Right Heart Evaluation: A Tough Challenge for Clinicians
Abstract
1. Introduction
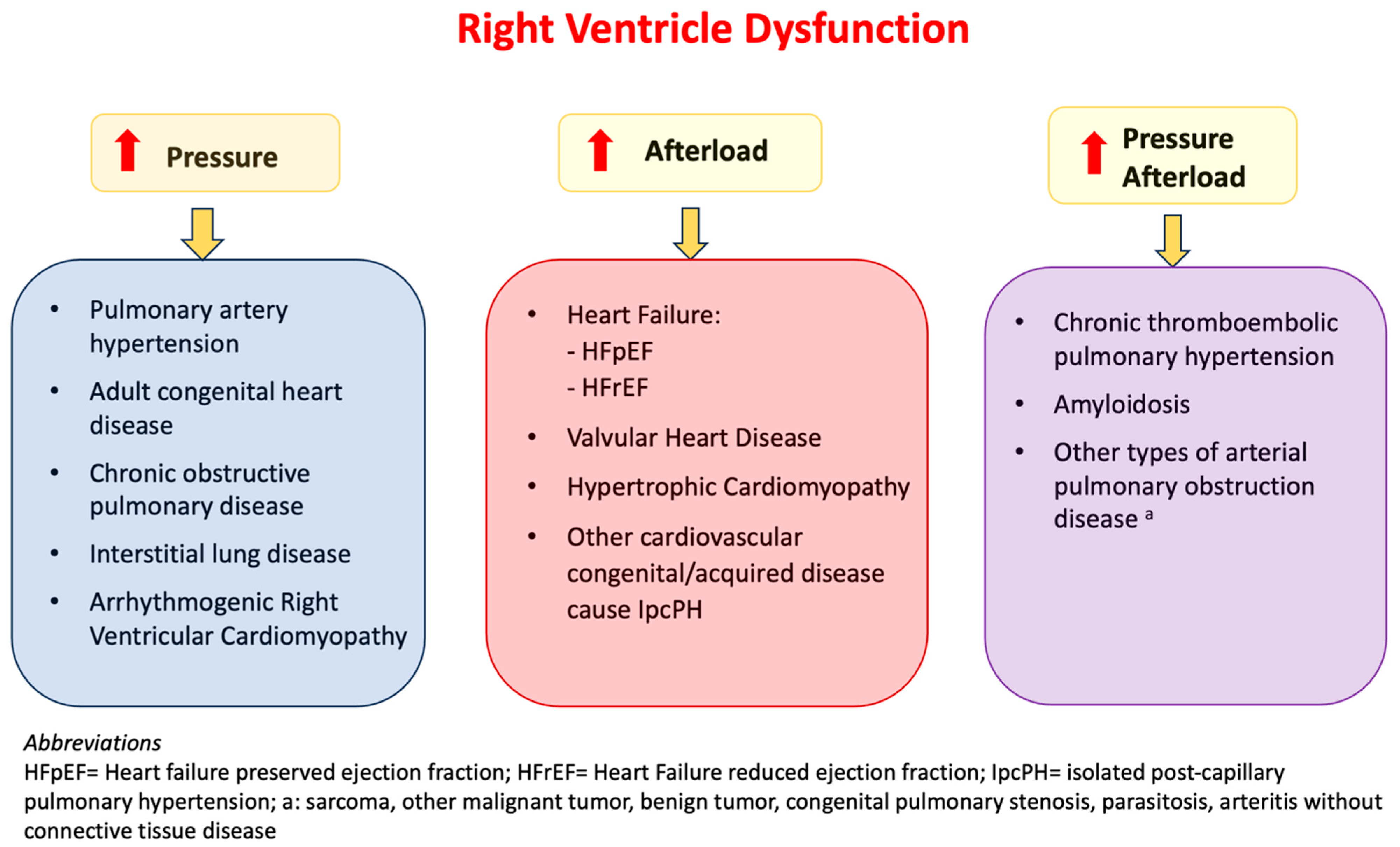
1.1. Pathophysiology
1.2. Clinical Presentation
2. Symptoms
3. Signs
Diagnosis
4. Lab Test
5. ECG
6. Chest X-Ray
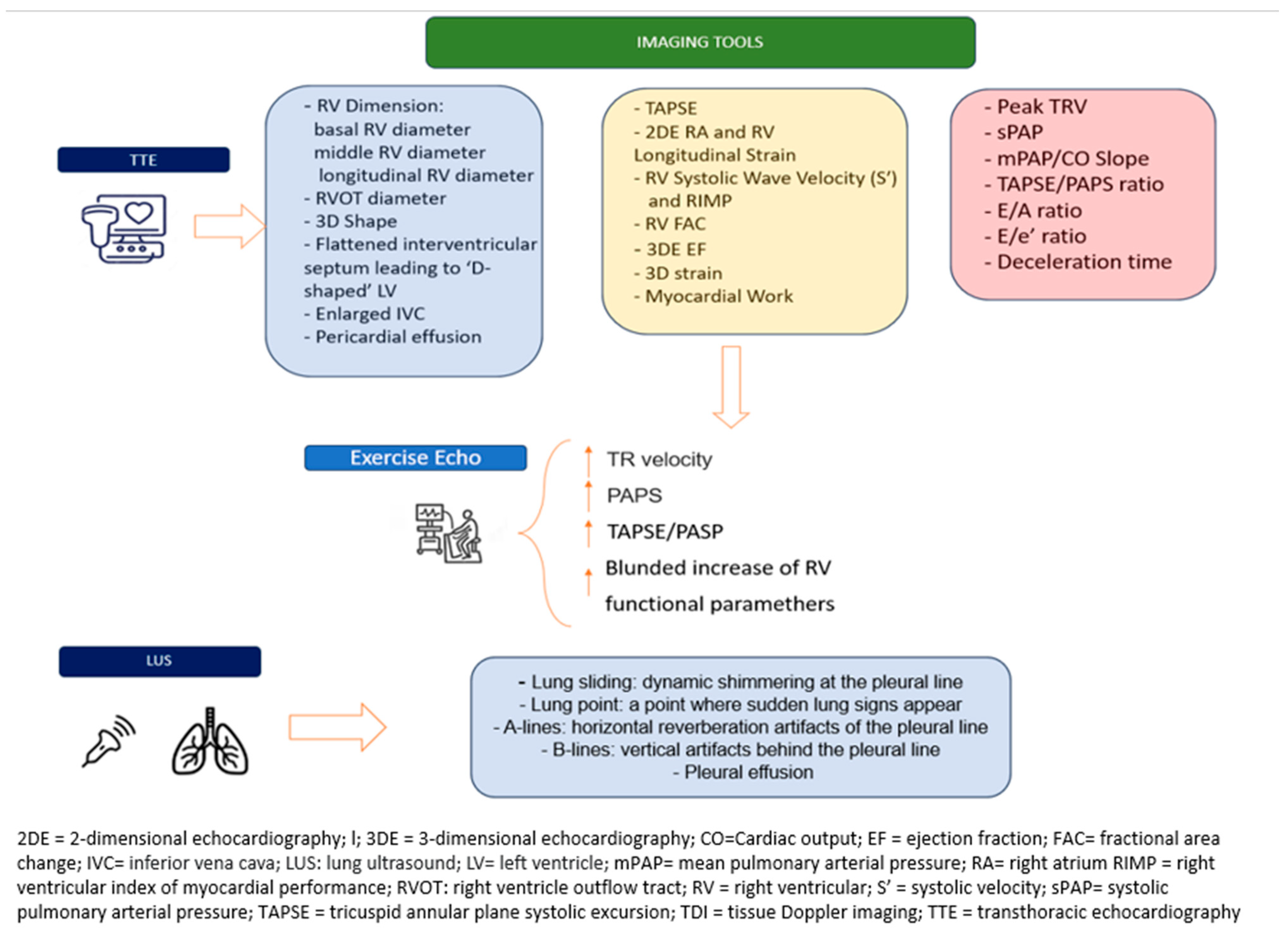
7. Lung Ultrasound
- -
- Lung sliding: Dynamic shimmering at the pleural line, producing the characteristic seashore sign on M-mode imaging.
- -
- Lung point: The transition zone where normal lung sliding intermittently appears, typically during inspiration, which is pathognomonic for a pneumothorax.
- -
- A-lines: Horizontal reverberation artifacts parallel to the pleural line, suggestive of a normally aerated lung.
- -
- B-lines: Vertical, hyperechoic artifacts originating from the pleural line associated with interstitial syndrome.
- -
- Pleural effusion: An anechoic or hypoechoic fluid collection in the pleural space [3].
