Reaven, P.D.; Emanuele, N.V.; Wiitala, W.L.; Bahn, G.D.; Reda, D.J.; McCarren, M.; Duckworth, W.C.; Hayward, R.A. Intensive Glucose Control in Patients with Type 2 Diabetes—15-Year Follow-up. N. Engl. J. Med. 2019, 380, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- The ACCORD Study Group. Long-Term Effects of Intensive Glucose Lowering on Cardiovascular Outcomes. N. Engl. J. Med. 2011, 364, 818–828. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H., Jr.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [PubMed]
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Nikolajczyk, B.S.; Jagannathan-Bogdan, M.; Shin, H.; Gyurko, R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011, 12, 239–250. [Google Scholar] [CrossRef]
- Girard, D.; Vandiedonck, C. How dysregulation of the immune system promotes diabetes mellitus and cardiovascular risk complications. Front. Cardiovasc. Med. 2022, 9, 991716. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Strissel, K.J.; Denis, G.V.; Nikolajczyk, B.S. Immune regulators of inflammation in obesity-associated type 2 diabetes and coronary artery disease. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Al-Ozairi, E. Inflammatory cytokines and the risk of cardiovascular complications in type 2 diabetes. Dis. Markers 2013, 35, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Clemente-Casares, X.; Zhou, A.C.; Lei, H.; Ahn, J.J.; Chan, Y.T.; Choi, O.; Luck, H.; Woo, M.; Dunn, S.E.; et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018, 28, 922–934. [Google Scholar] [CrossRef]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef]
- Ramos-Ramírez, P.; Malmhäll, C.; Tliba, O.; Rådinger, M.; Bossios, A. Adiponectin/AdipoR1 Axis Promotes IL-10 Release by Human Regulatory T Cells. Front. Immunol. 2021, 12, 677550. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J.P. The thrifty phenotype hypothesis: Type 2 diabetes. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef]
- Cruz-Pineda, W.D.; Parra-Rojas, I.; Rodríguez-Ruíz, H.A.; Illades-Aguiar, B.; Matia-García, I.; Garibay-Cerdenares, O.L. The regulatory role of insulin in energy metabolism and leukocyte functions. J. Leukoc. Biol. 2022, 111, 197–208. [Google Scholar] [CrossRef]
- Helderman, J.H.; Strom, T.B. Emergence of insulin receptors upon alloimmune T cells in the rat. J. Clin. Investig. 1977, 59, 338–344. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Apovian, C.M.; Aronne, L.J.; Astrup, A.; Cantley, L.C.; Ebbeling, C.B.; Heymsfield, S.B.; Johnson, J.D.; King, J.C.; Krauss, R.M.; et al. Competing paradigms of obesity pathogenesis: Energy balance versus carbohydrate-insulin models. Eur. J. Clin. Nutr. 2022, 76, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Aronne, L.J.; Astrup, A.; de Cabo, R.; Cantley, L.C.; Friedman, M.I.; Heymsfield, S.B.; Johnson, J.D.; King, J.C.; Krauss, R.M.; et al. The carbohydrate-insulin model: A physiological perspective on the obesity pandemic. Am. J. Clin. Nutr. 2021, 114, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, S.B.; Kahn, C.R. From mice to men: Insights into the insulin resistance syndromes. Annu. Rev. Physiol. 2006, 68, 123–158. [Google Scholar] [CrossRef]
- Bo, T.; Gao, L.; Yao, Z.; Shao, S.; Wang, X.; Proud, C.G.; Zhao, J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024, 36, 947–968. [Google Scholar] [CrossRef]
- Shimomura, I.; Matsuda, M.; Hammer, R.E.; Bashmakov, Y.; Brown, M.S.; Goldstein, J.L. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 2000, 6, 77–86. [Google Scholar] [CrossRef]
- Haas, J.T.; Miao, J.; Chanda, D.; Wang, Y.; Zhao, E.; Haas, M.E.; Hirschey, M.; Vaitheesvaran, B.; Farese, R.V., Jr.; Kurland, I.J.; et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012, 15, 873–884. [Google Scholar] [CrossRef]
- Gamble, J.-M.; Simpson, S.H.; Eurich, D.T.; Majumdar, S.R.; Johnson, J.A. Insulin use and increased risk of mortality in type 2 diabetes: A cohort study. Diabetes Obes. Metab. 2010, 12, 47–53. [Google Scholar] [CrossRef]
