Stereotactic Ablative Radiotherapy for Delayed Retrobulbar Metastasis of Renal Cell Carcinoma: Therapeutic Outcomes and Practical Insights
Abstract
1. Introduction
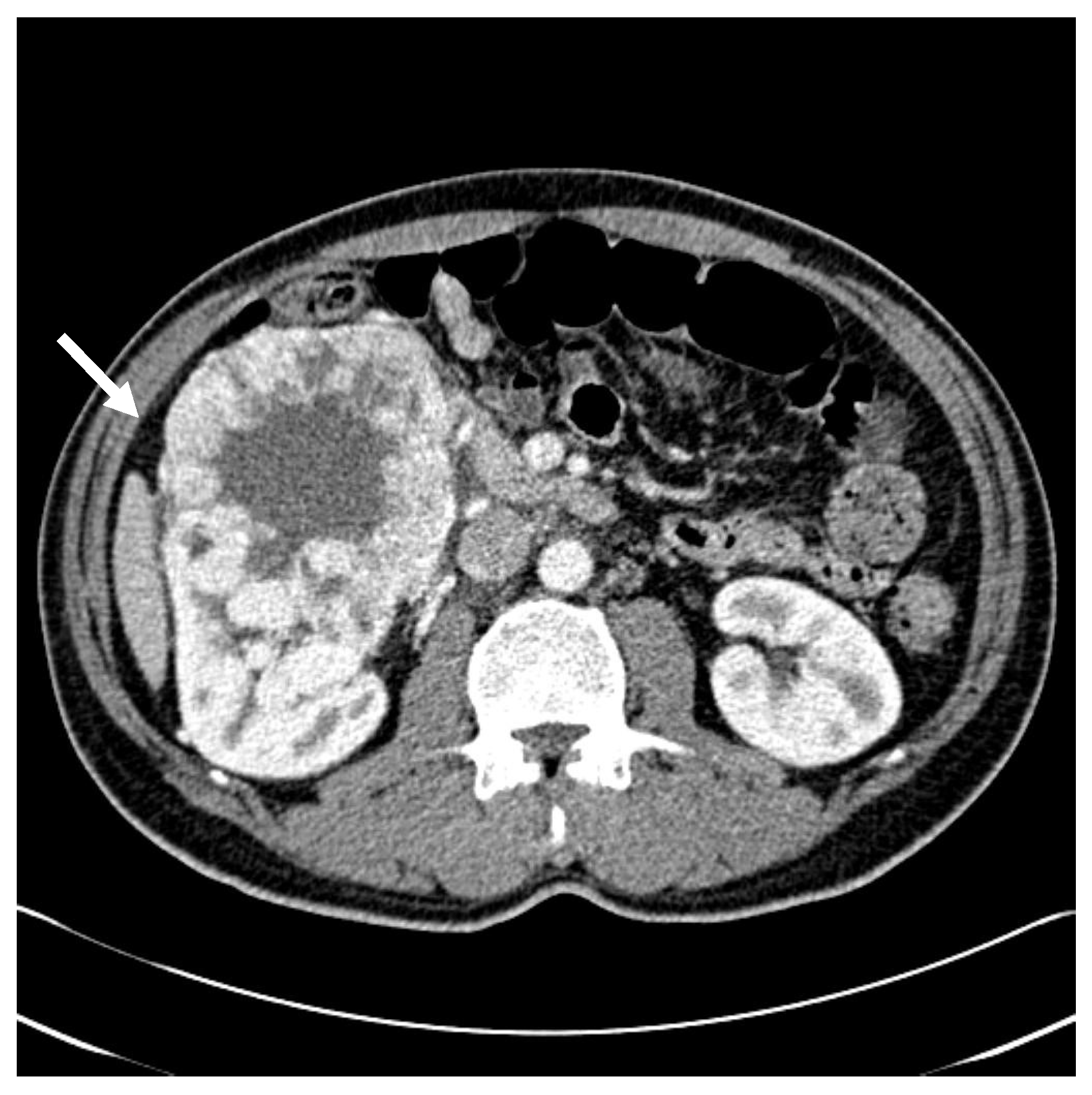
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.; Sharma, U.; Makkar, A.; Masood, P.F.; Goel, H.K.; Sood, R.; Ahuja, A.; Singh, R. Rare metastatic sites of renal cell carcinoma: A case series. Pan. Afr. Med. J. 2022, 42, 26. [Google Scholar] [CrossRef] [PubMed]
- Shome, D.; Honavar, S.G.; Gupta, P.; Vemuganti, G.K.; Reddy, P.V. Metastasis to the eye and orbit from renal cell carcinoma--a report of three cases and review of literature. Surv. Ophthalmol. 2007, 52, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Palmisciano, P.; Ferini, G.; Ogasawara, C.; Wahood, W.; Bin Alamer, O.; Gupta, A.D.; Scalia, G.; Larsen, A.M.G.; Yu, K.; Umana, G.E.; et al. Orbital Metastases: A Systematic Review of Clinical Characteristics, Management Strategies, and Treatment Outcomes. Cancers 2021, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.M.; Esmaeli, B. Metastatic tumors of the orbit and ocular adnexa. Curr. Opin. Ophthalmol. 2007, 18, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.H.; Seo, Y.; Choi, J. Extremely delayed solitary cerebral metastasis in patient with T1N0M0 renal cell carcinoma after radical nephrectomy: Case report and literature review. Medicine 2021, 100, e25586. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Louie, A.V.; Warner, A.; Muacevic, A.; Gandhidasan, S.; Ponsky, L.; Ellis, R.; Kaplan, I.; Mahadevan, A.; Chu, W.; et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK). Cancer 2018, 124, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Greco, C.; Motzer, R.; Magsanoc, J.M.; Pei, X.; Lovelock, M.; Mechalakos, J.; Zatcky, J.; Fuks, Z.; Yamada, Y. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Bentahila, R.; Bensalah, K.; Benziane-Ouaritini, N.; Barthelemy, P.; Rioux-Leclerc, N.; Correas, J.M.; Belhomme, S.; Bigot, P.; Sargos, P. Stereotactic body radiation therapy for primary renal cell carcinoma: A review on behalf of the CC-AFU. Fr. J. Urol. 2024, 34, 102660. [Google Scholar] [CrossRef] [PubMed]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, B.; Evans, T.R. Late recurrences of renal cell carcinoma at unusual sites: Implications for patient management. Clin. Adv. Hematol. Oncol. 2012, 10, 126–128. [Google Scholar] [PubMed]
- Marra, C.; Losco, L.; Ceccaroni, A.; Pentangelo, P.; Troisi, D.; Alfano, C. Metastatic Renal Cell Carcinoma to the Soft Tissue 27 Years after Radical Nephrectomy: A Case Report. Medicina 2023, 59, 150. [Google Scholar] [CrossRef] [PubMed]