8. Computed Tomography
9. Standard Echocardiography
- -
- Left Heart Disease Assessment
- -
- Right Heart Size and Function Assessment
- -
- Hemodynamics Parameter Assessment
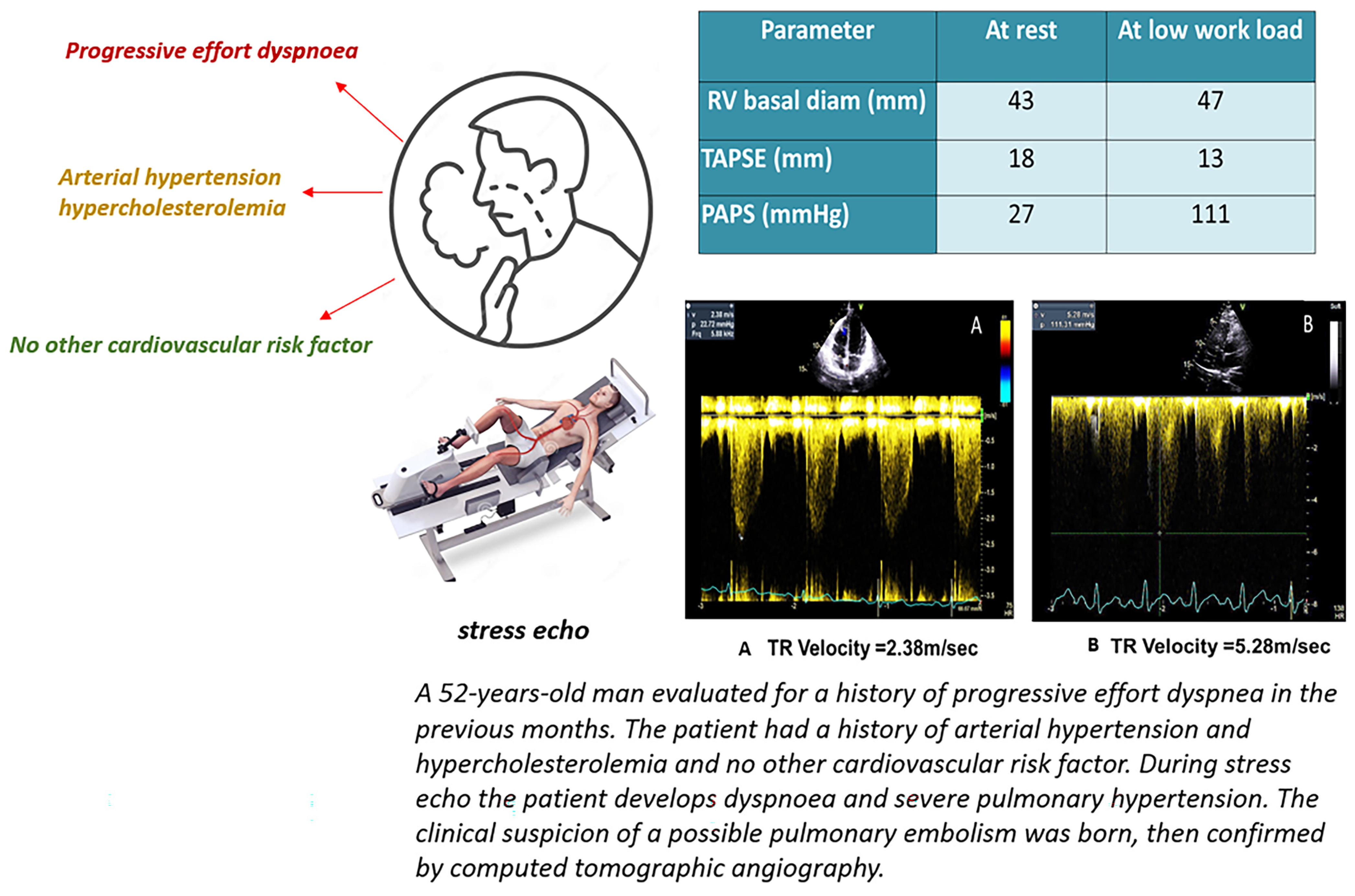
10. Stress Echocardiography
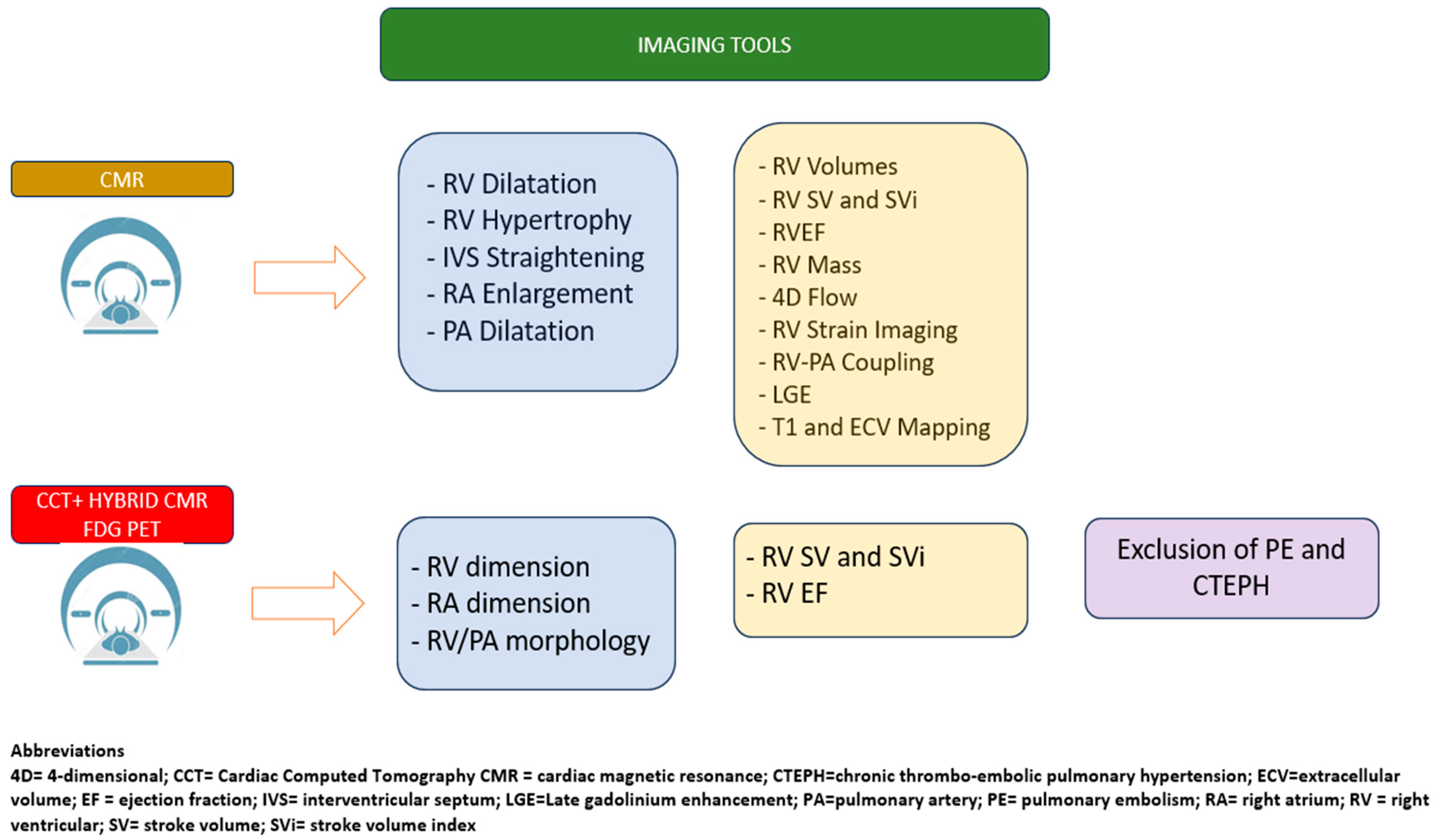
11. Cardiac Magnetic Resonance
12. Cardiac Computed Tomography and Nuclear Imaging
13. Right Heart Catheterization
14. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABG | Arterial Blood Gas |
| ARDS | Acute Respiratory Distress Syndrome |
| BNP | B-type Natriuretic Peptide |
| CHD | Coronary Heart Disease/Congenital Heart Disease (ambiguità) |
| CMR | Cardiac Magnetic Resonance |
| CMR-FT | Cardiac Magnetic Resonance Feature Tracking |
| CO | Cardiac Output |
| COPD | Chronic Obstructive Pulmonary Disease |
| CT | Computed Tomography |
| CTEPH | Chronic Thromboembolic Pulmonary Hypertension |
| DCM | Dilated Cardiomyopathy |
| DVT | Deep Vein Thrombosis |
| ECG | Electrocardiogram |
| ECV | Extracellular Volume |
| EF | Ejection Fraction |
| FAC | Fractional Area Change |
| HCM | Hypertrophic Cardiomyopathy |
| HF | Heart Failure |
| LA | Left Atrium |
| LAD | Left Anterior Descending (artery) |
| LGE | Late Gadolinium Enhancement |
| LUS | Lung Ultrasound |
| LV | Left Ventricle |
| LVEF | Left Ventricular Ejection Fraction |
| MRI | Magnetic Resonance Imaging |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| PA | Pulmonary Artery |
| PAH | Pulmonary Arterial Hypertension |
| PAP | Pulmonary Arterial Pressure |
| PASP | Pulmonary Artery Systolic Pressure |
| PCWP | Pulmonary Capillary Wedge Pressure |
| PH | Pulmonary Hypertension |
| PNX | Pneumothorax |
| PVR | Pulmonary Vascular Resistance |
| RA | Right Atrium |
| RAP | Right Atrial Pressure |
| RCM | Restrictive Cardiomyopathy |
| RH-PCU | Right Heart–Pulmonary Circulation Unit |
| RHC | Right Heart Catheterization |
| RIMP | Right Ventricular Index of Myocardial Performance |
| RV | Right Ventricle |
| RVEF | Right Ventricular Ejection Fraction |
| RVH | Right Ventricular Hypertrophy |
| RVMI | Right Ventricular Myocardial Infarction |
| RVMW | Right Ventricular Myocardial Work |
| SCD | Sudden Cardiac Death |
| SaO2 | Arterial Oxygen Saturation |
| SvO2 | Mixed Venous Oxygen Saturation |
| S′ | RV Systolic Wave Velocity |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TDE | Transthoracic Doppler Echocardiography |
| TRV | Tricuspid Regurgitation Velocity |
| VHD | Valvular Heart Disease |
| mPAP | Mean Pulmonary Artery Pressure |
| sPAP | Systolic Pulmonary Artery Pressure |
References