- Yu, B.; Li, C.; Sun, Y.; Wang, D.W. Insulin Treatment Is Associated with Increased Mortality in Patients with COVID-19 and Type 2 Diabetes. Cell Metab. 2021, 33, 65–77. [Google Scholar] [CrossRef]
- Manhiani, M.M.; Cormican, M.T.; Brands, M.W. Chronic sodium-retaining action of insulin in diabetic dogs. Am. J. Physiol. Renal Physiol. 2011, 300, F957–F965. [Google Scholar] [CrossRef][Green Version]
- Brands, M.W.; Hildebrandt, D.A.; Mizelle, H.L.; Hall, J.E. Sustained hyperinsulinemia increases arterial pressure in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1991, 260, R764–R768. [Google Scholar] [CrossRef]
- Bursztyn, M.; Ben-Ishay, D.; Mekler, J.; Raz, I. Chronic exogenous hyperinsulinaemia without sugar supplementation: Acute salt-sensitive hypertension without changes in resting blood pressure. J. Hypertens. 1993, 11, 703–707. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Cooke, C.R.; Andres, R.; Faloona, G.R.; Davis, P.J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J. Clin. Investig. 1975, 55, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Bie, P. Blood volume, blood pressure and total body sodium: Internal signalling and output control. Acta Physiol. 2009, 195, 187–196. [Google Scholar] [CrossRef]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular Actions of Insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Kintscher, U.; Hartge, M.; Hess, K.; Foryst-Ludwig, A.; Clemenz, M.; Wabitsch, M.; Fischer-Posovszky, P.; Barth, T.F.; Dragun, D.; Skurk, T.; et al. T-lymphocyte infiltration in visceral adipose tissue: A primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Cham, C.M.; Gajewski, T.F. Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 2005, 174, 4670–4677. [Google Scholar] [CrossRef]
- Bar, R.S.; Kahn, C.R.; Koren, H.S. Insulin inhibition of antibody-dependent cytoxicity and insulin receptors in macrophages. Nature 1977, 265, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Tessaro, F.H.G.; Ayala, T.S.; Nolasco, E.L.; Bella, L.M.; Martins, J.O. Insulin Influences LPS-Induced TNF-α and IL-6 Release Through Distinct Pathways in Mouse Macrophages from Different Compartments. Cell. Physiol. Biochem. 2017, 42, 2093–2104. [Google Scholar] [CrossRef]
- Tsiotra, P.C.; Boutati, E.; Dimitriadis, G.; Raptis, S.A. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. BioMed Res. Int. 2013, 2013, 487081. [Google Scholar] [CrossRef]
- Ratter, J.M.; van Heck, J.I.P.; Rooijackers, H.M.M.; Jansen, H.J.; van Poppel, P.C.M.; Tack, C.J.; Stienstra, R. Insulin acutely activates metabolism of primary human monocytes and promotes a proinflammatory phenotype. J. Leukoc. Biol. 2021, 110, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Baumgartl, J.; Baudler, S.; Scherner, M.; Babaev, V.; Makowski, L.; Suttles, J.; McDuffie, M.; Tobe, K.; Kadowaki, T.; Fazio, S.; et al. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab. 2006, 3, 247–256. [Google Scholar] [CrossRef]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef]
- Grundy, S.M. Adipose tissue and metabolic syndrome: Too much, too little or neither. Eur. J. Clin. Investig. 2015, 45, 1209–1217. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Almer, G.; Saba-Lepek, M.; Haj-Yahya, S.; Rohde, E.; Strunk, D.; Fröhlich, E.; Prassl, R.; Mangge, H. Globular domain of adiponectin: Promising target molecule for detection of atherosclerotic lesions. Biol. Targets Ther. 2011, 5, 95–105. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Shao, H.; Liu, S.; Niu, Y.; Fu, L. Globular adiponectin ameliorates insulin resistance in skeletal muscle by enhancing the LKB1-mediated AMPK activation via SESN2. Sports Med. Health Sci. 2023, 5, 34–41. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef]