- Bruckschen, F.; Gerharz, C.D.; Sagir, A. Renal cell carcinoma with unusual metachronous metastasis up to 22 years after nephrectomy: Two case reports. J. Med. Case. Rep. 2021, 15, 490. [Google Scholar] [CrossRef] [PubMed]
- Khalafi-Nezhad, A.; Zamani, A.; Amini, M.; Negahban, S. A case report of renal cell carcinoma metastasis revealed through late-onset thyroid nodules. Cancer Rep. 2024, 7, e2113. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Guo, K.; Zheng, S. Case Report: Pancreatic metastasis of renal cell carcinoma 16 years after nephrectomy. Front. Oncol. 2023, 13, 1091635. [Google Scholar] [CrossRef] [PubMed]
- Magara, N.; Takahashi, N.; Takano, Y.; Takeshita, K.; Toya, N.; Yano, F.; Eto, K. Gastric metastasis from renal cell carcinoma with submucosal invasion treated by surgical full-thickness resection: A case report. Surg. Case Rep. 2024, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yang, T.; Li, Y.; Qu, P.; Shao, Z.; Wang, Y.; Chang, W.; Umar, S.M.; Wang, J.; Ding, N.; et al. A future directions of renal cell carcinoma treatment: Combination of immune checkpoint inhibition and carbon ion radiotherapy. Front. Immunol. 2024, 15, 1428584. [Google Scholar] [CrossRef] [PubMed]
- Deschavanne, P.J.; Fertil, B. A review of human cell radiosensitivity in vitro. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sang, N. Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells. J. Cell. Biochem. 2016, 117, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Raval, R.R.; Lau, K.W.; Tran, M.G.; Sowter, H.M.; Mandriota, S.J.; Li, J.L.; Pugh, C.W.; Maxwell, P.H.; Harris, A.L.; Ratcliffe, P.J. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell. Biol. 2005, 25, 5675–5686. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J.; Fu, L.; Minton, D.R.; Mongan, N.P.; Nanus, D.M. The role of HIF1α in renal cell carcinoma tumorigenesis. J. Mol. Med. 2014, 92, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Mo, F.; Patel, G.; Butterworth, K.; Shao, C.; Prise, K.M. The Roles of HIF-1α in Radiosensitivity and Radiation-Induced Bystander Effects Under Hypoxia. Front. Cell Dev. Biol. 2021, 9, 637454. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Kim, H.; Kim, M.S.; Park, H.J.; Paek, S.H.; Terezakis, S.; Cho, L.C. Role of HIF-1α in the Responses of Tumors to Radiotherapy and Chemotherapy. Cancer Res. Treat. 2025, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Trisler, K.; Wessels, B.W.; Knox, S.J. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer 1997, 80, 2519–2528. [Google Scholar] [CrossRef]