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right ventricular function in cardiovascular disease, part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008, 117, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Gargani, L.; Ostenfeld, E.; D’Alto, M.; Kasprzak, J.; Voilliot, D.; Selton-Suty, C.; Vriz, O.; Marra, A.M.; Argiento, P.; et al. Imaging the right heart pulmonary circulation unit: Insights from advanced ultrasound techniques. Echocardiography 2017, 34, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D. Lung ultrasound in the critically ill. Ann. Intensive Care 2014, 20, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Caramello, V.; Cardinale, L.; Mussa, A.; Bar, F.; Frascisco, M.F. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am. J. Emerg. Med. 2008, 26, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picano, E.; Scali, M.C.; Ciampi, Q.; Lichtenstein, D. Lung Ultrasound for the Cardiologist. JACC Cardiovasc. Imaging 2018, 11, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Dellegrottaglie, S.; Patel, S.; Grunig, E.; D’Andrea, A.; Ferrara, F.; Gargiulo, P.; D’Alto, M.; Soricelli, A.; Cittadini, A.; et al. Multimodality imaging in pulmonary hypertension. Can. J. Cardiol. 2015, 31, 440–459. [Google Scholar] [CrossRef] [PubMed]
- Contractor, S.; Maldjian, P.D.; Sharma, V.K.; Gor, D.M. Role of helical CT in detecting right ventricular dysfunction secondary to acute pulmonary embolism. J. Comput. Assist. Tomogr. 2002, 26, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Wittram, C.; Kalra, M.K.; Maher, M.M.; Greenfield, A.; McLoud, T.C.; Shepard, J.A. Acute and chronic pulmonary emboli: Angiography-CT correlation. AJR Am. J. Roentgenol. 2006, 186 (Suppl. 2), S421–S429. [Google Scholar] [CrossRef] [PubMed]
- Nadrous, H.F.; Pellikka, P.A.; Krowka, M.J.; Swanson, K.L.; Chaowalit, N.; Decker, P.A.; Ryu, J.H. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest 2005, 128, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Motoji, Y.; Ferrara, F.; Bossone, E.; Naeije, R. The Right Heart-Pulmonary Circulation Unit in Systemic Hypertension. Heart Fail. Clin. 2018, 14, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Filippetti, L.; Voilliot, D.; Bellino, M.; Citro, R.; Go, Y.Y.; Lancellotti, P. The Right Heart-Pulmonary Circulation Unit and Left Heart Valve Disease. Heart Fail. Clin. 2018, 14, 431–442. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Formisano, T.; La Gerche, A.; Cardim, N.; Carbone, A.; Scarafile, R.; Martone, F.; D’Alto, M.; Bossone, E.; Galderisi, M. Right Heart-Pulmonary Circulation Unit in Cardiomyopathies and Storage Diseases. Heart Fail. Clin. 2018, 14, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Iadevaia, V.; Gammaldi, V.; Iervolino, A.; Capece, L.M.; Sciascia, D.; Cuomo, V.; Iacono, M.; Paoletta, D.; Santoro, C.; et al. Right Ventricular Myocardial Involvement in Anderson-Fabry Disease at Diagnosis: Evaluation with Three-Dimensional Strain Imaging. Life 2023, 13, 1571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Femia, G.; French, J.K.; Juergens, C.; Leung, D.; Lo, S. Right ventricular myocardial infarction: Pathophysiology, clinical implications and management. Rev. Cardiovasc. Med. 2021, 22, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Surkova, E.; Cosyns, B.; Gerber, B.; Gimelli, A.; la Gerche, A.; Ajmone, M.N. The dysfunctional right ventricle: The importance of multi-modality imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 885–897. [Google Scholar] [CrossRef]
