Impaired Coronary Microcirculation and Myocardial Systolic Function: A Narrative Review on Non-Invasive Assessment in Cardiovascular Diseases
Abstract
1. Introduction
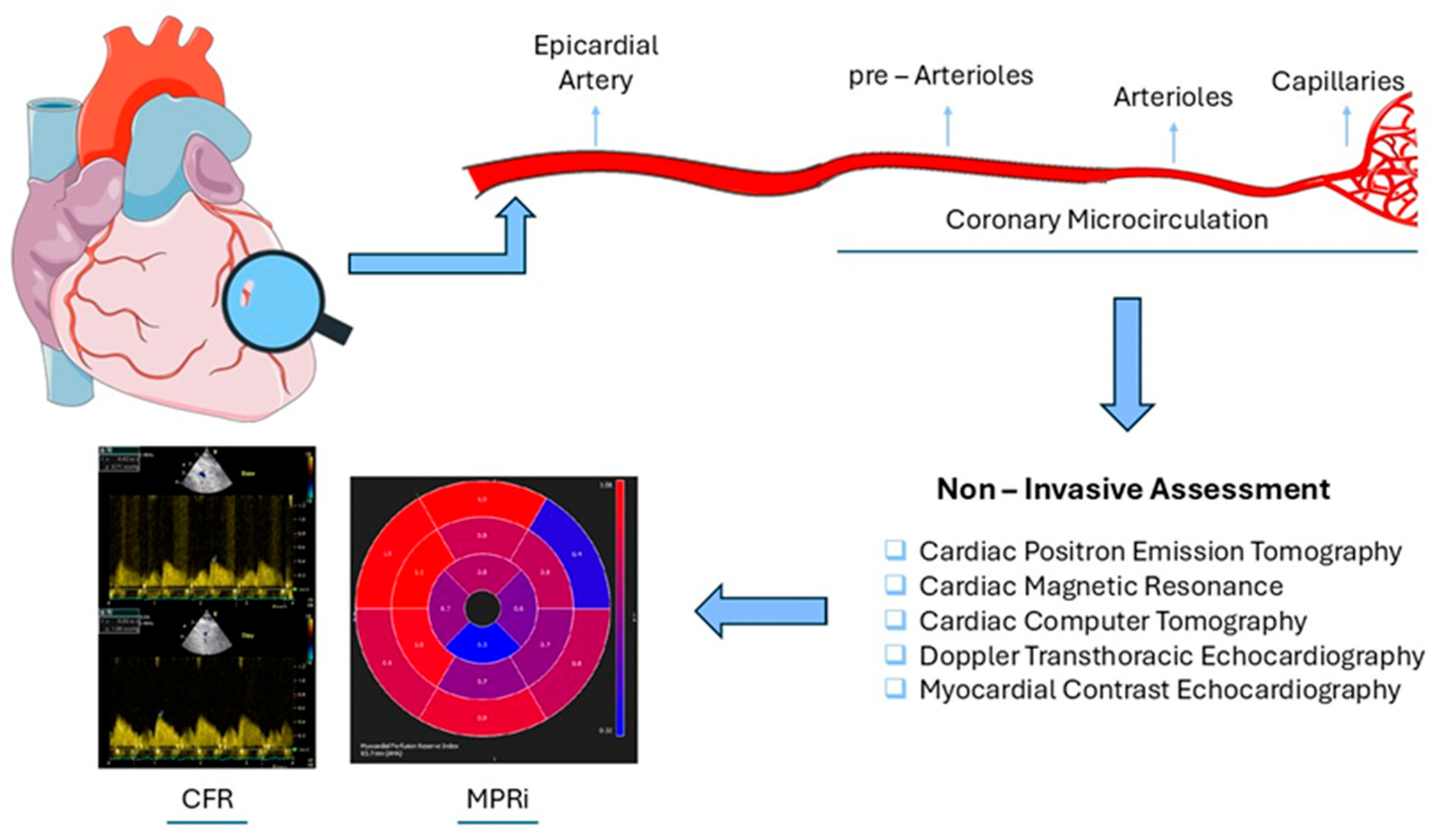
2. Coronary Microvascular Dysfunction
2.1. Invasive Assessment
2.2. Non-Invasive Assessment (Table 1)
2.2.1. Cardiac Positron Emission Tomography (PET)
| Imaging Modality | Methodology Protocol and Agents Used | Index Derived | Advantages | Disadvantages |
|---|---|---|---|---|
| PET |
| MPR < 2 |
|
|
| CMR |
| MPRI < 2 |
|
|
| CT |
| MPR < 2 |
|
|
| Doppler TTE |
| CFR < 2 |
|
|
| MCE |
| MBF < 2 |
|
|
2.2.2. Cardiac Magnetic Resonance (CMR)
2.2.3. Cardiac Computer Tomography (CT)
2.2.4. Transthoracic Echocardiography (TTE) and Myocardial Contrast Echocardiography (MCE)
3. Left Ventricular Systolic Function
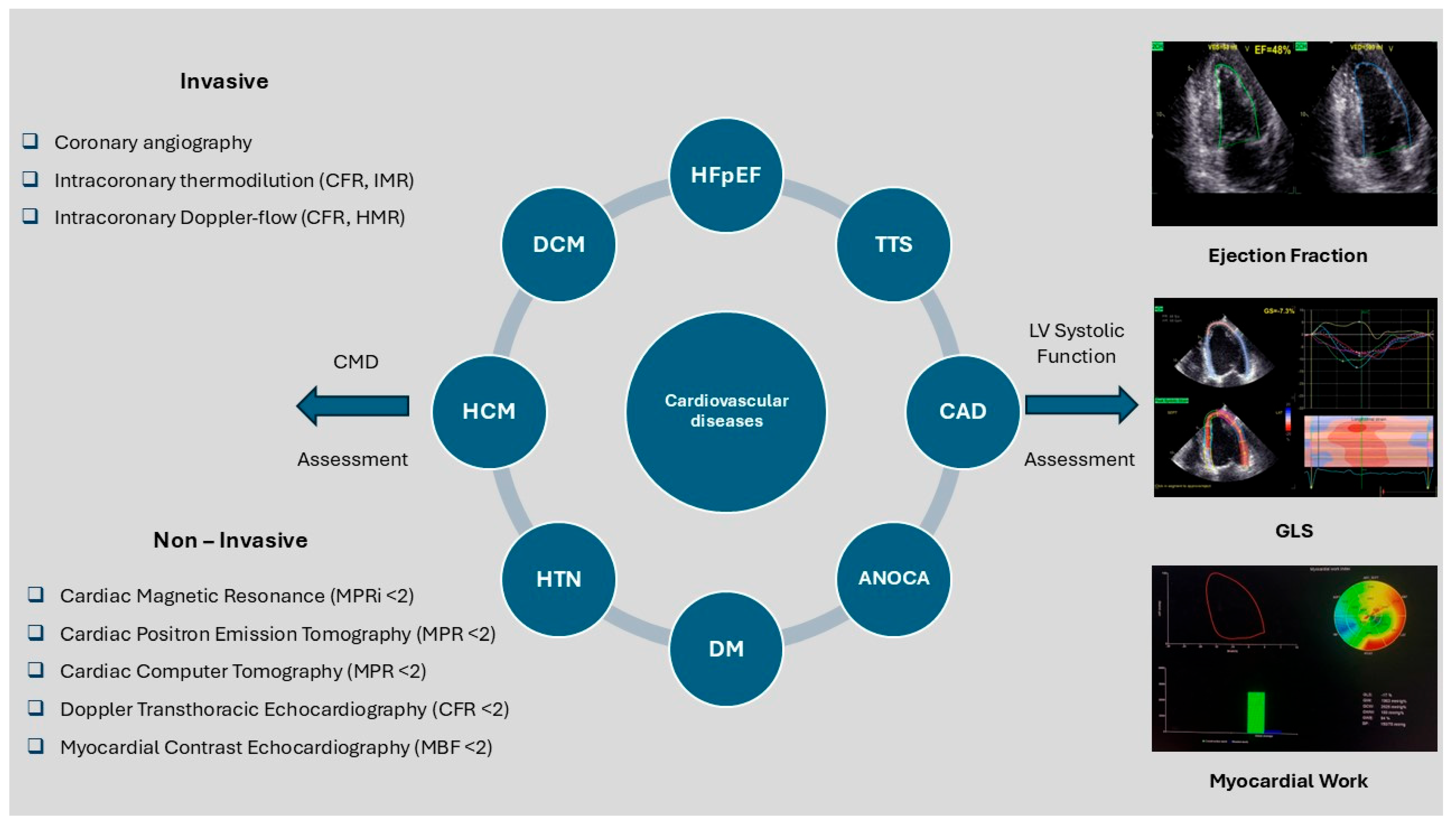
4. Coronary Microcirculation and Systolic Function in Cardiovascular Pathologies
4.1. Hypertension
4.2. Diabetes Mellitus (DM)
4.3. Dilated Cardiomyopathy (DCM)
4.4. Hypertrophic Cardiomyopathy (HCM)
4.5. Takotsubo Syndrome (TTS)
4.6. Obstructive CAD
4.7. Angina with No Obstructive Coronary Artery Disease (ANOCA)
4.8. Heart Failure with Preserved Ejection Fraction (HFpEF)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef]
- Shome, J.S.; Perera, D.; Plein, S.; Chiribiri, A. Current perspectives in coronary microvascular dysfunction. Microcirculation 2017, 24, e12340. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.; Samson, S.; Grover, A. Sarco/endoplasmic reticulum Ca2+-pump isoform SERCA3a is more resistant to superoxide damage than SERCA2b. Mol. Cell. Biochem. 2000, 203, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Talabieke, S.; Yang, X.; Yang, J.; Wan, Q.; Zhu, D.; Rao, H.; Wu, Y.; Chen, Z.; Li, H.; Xu, P.; et al. Arachidonic acid synergizes with aspirin preventing myocardial ischaemia–reperfusion injury and mitigates bleeding risk. Cardiovasc. Res. 2025, 121, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, J.J.; Liu, Y.J.; Mo, Z.W.; Wu, F.Y.; Hu, Z.J.; Peng, Y.M.; Zhang, X.Q.; Ma, Z.S.; Liu, Z.L.; et al. The oxidized phospholipid PGPC impairs endothelial function by promoting endothelial cell ferroptosis via FABP3. J. Lipid Res. 2024, 65, 100499. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October 2013; The GRADE Working Group: Freiburg, Germany, 2013. [Google Scholar]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef]
- Rehan, R.; Yong, A.; Ng, M.; Weaver, J.; Puranik, R. Coronary microvascular dysfunction: A review of recent progress and clinical implications. Front. Cardiovasc. Med. 2023, 10, 1111721. [Google Scholar] [CrossRef]
- Tonet, E.; Pompei, G.; Faragasso, E.; Cossu, A.; Pavasini, R.; Passarini, G.; Tebaldi, M.; Campo, G. Coronary Microvascular Dysfunction: PET, CMR and CT Assessment. J. Clin. Med. 2021, 10, 1848. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Mathew, R.C.; Bourque, J.M.; Salerno, M.; Kramer, C.M. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc. Imaging 2020, 13, 1577–1590. [Google Scholar] [CrossRef]