- Ma, M.W.; Li, H.Z.; Gao, X.S.; Liu, M.Z.; Yin, H.; Yang, K.W.; Chen, J.Y.; Ren, X.Y.; Wang, D. Outcomes of High-Dose Stereotactic Ablative Radiotherapy to All/Multiple Sites for Oligometastatic Renal Cell Cancer Patients. Curr. Oncol. 2022, 29, 7832–7841. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ansinelli, H.; Sharma, D.; Jenkins, J.; Davis, J.; Sharma, S.; Vargo, J.A. Stereotactic body radiation therapy (SBRT) for metastatic renal cell carcinoma: A multi-institutional experience. J. Radiosurg. SBRT 2020, 7, 29–37. [Google Scholar] [PubMed]
- Siva, S.; Ali, M.; Correa, R.J.M.; Muacevic, A.; Ponsky, L.; Ellis, R.J.; Lo, S.S.; Onishi, H.; Swaminath, A.; McLaughlin, M.; et al. 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: An individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney). Lancet Oncol. 2022, 23, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, C.J.Q.; Swaminath, A. Stereotactic Body Radiotherapy for Renal Cell Carcinoma-A Review of Use in the Primary, Cytoreductive and Oligometastatic Settings. Cancers 2024, 16, 3334. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Han, H.; Liu, Z.; Huang, S.; Cao, W.; Liu, B.; Qin, Z.; Guo, S.; Zhang, Z.; et al. Survival Outcomes After Adding Stereotactic Body Radiotherapy to Metastatic Renal Cell Carcinoma Patients Treated with Tyrosine Kinase Inhibitors. Am. J. Clin. Oncol. 2020, 43, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Rühle, A.; Andratschke, N.; Siva, S.; Guckenberger, M. Is there a role for stereotactic radiotherapy in the treatment of renal cell carcinoma? Clin. Transl. Radiat. Oncol. 2019, 18, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Schoenhals, J.E.; Mohamad, O.; Christie, A.; Zhang, Y.; Li, D.; Singla, N.; Bowman, I.; Arafat, W.; Hammers, H.; Courtney, K.; et al. Stereotactic Ablative Radiation Therapy for Oligoprogressive Renal Cell Carcinoma. Adv. Radiat. Oncol. 2021, 6, 100692. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Chargari, C.; Marabelle, A.; Perfettini, J.L.; Magné, N.; Deutsch, E. Can immunostimulatory agents enhance the abscopal effect of radiotherapy? Eur. J. Cancer 2016, 62, 36–45. [Google Scholar] [CrossRef] [PubMed]





| Time Point | Treatment | Key Clinical Events |
|---|---|---|
| Initial diagnosis | Initial presentation with gross hematuria Abdominal CT: 10.4 × 14.3 × 10.0 cm3 right renal mass | |
| Surgery | Radical nephrectomy performed Pathology: clear cell RCC, Grade III/IV, pT2b | |
| 1 and 6 months after surgery | Follow-up CT of brain, chest, and abdomen: No recurrence | |
| 6 months after surgery | axitinib initiated | |
| 9 months after surgery | axitinib discontinued at patient’s request → Lost to follow-up | |
| 5.5 years after surgery | Referred due to exophthalmos → CT/MRI: Right adrenal (2.9 × 3.0 × 2.6 cm3) and right retrobulbar (3.0 × 3.7 × 2.8 cm3) metastases | |