- Ho, S.Y.; Nihoyannopoulos, P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006, 92, 12–13. [Google Scholar] [CrossRef]
- Le Tourneau, T.; Piriou, N.; Donal, E.; Deswarte, G.; Topilsky, Y.; Lamblin, N.; Warin-Fresse, K.; Crochet, D.; Damy, T.; Trochu, J.N. Imaging and modern assessment of the right ventricle. Minerva Cardioangiol. 2011, 59, 349–373. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantifcation by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Damy, T.; Kallvikbacka-Bennett, A.; Goode, K.; Khaleva, O.; Lewinter, C.; Hobkirk, J.; Nikitin, N.P.; Dubois-Randé, J.-L.; Hittinger, L.; Clark, A.L.; et al. Prevalence of, Associations with, and Prognostic Value of Tricuspid Annular Plane Systolic Excursion (TAPSE) Among Out-Patients Referred for the Evaluation of Heart Failure. J. Card. Fail. 2012, 18, 216–225. [Google Scholar] [CrossRef]
- Aloia, E.; Cameli, M.; D’Ascenzi, F.; Sciaccaluga, C.; Mondillo, S. TAPSE: An old but useful tool in different diseases. Int. J. Cardiol. 2016, 225, 177–183. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; De Isla, L.P.; et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 3–46. [Google Scholar] [CrossRef]
- Otani, K.; Nabeshima, Y.; Kitano, T.; Takeuchi, M. Accuracy of fully automated right ventricular quantification software with 3D echocardiography: Direct comparison with cardiac magnetic resonance and semi-automated quantification software. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 787–795. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Cvijic, M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Mondillo, S.; Galderisi, M.; Mele, D.; Cameli, M.; Lomoriello, V.S.; Zacà, V.; Ballo, P.; D’ANdrea, A.; Muraru, D.; Losi, M.; et al. Speckle-tracking echocardiography: A new technique for assessing myocardial function. J. Ultrasound Med. 2011, 30, 71–83. [Google Scholar] [CrossRef]
- Butcher, S.C.; Fortuni, F.; Montero-Cabezas, J.M.; Abou, R.; El Mahdiui, M.; van der Bijl, P.; van der Velde, E.T.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Right ventricular myocardial work: Proof-of-concept for non-invasive assessment of right ventricular function. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, X.; Chen, W.; Tang, Y.; Chen, X.; Wang, X.; Jing, B.; Sun, Y.; Huang, K.; Gao, Q.; et al. Noninvasive right ventricular work in patients with atrial septal defects: A proof-of-concept study. Cardiovasc. Ultrasound 2023, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- DiLorenzo, M.P.; Bhatt, S.M.; Mercer-Rosa, L. How best to assess right ventricular function by echocardiography. Cardiol. Young 2015, 25, 1473–1481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panesar, D.K.; Burch, M. Assessment of Diastolic Function in Congenital Heart Disease. Front. Cardiovasc. Med. 2017, 4, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudski, L.G.; Gargani, L.; Armstrong, W.F.; Lancellotti, P.; Lester, S.J.; Grünig, E.; D’Alto, M.; Åström Aneq, M.; Ferrara, F.; Saggar, R. Stressing the Cardiopulmonary Vascular System: The Role of Echocardiography. J. Am. Soc. Echocardiogr. 2018, 31, 527–550.e11. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart inadults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [PubMed]