- Brown, L.A.E.; Gulsin, G.S.; Onciul, S.C.; Broadbent, D.A.; Yeo, J.L.; Wood, A.L.; Saunderson, C.E.D.; Das, A.; Jex, N.; Chowdhary, A.; et al. Sex- and age-specific normal values for automated quantitative pixel-wise myocardial perfusion cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2022, 24, 26–34. [Google Scholar] [CrossRef]
- Bechsgaard, D.F.; Hove, J.D.; Michelsen, M.M.; Mygind, N.D.; Pena, A.; Hansen, P.R.; Hansen, H.S.; Kastrup, J.; Høst, N.; Gustafsson, I.; et al. Myocardial CT perfusion compared with transthoracic Doppler echocardiography in evaluation of the coronary microvascular function: An iPOWERsubstudy. Clin. Physiol. Funct. Imaging 2021, 41, 85–94. [Google Scholar] [CrossRef]
- Schroder, J.; Prescott, E. Doppler Echocardiography Assessment of Coronary Microvascular Function in Patients With Angina and No Obstructive Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 723542. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Indermühle, A.; Reinhardt, J.; Meier, P.; Siegrist, P.T.; Namdar, M.; Kaufmann, P.A.; Seiler, C. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: Algorithm and validation. J. Am. Coll. Cardiol. 2005, 45, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.W.; Benjamins, J.W.; Knol, R.J.J.; van der Zant, F.M.; Asselbergs, F.W.; van der Harst, P.; van Es, R.; Piek, J.J.; Knuuti, J.; Kietselaer, B.; et al. Multi-task Deep Learning of Myocardial Blood Flow and Cardiovascular Risk Traits from PET Myocardial Perfusion Imaging. J. Nucl. Cardiol. 2022, 29, 3300–3310. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Seraphim, A.; Augusto, J.B.; Xue, H.; Chacko, L.; Aung, N.; Petersen, S.E.; Manisty, C.H.; Moon, J.C.; Treibel, T.A.; et al. The Prognostic Significance of Quantitative Myocardial Perfusion: An Artificial Intelligence-Based Approach Using Perfusion Mapping. Circulation 2020, 141, 1282–1291. [Google Scholar] [CrossRef]
- Scannell, C.M.; Alskaf, E.; Sharrack, N.; Razavi, R.; Ourselin, S.; Young, A.A.; Plein, S.; Chiribiri, A.; Villa, A.D.M.; Breeuwer, M.; et al. AI-AIF: Artificial Intelligence-Based Arterial Input Function for Quantitative Stress Perfusion Cardiac Magnetic Resonance. Eur. Heart J. Digit. Health 2022, 4, 12–21. [Google Scholar] [CrossRef]
- Costantini, P.; Groenhoff, L.; Ostillio, E.; Coraducci, F.; Secchi, F.; Carriero, A.; Albrecht, M.H.; Rotzinger, D.C.; De Cecco, C.N.; Varga-Szemes, A.; et al. Advancements in Cardiac CT Imaging: The Era of Artificial Intelligence. Echocardiography 2024, 41, e70042. [Google Scholar] [CrossRef]
- Sunyecz, I.L.; McCallinhart, P.E.; Patel, K.U.; McDermott, M.R.; Trask, A.J. Defining Coronary Flow Patterns: Comprehensive Automation of Transthoracic Doppler Coronary Blood Flow. Sci. Rep. 2018, 8, 17268. [Google Scholar] [CrossRef]
- Bossenbroek, J.; Ueyama, Y.; McCallinhart, P.E.; Bartlett, C.W.; Ray, W.C.; Trask, A.J. Improvement of automated analysis of coronary Doppler echocardiograms. Sci. Rep. 2022, 12, 7490. [Google Scholar] [CrossRef]
- Li, M.; Zeng, D.; Xie, Q.; Xu, R.; Wang, Y.; Ma, D.; Liu, Y.; Zhang, H.; Chen, J.; Sun, L.; et al. A Deep Learning Approach with Temporal Consistency for Automatic Myocardial Segmentation of Quantitative Myocardial Contrast Echocardiography. Int. J. Cardiovasc. Imaging 2021, 37, 1967–1978. [Google Scholar] [CrossRef]