- Denzel, M.S.; Scimia, M.-C.; Zumstein, P.M.; Walsh, K.; Ruiz-Lozano, P.; Ranscht, B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Investig. 2010, 120, 4342–4352. [Google Scholar] [CrossRef]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Combs, T.P.; Berg, A.H.; Obici, S.; Scherer, P.E.; Rossetti, L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Investig. 2001, 108, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, X.; You, L.; Li, F.; Lian, H.; Wang, J.; Mao, N.; Ren, M.; Li, Y.; Wang, C.; et al. Association between adiponectin and newly diagnosed type 2 diabetes in population with the clustering of obesity, dyslipidaemia and hypertension: A cross-sectional study. BMJ Open 2023, 13, e060377. [Google Scholar] [CrossRef]
- Luo, N.; Wang, X.; Zhang, W.; Garvey, W.T.; Fu, Y. AdR1-TG/TALLYHO mice have improved lipid accumulation and insulin sensitivity. Biochem. Biophys. Res. Commun. 2013, 433, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Imai, Y.; Shimozawa, N.; Hioki, K.; Uchida, S.; Ito, Y.; Takakuwa, K.; Matsui, J.; et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J. Biol. Chem. 2003, 278, 2461–2468. [Google Scholar] [CrossRef]
- Salvator, H.; Grassin-Delyle, S.; Brollo, M.; Couderc, L.J.; Abrial, C.; Victoni, T.; Naline, E.; Devillier, P. Adiponectin Inhibits the Production of TNF-α, IL-6 and Chemokines by Human Lung Macrophages. Front. Pharmacol. 2021, 12, 718929. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Luo, N.; Klein, R.L.; Chung, B.H.; Garvey, W.T.; Fu, Y. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis 2009, 202, 152–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhuang, R.; Ma, Y.; Zhang, C.; Tang, K.; Yi, H.; Jin, B. Adiponectin’s globular domain inhibits T cell activation by interacting with LAIR-1. Biochem. Biophys. Res. Commun. 2021, 573, 117–124. [Google Scholar] [CrossRef]
- Surendar, J.; Frohberger, S.J.; Karunakaran, I.; Schmitt, V.; Stamminger, W.; Neumann, A.L.; Wilhelm, C.; Hoerauf, A.; Hübner, M.P. Adiponectin Limits IFN-γ and IL-17 Producing CD4 T Cells in Obesity by Restraining Cell Intrinsic Glycolysis. Front. Immunol. 2019, 10, 2555. [Google Scholar] [CrossRef] [PubMed]
- Picó, C.; Palou, M.; Pomar, C.A.; Rodríguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef]
- Brydon, L.; O’Donnell, K.; Wright, C.E.; Wawrzyniak, A.J.; Wardle, J.; Steptoe, A. Circulating leptin and stress-induced cardiovascular activity in humans. Obesity 2008, 16, 2642–2647. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Sims, E.D.; Jennings, W.J.; Empringham, B.; Fleming, A.; Portwine, C.; Johnston, D.L.; Zelcer, S.M.; Rassekh, S.R.; Burrow, S.; Thabane, L.; et al. Circulating leptin levels are associated with adiposity in survivors of childhood brain tumors. Sci. Rep. 2020, 10, 4711. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Feitosa, M.F.; Wilk, J.B.; Laramie, J.M.; Yu, K.; Leiendecker-Foster, C.; Myers, R.H.; Province, M.A.; Borecki, I.B. Leptin Is Associated with Blood Pressure and Hypertension in Women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension 2009, 53, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Simonds, S.E.; Pryor, J.T.; Ravussin, E.; Greenway, F.L.; Dileone, R.; Allen, A.M.; Bassi, J.; Elmquist, J.K.; Keogh, J.M.; Henning, E.; et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 2014, 159, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Shinozaki, S.; Nakazawa, T.; Kawakami, A.; Ai, M.; Kaneko, E.; Kitagawa, M.; Kondo, K.; Chait, A.; Shimokado, K. Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis 2008, 196, 68–75. [Google Scholar] [CrossRef]
- Bodary, P.F.; Gu, S.; Shen, Y.; Hasty, A.H.; Buckler, J.M.; Eitzman, D.T. Recombinant Leptin Promotes Atherosclerosis and Thrombosis in Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, e119–e122. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.; Herbin, O.; Ait-Oufella, H.; Verreth, W.; Gourdy, P.; Barateau, V.; Merval, R.; Esposito, B.; Clément, K.; Holvoet, P.; et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J. Leptin and atherosclerosis. Atherosclerosis 2006, 189, 47–60. [Google Scholar] [CrossRef]
- De Rosa, V.; Procaccini, C.; Calì, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–255. [Google Scholar] [CrossRef]