- Ho, J.E.; Zern, E.K.; Lau, E.S.; Wooster, L.; Bailey, C.S.; Cunningham, T.; Eisman, A.S.; Hardin, K.M.; Farrell, R.; Sbarbaro, J.A.; et al. Exercise Pulmonary Hypertension Predicts Clinical Outcomes in Patients with Dyspnea on Effort. J. Am. Coll. Cardiol. 2020, 75, 17–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bidart, C.M.; Abbas, A.E.; Parish, J.M.; Chaliki, H.P.; Moreno, C.A.; Lester, S.J. The noninvasive evaluation of exercise-induced changes in pulmonary artery pressure and pulmonary vascular resistance. J. Am. Soc. Echocardiogr. 2007, 20, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Rubenfire, M.; Bach, D.S.; Ricciardi, M.; Armstrong, W.F. Range of tricuspid regurgitation velocity at rest and during exercise in normal adult men: Implications for the diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 1999, 33, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Argiento, P.; Chesler, N.; Mulè, M.; D’Alto, M.; Bossone, E.; Unger, P.; Naeije, R. Exercise stress echocardiography for the study of the pulmonary circulation. Eur. Respir. J. 2010, 35, 1273–1278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varga, A.; Garcia, M.A.; Picano, E. International Stress Echo Complication Registry. Safety of stress echocardiography (from the International Stress Echo Complication Registry). Am. J. Cardiol. 2006, 98, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Pugliese, N.R.; De Biase, N.; Mazzola, M.; Agoston, G.; Arcopinto, M.; Argiento, P.; Armstrong, W.F.; Bandera, F.; Cademartiri, F.; et al. RIGHT Heart International NETwork (RIGHT-NET) Investigators. Exercise Stress Echocardiography of the Right Ventricle and Pulmonary Circulation. J. Am. Coll. Cardiol. 2023, 82, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Addetia, K.; Pontone, G.; Torlasco, C.; Lang, R.M.; Parati, G.; Muraru, D. Advanced imaging of right ventricular anatomy and function. Heart 2020, 106, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.A.; Raman, S.V.; Simonetti, O.P. Basic Principles of Cardio Vascular MRI: Physics and Imaging Techniques; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Swift, A.J.; Rajaram, S.; Condliffe, R.; Capener, D.; Hurdman, J.; AElliot, C.; Wild, J.M.; Kiely, D.G. Diagnostic accuracy of cardiovascular mag netic resonance imaging of right ventricular morphology and function in the assessment of suspected pulmonary hypertension results from the ASPIRE registry. J. Cardiovasc. Magn. Reson. 2012, 14, 31–40. [Google Scholar] [CrossRef]
- Dellegrottaglie, S.; Sanz, J.; Poon, M.; Viles-Gonzalez, J.F.; Sulica, R.; Goyenechea, M.; Macaluso, F.; Fuster, V.; Rajagopalan, S. Pulmonary hypertension: Accuracy of detection with left ventricular septal-to-free wall curvature ratio measured at cardiac MR. Radiology 2007, 243, 63–69. [Google Scholar] [CrossRef]