- Marwick, T. Ejection Fraction Pros and Cons: JACC State-of-the-Art Review. JACC 2018, 72, 2360–2379. [Google Scholar] [CrossRef]
- Marzlin, N.; Hays, A.G.; Peters, M.; Kaminski, A.; Roemer, S.; O’Leary, P.; Schuster, A.; Nagel, E.; Kelle, S.; Morton, G.; et al. Myocardial Work in Echocardiography. Circ. Cardiovasc. Imaging 2023, 16, e014419. [Google Scholar]
- Völz, S.; Svedlund, S.; Andersson, B.; Li-Ming, G.; Rundqvist, B. Coronary flow reserve in patients with resistant hypertension. Clin. Res. Cardiol. 2017, 106, 151–157. [Google Scholar] [CrossRef]
- Zdravkovic, M.; Popadic, V.; Klasnja, S.; Klasnja, A.; Ivankovic, T.; Lasica, R.; Radovanovic, D.; Pavlovic, M.; Jankovic, R.; Savic, L.; et al. Coronary Microvascular Dysfunction and Hypertension: A Bond More Important than We Think. Med. Kaunas. Lith. 2023, 59, 2149. [Google Scholar] [CrossRef] [PubMed]
- Kozàkovà, M.; Ferrannini, E.; Palombo, C. Relation between left ventricular midwall function and coronary vasodilator capacity in arterial hypertension. Hypertension 2003, 42, 528–533. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lekakis, J.; Papadopoulos, C.; Triantafyllidi, H.; Paraskevaidis, I.; Georgoula, G.; Trivilou, P.; Stamatelopoulos, K.; Makavos, G.; Tsioufis, C.; et al. Incremental Value of Pulse Wave Velocity in the Determination of Coronary Microcirculatory Dysfunction in Never-Treated Patients with Essential Hypertension. Am. J. Hypertens. 2008, 21, 806–813. [Google Scholar] [CrossRef]
- Caliskan, M.; Caliskan, Z.; Gullu, H.; Keles, N.; Bulur, S.; Turan, Y.; Aksu, U.; Akyuz, A.; Ciftci, O.; Demirtas, E.; et al. Increased Morning Blood Pressure Surge and Coronary Microvascular Dysfunction in Patients with Early Stage Hypertension. J. Am. Soc. Hypertens. 2014, 8, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Tzortzis, S.; Paraskevaidis, I.; Triantafyllidi, H.; Papadopoulos, C.; Papadakis, I.; Stamatelopoulos, K.; Lekakis, J.; Kremastinos, D.; Makavos, G.; et al. Association of Abnormal Coronary Microcirculatory Function with Impaired Response of Longitudinal Left Ventricular Function during Adenosine Stress Echocardiography in Untreated Hypertensive Patients. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Voumvourakis, A.; Makavos, G.; Triantafyllidi, H.; Pavlidis, G.; Katogiannis, K.; Trivilou, P.; Parissis, J.; Lekakis, J.; Thymis, J.; et al. Association of Impaired Endothelial Glycocalyx with Arterial Stiffness, Coronary Microcirculatory Dysfunction, and Abnormal Myocardial Deformation in Untreated Hypertensives. J. Clin. Hypertens. 2018, 20, 672–679. [Google Scholar] [CrossRef]
- Evangelou, D.; Bechlioulis, A.; Tzeltzes, G.; Lakkas, L.; Theodorou, I.; Kalaitzidis, R.; Styliadis, I.; Tzimas, T.; Papanikolaou, V.; Fountoulakis, N.; et al. Myocardial Strain Indices and Coronary Flow Reserve Are Only Mildly Affected in Healthy Hypertensive Patients. Int. J. Cardiovasc. Imaging 2021, 37, 69–79. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.G.; Gao, Y.; Xie, L.J.; Jiang, L.; Hu, B.Y.; Zhang, Y.; Wang, J.; Li, R.; Chen, J.; et al. Left Ventricular Subclinical Myocardial Dysfunction in Uncomplicated Type 2 Diabetes Mellitus Is Associated with Impaired Myocardial Perfusion: A Contrast-Enhanced Cardiovascular Magnetic Resonance Study. Cardiovasc. Diabetol. 2018, 17, 139. [Google Scholar] [CrossRef]