- De Rosa, V.; Procaccini, C.; La Cava, A.; Chieffi, P.; Nicoletti, G.F.; Fontana, S.; Zappacosta, S.; Matarese, G. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J. Clin. Investig. 2006, 116, 447–455. [Google Scholar] [CrossRef]
- Gerriets, V.A.; Danzaki, K.; Kishton, R.J.; Eisner, W.; Nichols, A.G.; Saucillo, D.C.; Shinohara, M.L.; MacIver, N.J. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. 2016, 46, 1970–1983. [Google Scholar] [CrossRef]
- La Cava, A.; Matarese, G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004, 4, 371–379. [Google Scholar] [CrossRef]
- Saucillo, D.C.; Gerriets, V.A.; Sheng, J.; Rathmell, J.C.; MacIver, N.J. Leptin Metabolically Licenses T Cells for Activation to Link Nutrition and Immunity. J. Immunol. 2014, 192, 136–144. [Google Scholar] [CrossRef]
- Vaughan, T.; Li, L. Molecular mechanism underlying the inflammatory complication of leptin in macrophages. Mol. Immunol. 2010, 47, 2515–2518. [Google Scholar] [CrossRef]
- Li, Y.; Yao, L.; Huang, Y.; Pang, L.; Zhang, C.; Li, T.; Wang, D.; Zhou, K.; Li, J.; Tang, X. Leptin Enhances M1 Macrophage Polarization and Impairs Tendon-Bone Healing in Rotator Cuff Repair: A Rat Model. Clin. Orthop. Relat. Res. 2025, 483, 939–951. [Google Scholar] [CrossRef]
- Stentz, F.B.; Kitabchi, A.E. Activated T lymphocytes in Type 2 diabetes: Implications from in vitro studies. Curr. Drug Targets 2003, 4, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 7457269. [Google Scholar] [CrossRef]
- Jagannathan-Bogdan, M.; McDonnell, M.E.; Shin, H.; Rehman, Q.; Hasturk, H.; Apovian, C.M.; Nikolajczyk, B.S. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J. Immunol. 2011, 186, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Liu, L.F.; Lamendola, C.; Shen, L.; Morton, J.; Rivas, H.; Winer, D.; Tolentino, L.; Choi, O.; Zhang, H.; et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- DeFuria, J.; Belkina, A.C.; Jagannathan-Bogdan, M.; Snyder-Cappione, J.; Carr, J.D.; Nersesova, Y.R.; Markham, D.; Strissel, K.J.; Watkins, A.A.; Zhu, M.; et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA 2013, 110, 5133–5138. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): Implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar] [CrossRef]
- Fadini, G.P.; de Kreutzenberg, S.V.; Boscaro, E.; Albiero, M.; Cappellari, R.; Kränkel, N.; Landmesser, U.; Toniolo, A.; Bolego, C.; Cignarella, A.; et al. An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia 2013, 56, 1856–1866. [Google Scholar] [CrossRef]
- Surmi, B.K.; Hasty, A.H. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008, 3, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Lynch, L.; Nowak, M.; Varghese, B.; Clark, J.; Hogan, A.E.; Toxavidis, V.; Balk, S.P.; O’Shea, D.; O’Farrelly, C.; Exley, M.A. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 2012, 37, 574–587. [Google Scholar] [CrossRef]
- Lynch, L.; O’Shea, D.; Winter, D.C.; Geoghegan, J.; Doherty, D.G.; O’Farrelly, C. Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 2009, 39, 1893–1901. [Google Scholar] [CrossRef]
- Gupta, S.; Maratha, A.; Siednienko, J.; Natarajan, A.; Gajanayake, T.; Hoashi, S.; Miggin, S. Analysis of inflammatory cytokine and TLR expression levels in Type 2 Diabetes with complications. Sci. Rep. 2017, 7, 7633. [Google Scholar] [CrossRef]
- Josefs, T.; Barrett, T.J.; Brown, E.J.; Quezada, A.; Wu, X.; Voisin, M.; Amengual, J.; Fisher, E.A. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight 2020, 5, e134796. [Google Scholar] [CrossRef] [PubMed]
- Chatzigeorgiou, A.; Mitroulis, I.; Chrysanthopoulou, A.; Legaki, A.I.; Ritis, K.; Tentolouris, N.; Protogerou, A.D.; Koutsilieris, M.; Sfikakis, P.P. Increased Neutrophil Extracellular Traps Related to Smoking Intensity and Subclinical Atherosclerosis in Patients with Type 2 Diabetes. Thromb. Haemost. 2020, 120, 1587–1589. [Google Scholar] [CrossRef]
- Restrepo, B.I.; Twahirwa, M.; Rahbar, M.H.; Schlesinger, L.S. Phagocytosis via complement or Fc-γ receptors is compromised in monocytes from type 2 diabetes patients with chronic hyperglycemia. PLoS ONE 2014, 9, e92977. [Google Scholar] [CrossRef]