| sunitinib initiated → Surgical resection recommended but declined → SABR (40 Gy in 5 fractions) | ||
| 2 months after SABR | Continued sunitinib | Decrease in orbital lesion size (2.1 × 3.0 × 2.2 cm3) on MRI |
| 3 months after SABR | Continued sunitinib | Decrease in adrenal metastasis size (1.6 × 1.4 × 1.5 cm3) on CT |
| 7 months after SABR | Continued sunitinib | Continued regression of orbital lesion (1.9 × 1.8 × 1.7 cm3) |
| Up to 4 years after SABR | Continued sunitinib | Stable disease confirmed on serial imaging every 6 months |
| 4 years 2 months after SABR | Acute mental status change → Brain MRI: Hemorrhagic lesion in left frontoparietal lobe (anatomically distinct from the prior SABR) | |
| After acute care | Transferred to rehabilitation facility |
| Author (Year) | Site of Metastasis | Interval After Nephrectomy | Treatment | Outcome and Significance |
|---|---|---|---|---|
| Marra et al. (2023) [12] | Scalp soft tissue | 27 years | Surgical resection + histopathologic confirmation | Demonstrates the potential for extremely delayed RCC metastasis to soft tissue. |
| Lou et al. (2023) [15] | Pancreas | 16 years | Pancreaticoduodenectomy (Whipple) | Isolated pancreatic metastasis from RCC can occur after long latency; surgery is effective. |
| Khalafi-Nezhad et al. (2024) [14] | Thyroid gland | 13 years | Thyroidectomy + immunohistochemistry | Late-onset thyroid nodules can be first indicator of RCC metastasis. |
| Magara et al. (2024) [16] | Stomach (submucosal) | 12 years | Full-thickness gastric resection | Rare gastric metastasis; surgical management led to favorable prognosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, S.J.; Kim, B.H.; Park, S.G.; Choi, E. Stereotactic Ablative Radiotherapy for Delayed Retrobulbar Metastasis of Renal Cell Carcinoma: Therapeutic Outcomes and Practical Insights. Life 2025, 15, 1176. https://doi.org/10.3390/life15081176
Byun SJ, Kim BH, Park SG, Choi E. Stereotactic Ablative Radiotherapy for Delayed Retrobulbar Metastasis of Renal Cell Carcinoma: Therapeutic Outcomes and Practical Insights. Life. 2025; 15(8):1176. https://doi.org/10.3390/life15081176
Chicago/Turabian StyleByun, Sang Jun, Byung Hoon Kim, Seung Gyu Park, and Euncheol Choi. 2025. "Stereotactic Ablative Radiotherapy for Delayed Retrobulbar Metastasis of Renal Cell Carcinoma: Therapeutic Outcomes and Practical Insights" Life 15, no. 8: 1176. https://doi.org/10.3390/life15081176
APA StyleByun, S. J., Kim, B. H., Park, S. G., & Choi, E. (2025). Stereotactic Ablative Radiotherapy for Delayed Retrobulbar Metastasis of Renal Cell Carcinoma: Therapeutic Outcomes and Practical Insights. Life, 15(8), 1176. https://doi.org/10.3390/life15081176