- Halabi, A.; Nolan, M.; Potter, E.; Wright, L.; Asham, A.; Marwick, T.H. Role of microvascular dysfunction in left ventricular dysfunction in type 2 diabetes mellitus. J. Diabetes Complicat. 2021, 35, 107907. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Nistri, S.; Castaldo, F.; Galderisi, M.; Mele, D.; Agricola, E.; Mondillo, S.; Caso, P.; D’Errico, A.; Russo, M.G.; et al. The Relationship between Early Left Ventricular Myocardial Alterations and Reduced Coronary Flow Reserve in Non-Insulin-Dependent Diabetic Patients with Microvascular Angina. Int. J. Cardiol. 2012, 154, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Pavlidis, G.; Tsoumani, M.; Kousathana, F.; Katogiannis, K.; Tsilivarakis, D.; Trivilou, P.; Papaioannou, T.G.; Lekakis, J.; Makavos, G.; et al. Endothelial Dysfunction Is Associated with Decreased Nitric Oxide Bioavailability in Dysglycaemic Subjects and First-Degree Relatives of Type 2 Diabetic Patients. J. Clin. Med. 2022, 11, 3299. [Google Scholar] [CrossRef] [PubMed]
- Dattani, A.; Marrow, B.A.; Gulsin, G.S.; Yeo, J.L.; Puranik, A.; Brady, E.M.; McCann, G.P.; Levelt, E.; Rider, O.J.; Brown, L.A.E.; et al. Association between Coronary Microvascular Dysfunction and Exercise Capacity in Dilated Cardiomyopathy. J. Soc. Cardiovasc. Magn. Reason. 2024, 26, 101108. [Google Scholar] [CrossRef]
- Ma, J.; Guan, L.; Yang, L.; Mahemuti, A.; Mu, Y. Relationship Between Myocardial Perfusion and Myocardial Function in Dilated Cardiomyopathy by Shown Ultrasonography. Int. Heart J. 2021, 62, 792–800. [Google Scholar] [CrossRef]
- Takafuji, M.; Ishida, M.; Nakamura, S.; Nakata, K.; Ito, H.; Kokawa, T.; Kido, T.; Kido, T.; Kurata, A.; Kitagawa, K.; et al. Microvascular Dysfunction in Patients with Idiopathic Dilated Cardiomyopathy: Quantitative Assessment with Phase Contrast Cine MR Imaging of the Coronary Sinus. Magn. Reson. Med. Sci. 2025, 24, 10–19. [Google Scholar] [CrossRef]
- Ohba, M.; Hosokawa, R.; Kambara, N.; Tadamura, E.; Mamede, M.; Kubo, S.; Higashi, M.; Nishimura, K.; Inoue, K.; Nakamura, T.; et al. Difference in Myocardial Flow Reserve between Patients with Dilated Cardiomyopathy and Those with Dilated Phase of Hypertrophic Cardiomyopathy: Evaluation by 15O-Water PET. Circ. J. 2007, 71, 884–890. [Google Scholar] [CrossRef]
- Raphael, C.E.; Mitchell, F.; Kanaganayagam, G.S.; Liew, A.C.; Di Pietro, E.; Vieira, M.S.; Dweck, M.R.; Semple, T.R.; Prasad, S.; Pennell, D.J.; et al. Cardiovascular Magnetic Resonance Predictors of Heart Failure in Hypertrophic Cardiomyopathy: The Role of Myocardial Replacement Fibrosis and the Microcirculation. J. Cardiovasc. Magn. Reson. 2021, 23, 26. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Girolami, F.; Torricelli, F.; Yacoub, M.H.; Spirito, P.; Poggesi, C.; et al. Relevance of Coronary Microvascular Flow Impairment to Long-Term Remodeling and Systolic Dysfunction in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 1043–1048. [Google Scholar] [CrossRef]