- Pavlou, S.; Lindsay, J.; Ingram, R.; Xu, H.; Chen, M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Berrou, J.; Fougeray, S.; Venot, M.; Chardiny, V.; Gautier, J.F.; Dulphy, N.; Toubert, A.; Peraldi, M.N. Natural killer cell function, an important target for infection and tumor protection, is impaired in type 2 diabetes. PLoS ONE 2013, 8, e62418. [Google Scholar] [CrossRef]
- Kumar, M.; Roe, K.; Nerurkar, P.V.; Orillo, B.; Thompson, K.S.; Verma, S.; Nerurkar, V.R. Reduced immune cell infiltration and increased pro-inflammatory mediators in the brain of Type 2 diabetic mouse model infected with West Nile virus. J. Neuroinflammation 2014, 11, 80. [Google Scholar] [CrossRef]
- Altan-Bonnet, G.; Mukherjee, R. Cytokine-mediated communication: A quantitative appraisal of immune complexity. Nat. Rev. Immunol. 2019, 19, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Beutler, B.; Mahoney, J.; Le Trang, N.; Pekala, P.; Cerami, A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J. Exp. Med. 1985, 161, 984–995. [Google Scholar] [CrossRef]
- Borsotti, C.; Franklin, A.R.; Lu, S.X.; Kim, T.D.; Smith, O.M.; Suh, D.; King, C.G.; Chow, A.; Liu, C.; Alpdogan, O.; et al. Absence of donor T-cell-derived soluble TNF decreases graft-versus-host disease without impairing graft-versus-tumor activity. Blood 2007, 110, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Barath, P.; Fishbein, M.C.; Cao, J.; Berenson, J.; Helfant, R.H.; Forrester, J.S. Tumor necrosis factor gene expression in human vascular intimal smooth muscle cells detected by in situ hybridization. Am. J. Pathol. 1990, 137, 503–509. [Google Scholar]
- Ohta, H.; Wada, H.; Niwa, T.; Kirii, H.; Iwamoto, N.; Fujii, H.; Saito, K.; Sekikawa, K.; Seishima, M. Disruption of tumor necrosis factor-α gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005, 180, 11–17. [Google Scholar] [CrossRef]
- Persson, J.; Nilsson, J.; Lindholm, M.W. Interleukin-1beta and tumour necrosis factor-alpha impede neutral lipid turnover in macrophage-derived foam cells. BMC Immunol. 2008, 9, 70. [Google Scholar] [CrossRef]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-α in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Picchi, A.; Gao, X.; Belmadani, S.; Potter, B.J.; Focardi, M.; Chilian, W.M.; Zhang, C. Tumor necrosis factor-α induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 2006, 99, 69–77. [Google Scholar] [CrossRef]
- Gao, X.; Belmadani, S.; Picchi, A.; Xu, X.; Potter, B.J.; Tewari-Singh, N.; Capobianco, S.; Chilian, W.M.; Zhang, C. Tumor necrosis factor-α induces endothelial dysfunction in Leprdb mice. Circulation 2007, 115, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.F.; Hassan, A.H.; El-Ansary, M.R. Cytokine modulation by etanercept ameliorates metabolic syndrome and its related complications induced in rats administered a high-fat high-fructose diet. Sci. Rep. 2022, 12, 20227. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, M.; Perrella, M.A.; Burnett, J.C., Jr.; Lee, M.E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ. Res. 1993, 73, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Andresen, B.T.; Hill, M.; Zhang, J.; Booth, F.; Zhang, C. Role of Reactive Oxygen Species in Tumor Necrosis Factor-α Induced Endothelial Dysfunction. Curr. Hypertens. Rev. 2008, 4, 245–255. [Google Scholar] [CrossRef]
- El-Edel, R.; Fathy, W.; Abou-Elela, D.; Emara, M.; Abo El-Khair, N. Role of tumor necrosis factor α in type 2 diabetic nephropathy. Menoufia Med. J. 2020, 33, 920–925. [Google Scholar]
- Navarro, J.F.; Mora-Fernández, C. The role of TNF-α in diabetic nephropathy: Pathogenic and therapeutic implications. Cytokine Growth Factor. Rev. 2006, 17, 441–450. [Google Scholar] [CrossRef]
- Mehaffey, E.; Majid, D.S.A. Tumor necrosis factor-α, kidney function, and hypertension. Am. J. Physiol. Ren. Physiol. 2017, 313, F1005–F1008. [Google Scholar] [CrossRef]