- Boerrigter, B.; Mauritz, G.J.; Marcus, J.T.; Helderman, F.; Postmus, P.E.; Westerhof, N.; Vonk-Noordegraaf, A. Progressive dilatation of the main pul monary artery in pulmonary arterial hypertension is irrespec tive of changes in pulmonary artery pressure. In B59. Pulmonary Arterial Hypertension: Diagnosis, Hemodynamic Assessment, and Imaging; American Thoracic Society: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Doyle, C.M.; Orr, J.; Greenwood, J.P.; Plein, S.; Tsoumpas, C.; Bissell, M.M. Four-dimensional flow magnetic resonance imaging in the assessment of blood flow in the heart and great vessels: A systematic review. J. Magn. Reason. Imaging 2021, 55, 1301–1321. [Google Scholar] [CrossRef]
- Markl, M.; Kilner, P.J.; Ebbers, T. Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reason. 2011, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Azarine, A.; Garcon, P.; Stansal, A.; Canepa, N.; Angelopoulos, G.; Silvera, S.; Sidi, D.; Marteau, V.; Zins, M. Four-dimensional Flow MRI: Principles and Cardiovascular Applications. Radiographics 2019, 39, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Leng, S.; Tan, R.-S.; Chai, P.; Yeo, T.J.; Bryant, J.A.; Teo, L.L.S.; Fortier, M.V.; Ruan, W.; Low, T.T.; et al. Right ventricular energetic biomarkers from 4D Flow CMR are associated with exertional capacity in pulmonary arterial hypertension. J. Cardiovasc. Magn. Reson. 2022, 24, 61. [Google Scholar] [CrossRef]
- Scatteia, A.; Baritussio, A.; Bucciarelli-Ducci, C. Strain imaging using cardiac magnetic resonance. Heart Fail. Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedrizzetti, G.; Claus, P.; Kilner, P.J.; Nagel, E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J. Cardiovasc. Magn. Reson. 2016, 18, 51. [Google Scholar] [CrossRef]
- Morais, P.; Marchi, A.; Bogaert, J.A.; Dresselaers, T.; Heyde, B.; D’hooge, J.; Bogaert, J. Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm: Assessment of variability in a real-life clinical setting. J. Cardiovasc. Magn. Reson. 2017, 19, 24. [Google Scholar] [CrossRef]
- de Siqueira, M.E.M.; Pozo, E.; Fernandes, V.R.; Sengupta, P.P.; Modesto, K.; Gupta, S.S.; Barbeito-Caamaño, C.; Narula, J.; Fuster, V.; Caixeta, A.; et al. Characterization and clinical significance of right ventricular mechanics in pulmonary hypertension evaluated with cardiovascular magnetic resonance feature tracking. J. Cardiovasc. Magn. Reson. 2016, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Freed, B.H.; Gomberg-Maitland, M.; Chandra, S.; Mor-Avi, V.; Rich, S.; Archer, S.L.; Jamison, E.B., Jr.; Lang, R.M.; Patel, A.R. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J. Cardiovasc. Magn. Reson. 