- Garcia Brás, P.; Rosa, S.A.; Cardoso, I.; Branco, L.M.; Galrinho, A.; Gonçalves, A.V.; Almeida, A.G.; Silva, D.; Pereira, A.; Sousa, L.; et al. Microvascular Dysfunction Is Associated with Impaired Myocardial Work in Obstructive and Nonobstructive Hypertrophic Cardiomyopathy: A Multimodality Study. J. Am. Heart Assoc. 2023, 12, e028857. [Google Scholar] [CrossRef]
- Ekenbäck, C.; Nickander, J.; Jokhaji, F.; Tornvall, P.; Engblom, H.; Spaak, J.; Frick, M.; Carlsson, M.; Aletras, A.H.; Ubachs, J.; et al. Coronary Microvascular Dysfunction in Takotsubo Syndrome and Associations with Left Ventricular Function. ESC Heart Fail. 2023, 10, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Yin, L.; Sisakian, H.; Hakobyan, T.; Jeong, L.S.; Joshi, H.; Kim, Y.J.; Zhang, Y.; Chen, W.; Chen, H.; et al. Takotsubo Syndrome Is a Coronary Microvascular Disease: Experimental Evidence. Eur. Heart J. 2023, 44, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.; Sicari, R.; Citro, R.; Ossena, G.; Buja, P.; Picano, E. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: A Doppler transthoracic echo study. Ann. Med. 2009, 41, 462–470. [Google Scholar] [CrossRef]
- Meimoun, P.; Malaquin, D.; Benali, T.; Boulanger, J.; Zemir, H.; Tribouilloy, C. Transient impairment of coronary flow reserve in tako-tsubo cardiomyopathy is related to left ventricular systolic parameters. Eur. J. Echocardiogr. 2009, 10, 265–270. [Google Scholar] [CrossRef]
- Remmelink, M.; Sjauw, K.D.; Yong, Z.Y.; Haeck, J.D.E.; Vis, M.M.; Koch, K.T.; Baan, J.; de Winter, R.J.; Tijssen, J.G.; Piek, J.J.; et al. Coronary Microcirculatory Dysfunction Is Associated with Left Ventricular Dysfunction during Follow-Up after STEMI. Neth. Heart J. 2013, 21, 238–244. [Google Scholar] [CrossRef]
- Frișan, A.C.; Mornoș, C.; Lazăr, M.A.; Șoșdean, R.; Crișan, S.; Ionac, I.; Tudoran, M.; Pop, D.; Petrescu, L.; Florescu, M.; et al. Echocardiographic Myocardial Work: A Novel Method to Assess Left Ventricular Function in Patients with Coronary Artery Disease and Diabetes Mellitus. Med. Kaunas. Lith. 2024, 60, 199. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Shah, A.M.; Everett, B.M.; Pradhan, A.D.; Piazza, G.; Bibbo, C.; Vasquez, N.; Murphy, S.A.; Skali, H.; Januzzi, J.L.; et al. Coronary Flow Reserve, Inflammation, and Myocardial Strain: The CIRT-CFR Trial. JACC Basic Transl. Sci. 2022, 8, 141–151. [Google Scholar] [CrossRef]
- Løgstrup, B.B.; Høfsten, D.E.; Christophersen, T.B.; Møller, J.E.; Bøtker, H.E.; Pellikka, P.A.; Dahl, J.S.; Møller, J.K.; Jensen, H.K.; Olsen, M.H.; et al. Correlation between Left Ventricular Global and Regional Longitudinal Systolic Strain and Impaired Microcirculation in Patients with Acute Myocardial Infarction. Echocardiography 2012, 29, 1181–1190. [Google Scholar] [CrossRef]
- Jin, W.; Wang, L.; Zhu, T.; Ma, Y.; Yu, C.; Zhang, F. Usefulness of echocardiographic myocardial work in evaluating the microvascular perfusion in STEMI patients after revascularization. BMC Cardiovasc. Disord. 2022, 22, 218. [Google Scholar] [CrossRef]