- Majid, D.S. Tumor necrosis factor-α and kidney function: Experimental findings in mice. Adv. Exp. Med. Biol. 2011, 691, 471–480. [Google Scholar]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Folco, E.J.; Sukhova, G.; Shimizu, K.; Gotsman, I.; Vernon, A.H.; Libby, P. Interferon-γ, a Th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ. Res. 2008, 103, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Potter, B.J.; Cao, J.M.; Zhang, C. Interferon-γ induced adipose tissue inflammation is linked to endothelial dysfunction in type 2 diabetic mice. Basic Res. Cardiol. 2011, 106, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Oldham, W.M.; He, H.; Wang, R.; Mulhern, R.; Handy, D.E.; Loscalzo, J. Interferon-γ Impairs Human Coronary Artery Endothelial Glucose Metabolism by Tryptophan Catabolism and Activates Fatty Acid Oxidation. Circulation 2021, 144, 1612–1628. [Google Scholar] [CrossRef]
- Gupta, S.; Pablo, A.M.; Jiang, X.; Wang, N.; Tall, A.R.; Schindler, C. IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Investig. 1997, 99, 2752–2761. [Google Scholar] [CrossRef]
- Whitman, S.C.; Ravisankar, P.; Elam, H.; Daugherty, A. Exogenous interferon-γ enhances atherosclerosis in apolipoprotein E−/− mice. Am. J. Pathol. 2000, 157, 1819–1824. [Google Scholar] [CrossRef]
- Benson, L.; Liu, Y.; Guo, Y.; Xiong, Y.; Mu, S. The IFNγ-PDL1 Pathway Enhances the Interaction Between CD8+ T Cells and Distal Convoluted Tubules to Promote Salt-Sensitive Hypertension. FASEB J. 2022, 36, R513. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; Semmler, M.; Abdel-Reheim, E.S.; Zanaty, M.I.; Addaleel, W. Association of glycemic status and interferon-γ production with leukocytes and platelet indices alterations in type2 diabetes. Diabetes Metab. Syndr. 2019, 13, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Kouri, V.-P.; Olkkonen, J.; Nurmi, K.; Peled, N.; Ainola, M.; Mandelin, J.; Nordström, D.C.; Eklund, K.K. IL-17A and TNF synergistically drive expression of proinflammatory mediators in synovial fibroblasts via IκBζ-dependent induction of ELF3. Rheumatology 2022, 62, 872–885. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S. The IL-17 family in diseases: From bench to bedside. Signal Transduct. Target. Ther. 2023, 8, 402. [Google Scholar] [CrossRef]
- Chang, Y.C.; Hee, S.W.; Chuang, L.M. T helper 17 cells: A new actor on the stage of type 2 diabetes and aging? J. Diabetes Investig. 2021, 12, 909–913. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Wang, M.; Wang, J.; Wu, Y.; Qi, X. Clinical significance of Interleukin 17 receptor E in diabetic nephropathy. Int. Immunopharmacol. 2023, 120, 110324. [Google Scholar] [CrossRef]
- Ohshima, K.; Mogi, M.; Jing, F.; Iwanami, J.; Tsukuda, K.; Min, L.J.; Higaki, J.; Horiuchi, M. Roles of interleukin 17 in angiotensin II type 1 receptor-mediated insulin resistance. Hypertension 2012, 59, 493–499. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.J.; Chen, X.; Kwan, T.; Chadban, S.J.; Wu, H. Interleukin 17A promotes diabetic kidney injury. Sci. Rep. 2019, 9, 2264. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Prasad, K.M.; Butcher, M.; Dobrian, A.; Kolls, J.K.; Ley, K.; Galkina, E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 2010, 121, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- van Es, T.; van Puijvelde, G.H.; Ramos, O.H.; Segers, F.M.; Joosten, L.A.; van den Berg, W.B.; Michon, I.M.; de Vos, P.; van Berkel, T.J.; Kuiper, J. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem. Biophys. Res. Commun. 2009, 388, 261–265. [Google Scholar] [CrossRef]
- Chen, S.; Shimada, K.; Zhang, W.; Huang, G.; Crother, T.R.; Arditi, M. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J. Immunol. 2010, 185, 5619–5627. [Google Scholar] [CrossRef]