2012, 14, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swift, A.J.; Rajaram, S.; Capener, D.; Elliot, C.; Condliffe, R.; Wild, J.M.; Kiely, D.G. LGE patterns in pulmonary hypertension do not impact overall mortality. JACC Cardiovasc. Imaging 2014, 7, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.C.; Johns, C.S.; Stewart, N.J.; Oram, C.J.E.; Capener, D.A.; Puntmann, V.O.; Elliot, C.A.; Condliffe, R.C.; Kiely, D.G.; Graves, M.J.; et al. Diagnostic and prognostic significance of cardiovascular magnetic resonance native myocardial T1 mapping in patients with pulmonary hypertension. J. Cardiovasc. Magn. Reson. 2018, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Li, E.; Benefield, B.C.; Swat, S.A.; Polsinelli, V.B.; Carr, J.C.; Shah, S.J.; Markl, M.; Collins, J.D.; Freed, B.H. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. 2020, 7, 254–264. [Google Scholar] [CrossRef]
- Guo, Y.K.; Gao, H.L.; Zhang, X.C.; Wang, Q.L.; Yang, Z.G.; Ma, E.S. Accuracy and reproducibility of assessing right ventricular function with 64 section multi-detector row CT: Comparison with magnetic resonance imaging. Int. J. Cardiol. 2010, 139, 254–262. [Google Scholar] [CrossRef]
- Pickett, C.A.; Cheezum, M.K.; Kassop, D.; Villines, T.C.; Hulten, E.A. Accuracy of cardiac CT, radionucleotide and invasive ventriculography, two- and three-dimensional echocardiography, and SPECT for left and right ventricular ejection fraction compared with cardiac MRI: A meta analysis. Eur. Heart. J. Cardiovasc. Imaging 2015, 16, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Lerakis, S.; Delgado, V.; Addetia, K.; Burkhoff, D.; Muraru, D.; Pinney, S.; Friedberg, M.K. Multimodality Imaging of Right Heart Function: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1954–1973. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation:a10-yearupdate. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Gonzalez-Hermosillo, L.M.; Cueto-Robledo, G.; Roldan-Valadez, E.; Graniel-Palafox, L.E.; Garcia-Cesar, M.; Torres-Rojas, M.B.; Romero-Martinez, B.; Castro-Escalante, K.Y. Right Heart Catheterization (RHC): A Comprehensive Review of Provocation Tests and Hepatic Hemodynamics in Patients with Pulmonary Hypertension (PH). Curr. Probl. Cardiol. 2022, 47, 101351. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucci, M.; Capece, L.M.; Pontoriero, M.; Paoletta, D.; Iacono, M.; La Rocca, F.; Luise, R.; Esposito, R. Right Heart Evaluation: A Tough Challenge for Clinicians. Life 2025, 15, 1194. https://doi.org/10.3390/life15081194
Pucci M, Capece LM, Pontoriero M, Paoletta D, Iacono M, La Rocca F, Luise R, Esposito R. Right Heart Evaluation: A Tough Challenge for Clinicians. Life. 2025; 15(8):1194. https://doi.org/10.3390/life15081194
Chicago/Turabian StylePucci, Martina, Luca Maria Capece, Mariateresa Pontoriero, Daniele Paoletta, Marina Iacono, Francesca La Rocca, Roberto Luise, and Roberta Esposito. 2025. "Right Heart Evaluation: A Tough Challenge for Clinicians" Life 15, no. 8: 1194. https://doi.org/10.3390/life15081194
APA StylePucci, M., Capece, L. M., Pontoriero, M., Paoletta, D., Iacono, M., La Rocca, F., Luise, R., & Esposito, R. (2025). Right Heart Evaluation: A Tough Challenge for Clinicians. Life, 15(8), 1194. https://doi.org/10.3390/life15081194