- Liu, T.; Ding, M.; Sun, D.; Zhang, H.; Guo, L.; Li, Y.; Zhao, X.; Chen, Q.; Wang, J.; Liu, X.; et al. The Association between Heart Rate Reserve and Impaired Coronary Flow Velocity Reserve: A Study Based on Adenosine Stress Echocardiography. Int. J. Cardiovasc. Imaging 2022, 38, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Ding, M.Y.; Sun, D.D.; Ji, W.; Zhang, H.H.; Li, Y.; Zhao, X.; Xu, L.; Zhou, J.; Wang, H.; et al. Clinical Value of TDI Combined with 2D-STI on Evaluating the Microcirculation Dysfunction and Left Ventricular Dysfunction in Patients with Non-Obstructive Coronary Angina. Chin. J. Cardiovasc. Dis. 2021, 49, 1191–1197. [Google Scholar]
- Jovanovic, I.; Tesic, M.; Giga, V.; Dobric, M.; Boskovic, N.; Vratonjic, J.; Radovanovic, S.; Nedeljkovic, I.; Milasinovic, D.; Obradovic, S.; et al. Impairment of Coronary Flow Velocity Reserve and Global Longitudinal Strain in Women with Cardiac Syndrome X and Slow Coronary Flow. J. Cardiol. 2020, 76, 1–8. [Google Scholar] [CrossRef]
- Xing, X.; Li, D.; Chen, S.; Wang, L.; Li, Z.; He, L. Evaluation of left ventricular systolic function in patients with different types of ischemic heart disease by two-dimensional speckle tracking imaging. J. Cardiothorac. Surg. 2020, 15, 325. [Google Scholar] [CrossRef]
- Michelsen, M.M.; Pena, A.; Mygind, N.D.; Bech, J.; Gustafsson, I.; Kastrup, J.; Prescott, E.; Hansen, P.R.; Bøtker, H.E.; Høst, N.; et al. Coronary Microvascular Dysfunction and Myocardial Contractile Reserve in Women with Angina and No Obstructive Coronary Artery Disease. Echocardiography 2018, 35, 196–203. [Google Scholar] [CrossRef]
- Tagliamonte, E.; Sperlongano, S.; Montuori, C.; Riegler, L.; Scarafile, R.; Carbone, A.; D’Andrea, A.; D’Alto, M.; Sarubbi, B.; Russo, M.G.; et al. Coronary Microvascular Dysfunction Affects Left Ventricular Global Longitudinal Strain Response to Dipyridamole Stress Echocardiography: A Pilot Study. Heart Vessel. 2023, 38, 470–477. [Google Scholar] [CrossRef]
- Li, Y.; Sun, D.; Zhao, H.; Qin, Z.; Ji, W.; Zhang, H.; Liu, T.; Ding, M.; Xu, L.; Zhou, J.; et al. Incremental Value of Non-Invasive Myocardial Work for the Evaluation and Prediction of Coronary Microvascular Dysfunction in Angina with No Obstructive Coronary Artery Disease. Front. Cardiovasc. Med. 2023, 10, 1209122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Q.; Wan, X.; Xu, M.; Pan, J.; Zhang, Y.; Zhao, X.; Chen, W.; Zhou, Y.; Wang, L.; et al. The Value of Myocardial Work in the Estimation of Left Ventricular Systolic Function in Patients with Coronary Microvascular Disease: A Study Based on Adenosine Stress Echocardiography. Front. Cardiovasc. Med. 2023, 10, 1119785. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Banovic, M.; Bosiljka, V.T.; Voin, B.; Milan, P.; Ivana, N.; Dejana, P.; Djordje, G.; Jelena, M.; Aleksandar, P.; Slobodan, P.; et al. Prognostic Value of Coronary Flow Reserve in Asymptomatic Moderate or Severe Aortic Stenosis with Preserved Ejection Fraction and Nonobstructed Coronary Arteries. Echocardiography 2014, 31, 428–433. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, Z.; Krljanac, G.; Zdravkovic, M.; Lasica, R.; Trifunovic, D.; Asanin, M. Coronary Microcirculation in Heart Failure with Preserved Systolic Function. Curr. Pharm. Des. 2018, 24, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- Miličić, D.; Jakuš, N.; Fabijanović, D. Microcirculation and Heart Failure. Curr. Pharm. Des. 2018, 24, 2954–2959. [Google Scholar] [CrossRef]