- Eid, R.E.; Rao, D.A.; Zhou, J.; Lo, S.F.; Ranjbaran, H.; Gallo, A.; Sokol, S.I.; Pfau, S.; Pober, J.S.; Tellides, G. Interleukin-17 and interferon-γ are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 2009, 119, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Erbel, C.; Chen, L.; Bea, F.; Wangler, S.; Celik, S.; Lasitschka, F.; Wang, Y.; Böckler, D.; Katus, H.A.; Dengler, T.J. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 2009, 183, 8167–8175. [Google Scholar] [CrossRef]
- Chang, S.L.; Hsiao, Y.W.; Tsai, Y.N.; Lin, S.F.; Liu, S.H.; Lin, Y.J.; Lo, L.W.; Chung, F.P.; Chao, T.F.; Hu, Y.F.; et al. Interleukin-17 enhances cardiac ventricular remodeling via activating MAPK pathway in ischemic heart failure. J. Mol. Cell Cardiol. 2018, 122, 69–79. [Google Scholar] [CrossRef]
- Kamat, N.V.; Thabet, S.R.; Xiao, L.; Saleh, M.A.; Kirabo, A.; Madhur, M.S.; Delpire, E.; Harrison, D.G.; McDonough, A.A. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ−/− and interleukin-17A−/− mice. Hypertension 2015, 65, 569–576. [Google Scholar] [CrossRef]
- Norlander, A.E.; Saleh, M.A.; Kamat, N.V.; Ko, B.; Gnecco, J.; Zhu, L.; Dale, B.L.; Iwakura, Y.; Hoover, R.S.; McDonough, A.A.; et al. Interleukin-17A Regulates Renal Sodium Transporters and Renal Injury in Angiotensin II–Induced Hypertension. Hypertension 2016, 68, 167–174. [Google Scholar] [CrossRef]
- Podor, T.J.; Jirik, F.R.; Loskutoff, D.J.; Carson, D.A.; Lotz, M. Human Endothelial Cells Produce IL-6. Ann. N. Y. Acad. Sci. 1989, 557, 374–387. [Google Scholar] [CrossRef]
- O’Brien, S.A.; Zhu, M.; Zhang, W. The Importance of IL-6 in the Development of LAT-Mediated Autoimmunity. J. Immunol. 2015, 195, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Liu, J.; Lau, C.W.; Huang, Y. IL-6 in diabetes and cardiovascular complications. Br. J. Pharmacol. 2014, 171, 3595–3603. [Google Scholar] [CrossRef]
- Koshino, A.; Schechter, M.; Sen, T.; Vart, P.; Neuen, B.L.; Neal, B.; Arnott, C.; Perkovic, V.; Ridker, P.M.; Tuttle, K.R.; et al. Interleukin-6 and Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: New Insights From CANVAS. Diabetes Care 2022, 45, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Reeh, H.; Rudolph, N.; Billing, U.; Christen, H.; Streif, S.; Bullinger, E.; Schliemann-Bullinger, M.; Findeisen, R.; Schaper, F.; Huber, H.J.; et al. Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: Fusing experimental insights and dynamic modelling. Cell Commun. Signal. 2019, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.; Tam, F.; Gursche, C.; Cheneval, C.; Besler, K.; Enns, W.; Manku, S.; Rey, K.; Hanson, P.J.; Rose-John, S.; et al. Overlapping and distinct biological effects of IL-6 classic and trans-signaling in vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2021, 320, C554–C565. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Wallenius, V.; Wallenius, K.; Ahrén, B.; Rudling, M.; Carlsten, H.; Dickson, S.L.; Ohlsson, C.; Jansson, J.-O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002, 8, 75–79. [Google Scholar] [CrossRef]
- van Hall, G.; Steensberg, A.; Sacchetti, M.; Fischer, C.; Keller, C.; Schjerling, P.; Hiscock, N.; Møller, K.; Saltin, B.; Febbraio, M.A.; et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J. Clin. Endocrinol. Metab. 2003, 88, 3005–3010. [Google Scholar] [CrossRef] [PubMed]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and α cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Peppler, W.T.; Townsend, L.K.; Meers, G.M.; Panasevich, M.R.; MacPherson, R.E.K.; Rector, R.S.; Wright, D.C. Acute administration of IL-6 improves indices of hepatic glucose and insulin homeostasis in lean and obese mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 316, G166–G178. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Robinson, R.; Greenway, G.; Glass, J.; Budkin, S.; Sharma, S. Blockade of interleukin-6 trans-signaling prevents mitochondrial dysfunction and cellular senescence in retinal endothelial cells. Exp. Eye Res. 2023, 237, 109721. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Inoue, H.; Ono, C.; Hashimoto, S.; Kioi, Y.; Matsumoto, H.; Matsuura, H.; Matsubara, T.; Shimizu, K.; et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 22351–22356. [Google Scholar] [CrossRef]