- Lakkas, L.; Naka, K.K.; Bechlioulis, A.; Duni, A.; Moustakli, M.; Balafa, O.; Theodorou, I.; Katsouras, C.S.; Dounousi, E.; Michalis, L.K. Coronary microcirculation and left ventricular diastolic function but not myocardial deformation indices are impaired early in patients with chronic kidney disease. Echocardiography 2023, 40, 600–607. [Google Scholar] [CrossRef]
- Gkirdis, I.; Naka, K.K.; Lakkas, L.; Manolakaki, P.; Duni, A.; Koulousios, K.; Kalaitzidis, R.; Dounousi, E.; Michalis, L.K.; Katsouras, C.S. Coronary microcirculation and left ventricular diastolic function: Comparison between patients on hemodialysis and peritoneal dialysis. J. Echocardiogr. 2021, 19, 103–112. [Google Scholar] [CrossRef]
- Dykun, I.; Kärner, L.; Mahmoud, I.; Hendricks, S.; Totzeck, M.; Al-Rashid, F.; Marx, N.; Rassaf, T.; Stegbauer, J.; Luedike, P.; et al. Association of Echocardiographic Measures of Left Ventricular Diastolic Dysfunction and Hypertrophy with Presence of Coronary Microvascular Dysfunction. Int. J. Cardiol. Heart Vasc. 2020, 27, 100493. [Google Scholar] [CrossRef]
- Yahia, M.; Emara, A.; Abdou, W.; Ewis, M.F. Impact of Coronary Microvascular Dysfunction on Myocardial Strain in Patients with Heart Failure and Preserved Ejection Fraction. J. Cardiovasc. Echogr. 2023, 33, 133–138. [Google Scholar] [CrossRef]
- Kato, S.; Fukui, K.; Kodama, S.; Azuma, M.; Nakayama, N.; Iwasawa, T.; Oda, S.; Nishida, M.; Takahashi, T.; Ueno, T.; et al. Cardiovascular Magnetic Resonance Assessment of Coronary Flow Reserve Improves Risk Stratification in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Magn. Reson. 2021, 23, 112. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxén, U.; Fermer, M.L.; Broberg, M.; et al. Prevalence and Correlates of Coronary Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef]
- Lin, M.; Qin, Y.; Ding, X.; Zhang, M.; Zhu, W.; Wang, J.; Leng, C.; Lu, X.; Cai, Q. Association between left ventricular geometry and global myocardial work in patients with heart failure with preserved ejection fraction: Assessment using strain-pressure loop. Int. J. Cardiovasc. Imaging 2023, 39, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Wang, Y.; Zhang, R.; Li, J.; Yu, T.; Yin, L.; Chen, X.; Huang, Z.; Zhao, J.; Xu, F.; et al. The Value of Speckle-Tracking Stratified Strain Combined with Myocardial Work Measurement in Evaluating Left Ventricular Function in Patients with Heart Failure with Preserved Ejection Fraction. Quant. Imaging Med. Surg. 2024, 14, 2514–2527. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M.; Koskenvuo, J.; Knuuti, J.; Toikka, J.; Laine, H.; Niemi, P.; Hesse, B.; Ukkonen, H.; Någren, K.; Nuutila, P.; et al. Coronary Flow Reserve: Measurement with Transthoracic Doppler Echocardiography Is Reproducible and Comparable with Positron Emission Tomography. Clin. Physiol. 2001, 21, 114–122. [Google Scholar] [CrossRef] [PubMed]
| Imaging Modality | AI Tasks | Advantages |
|---|---|---|
| PET |
|
|
| CMR |
|
|
| CT |
|
|
| Doppler TTE |
|
|
| MCE |
|
|
| Study | ANOCA Patients (N) | Technique | Agent | CMD Index | Systolic Function Index |
|---|---|---|---|---|---|
| Liu et al., 2021 | 78 | Doppler TTE | Adenosine | CFR < 2 | GLS |
| Xing et al., 2020 | 67 | Doppler TTE | Adenosine | CFR < 2.5 | GLS |
| Jovanovic et al., 2020 | 70 | Doppler TTE | Adenosine | CFR < 2 | GLS |
| Michelsen et al., 2018 | 963 | Doppler TTE | Dipyridamole | CFR < 2 | GLS |
| Tagliamonte et al., 2023 | 59 | Doppler TTE | Dipyridamole | CFR < 2 | GLS |
| Li et al., 2023 | 97 | Doppler TTE | Adenosine | CFR < 2 | MW |
| Liu et al., 2023 | 78 | Doppler TTE | Adenosine | CFR < 2.5 | MW |
| Study | Patients (N) | CVD | Technique | Level of Evidence * |
|---|---|---|---|---|
| Kozàkovà et al. (2003) | 60 | HTN | Doppler TEE | Low |
| Ikonomidis et al. (2008) | 100 | HTN | Doppler TTE | Low |
| Ikonomidis et al. (2012) | 90 | HTN | Doppler TTE | Low |
| Ikonomidis et al. (2018) | 320 | HTN | Doppler TTE | Low |
| Evangelou et al. (2020) | 47 | HTN | Doppler TTE | Low |
| Ikonomidis et al. (2022) | 80 | DM | Doppler TTE | Low |
| Liu et al. (2018) | 78 | DM | CMR | Low |
| D’ Andrea et al. (2010) | 45 | DM | Doppler TTE | Low |
| M. Takafuji et al. (2023) | 26 | DCM | CMR | Low |
| Olivotto et al. (2006) | 51 | HCM | PET | Low |
| Bras et al. (2023) | 75 | HCM | CMR | Moderate |
| Meimoun et al. (2009) | 20 | TTS | Doppler TTE | Low |
| Tanqueri et al. (2023) | 50 | CAD | PET | Moderate |
| Løgstrup et al. (2012) | 183 | CAD | Doppler TTE | Low |
| Jin et al. (2022) | 160 | CAD | MCE | Low |
| Liu et al., 2021 | 78 | ANOCA | Doppler TTE | Moderate |
| Xing et al., 2020 | 67 | ANOCA | Doppler TTE | Low |
| Jovanovic et al., 2020 | 70 | ANOCA | Doppler TTE | Low |
| Michelsen et al., 2018 | 963 | ANOCA | Doppler TTE | Moderate |
| Tagliamonte et al., 2023 | 59 | ANOCA | Doppler TTE | Low |
| Li et al., 2023 | 97 | ANOCA | Doppler TTE | Moderate |
| Liu et al., 2023 | 78 | ANOCA | Doppler TTE | Moderate |
| Kato et al., 2021 | 163 | HFpEF | CMR | Moderate |
| Shah et al., 2018 | 202 | HFpEF | Doppler TTE | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsis, E.; Papadopoulos, C.; Oikonomidis, D.; Lakkas, L.; Naka, K.K. Impaired Coronary Microcirculation and Myocardial Systolic Function: A Narrative Review on Non-Invasive Assessment in Cardiovascular Diseases. Life 2025, 15, 1350. https://doi.org/10.3390/life15091350
Tatsis E, Papadopoulos C, Oikonomidis D, Lakkas L, Naka KK. Impaired Coronary Microcirculation and Myocardial Systolic Function: A Narrative Review on Non-Invasive Assessment in Cardiovascular Diseases. Life. 2025; 15(9):1350. https://doi.org/10.3390/life15091350
Chicago/Turabian StyleTatsis, Evangelos, Constantinos Papadopoulos, Dimitrios Oikonomidis, Lampros Lakkas, and Katerina K. Naka. 2025. "Impaired Coronary Microcirculation and Myocardial Systolic Function: A Narrative Review on Non-Invasive Assessment in Cardiovascular Diseases" Life 15, no. 9: 1350. https://doi.org/10.3390/life15091350
APA StyleTatsis, E., Papadopoulos, C., Oikonomidis, D., Lakkas, L., & Naka, K. K. (2025). Impaired Coronary Microcirculation and Myocardial Systolic Function: A Narrative Review on Non-Invasive Assessment in Cardiovascular Diseases. Life, 15(9), 1350. https://doi.org/10.3390/life15091350