- Lamertz, L.; Rummel, F.; Polz, R.; Baran, P.; Hansen, S.; Waetzig, G.H.; Moll, J.M.; Floss, D.M.; Scheller, J. Soluble gp130 prevents interleukin-6 and interleukin-11 cluster signaling but not intracellular autocrine responses. Sci. Signal. 2018, 11, eaar7388. [Google Scholar] [CrossRef]
- Korotaeva, A.A.; Samoilova, E.V.; Chepurnova, D.A.; Zhitareva, I.V.; Shuvalova, Y.A.; Prokazova, N.V. Soluble glycoprotein 130 is inversely related to severity of coronary atherosclerosis. Biomarkers 2018, 23, 527–532. [Google Scholar] [CrossRef]
- Schuett, H.; Oestreich, R.; Waetzig, G.H.; Annema, W.; Luchtefeld, M.; Hillmer, A.; Bavendiek, U.; von Felden, J.; Divchev, D.; Kempf, T.; et al. Transsignaling of Interleukin-6 Crucially Contributes to Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 281–290. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef]
- Baumgartner, F.; Bamopoulos, S.A.; Faletti, L.; Hsiao, H.J.; Holz, M.; Gonzalez-Menendez, I.; Solé-Boldo, L.; Horne, A.; Gosavi, S.; Özerdem, C.; et al. Activation of gp130 signaling in T cells drives TH17-mediated multi-organ autoimmunity. Sci. Signal 2024, 17, eadc9662. [Google Scholar] [CrossRef]
- Tian, R.; Tian, M.; Wang, L.; Qian, H.; Zhang, S.; Pang, H.; Liu, Z.; Fang, L.; Shen, Z. C-reactive protein for predicting cardiovascular and all-cause mortality in type 2 diabetic patients: A meta-analysis. Cytokine 2019, 117, 59–64. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Teupser, D.; Weber, O.; Rao, T.N.; Sass, K.; Thiery, J.; Fehling, H.J. No reduction of atherosclerosis in C-reactive protein (CRP)-deficient mice. J. Biol. Chem. 2011, 286, 6272–6279. [Google Scholar] [CrossRef] [PubMed]
- Kirkgöz, K. C-Reactive Protein in Atherosclerosis—More than a Biomarker, but not Just a Culprit. Rev. Cardiovasc. Med. 2023, 24, 297. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; Siddiqui, S.A.; Ibrahim, M.; Hakim, M.L.; Ahammed, M.S.; Kabir, A.; Sultana, F. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020, 8, 2050312120965752. [Google Scholar] [CrossRef] [PubMed]
- Mehra, V.C.; Ramgolam, V.S.; Bender, J.R. Cytokines and cardiovascular disease. J. Leukoc. Biol. 2005, 78, 805–818. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adiponectin: Friend or foe in obesity and inflammation. Med. Rev. 2022, 2, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martín-Romero, C.; Pérez-Pérez, A.; González-Yanes, C.; Sánchez-Margalet, V. Role of leptin in the activation of immune cells. Mediat. Inflamm. 2010, 2010, 568343. [Google Scholar] [CrossRef] [PubMed]
- Makhijani, P.; Basso, P.J.; Chan, Y.T.; Chen, N.; Baechle, J.; Khan, S.; Furman, D.; Tsai, S.; Winer, D.A. Regulation of the immune system by the insulin receptor in health and disease. Front. Endocrinol. 2023, 14, 1128622. [Google Scholar] [CrossRef]
- Gao, M.; Cui, D.; Xie, J. The role of adiponectin for immune cell function in metabolic diseases. Diabetes Obes. Metab. 2023, 25, 2427–2438. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deck, K.; Mora, C.; Deng, S.; Rogers, P.; Rafferty, T.; Palade, P.T.; Mu, S.; Liu, Y. Immune Dysregulation Connecting Type 2 Diabetes and Cardiovascular Complications. Life 2025, 15, 1241. https://doi.org/10.3390/life15081241
Deck K, Mora C, Deng S, Rogers P, Rafferty T, Palade PT, Mu S, Liu Y. Immune Dysregulation Connecting Type 2 Diabetes and Cardiovascular Complications. Life. 2025; 15(8):1241. https://doi.org/10.3390/life15081241
Chicago/Turabian StyleDeck, Katherine, Christoph Mora, Shuoqiu Deng, Pamela Rogers, Tonya Rafferty, Philip T. Palade, Shengyu Mu, and Yunmeng Liu. 2025. "Immune Dysregulation Connecting Type 2 Diabetes and Cardiovascular Complications" Life 15, no. 8: 1241. https://doi.org/10.3390/life15081241
APA StyleDeck, K., Mora, C., Deng, S., Rogers, P., Rafferty, T., Palade, P. T., Mu, S., & Liu, Y. (2025). Immune Dysregulation Connecting Type 2 Diabetes and Cardiovascular Complications. Life, 15(8), 1241. https://doi.org/10.3390/life15081241






