Effects of Vitamin D3 Supplementation on Inflammatory Markers in Overweight and Obese Children and Adolescents: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- (1)
- Population: overweight or obese children and adolescents at the age of 9–19 years, based on the 2000 Center for Disease Control growth charts [25], a body mass index (BMI) ≥ 85th percentile was defined as overweight, and a BMI ≥ 95th percentile as obesity
- (2)
- Intervention: oral supplementation of vitamin D3 (cholecalciferol), regardless of the dose or duration of administration
- (3)
- Comparison: placebo
- (4)
- Outcome: the effects of oral vitamin D3 supplementation on inflammatory markers in the blood
- (5)
- Study: RCT
- (1)
- Case reports, case series, and observational studies
- (2)
- Vitamin D2 (ergocalciferol) or 1,25(OH)2D3 (calcitriol) supplementation
- (3)
- Studies focused on participants with acute or chronic conditions (e.g., endogenous obesity, Cushing’s disease, asthma)
- (4)
- Number of participants less than 10
- (5)
- Not English articles
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
3. Results
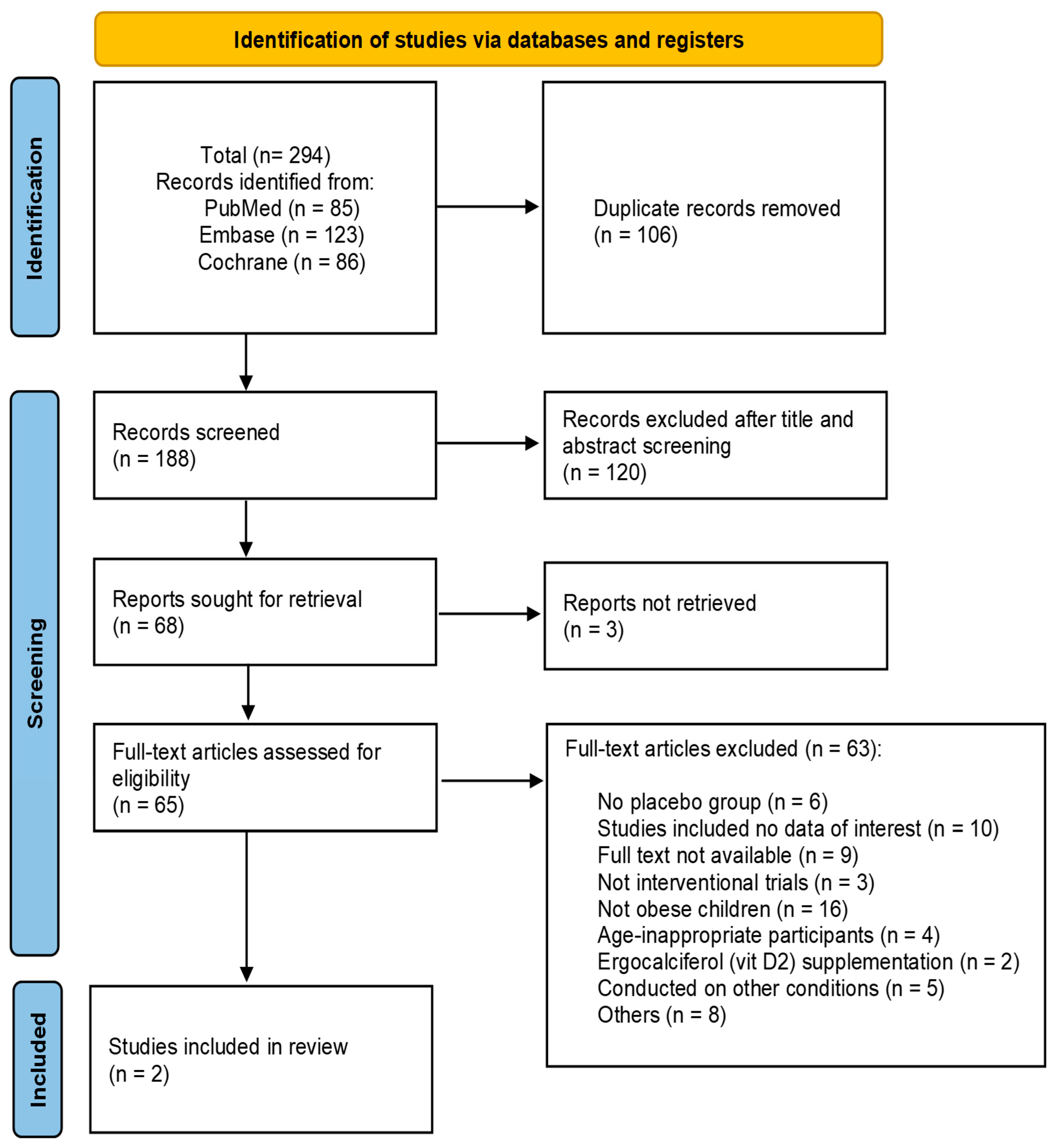
3.1. Study Selection
3.2. Characteristics of Included Studies
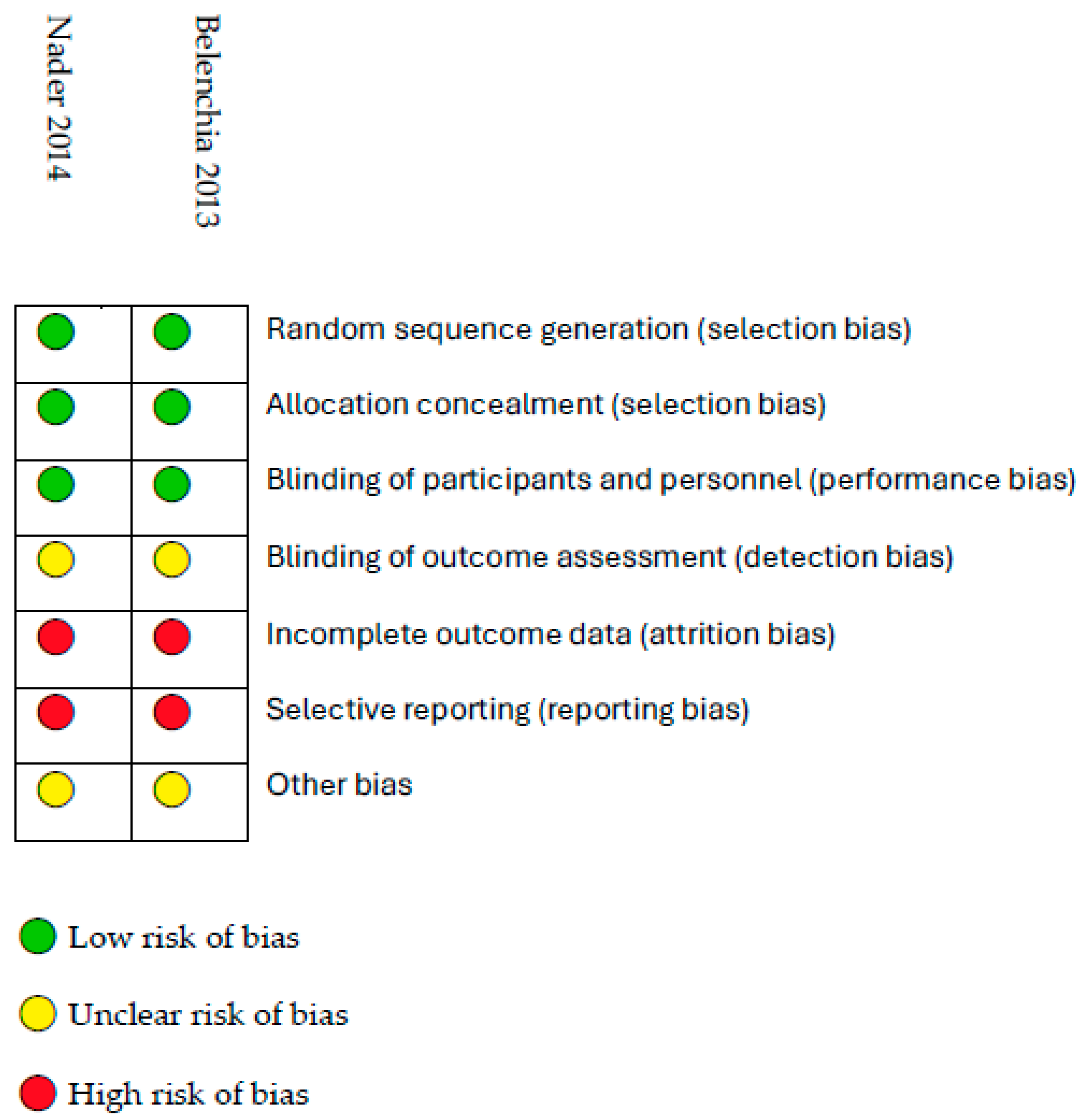
3.3. Risk of Bias Assessment
3.4. Effects of Cholecalciferol on C-Reactive Protein Levels
3.5. Effects of Cholecalciferol on IL-6 and TNF-α
3.6. Effects of Cholecalciferol on Leptin and Adiponectin Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCT | randomized controlled trial |
| CRP | C-reactive protein |
| hs-CRP | high sensitivity C-reactive protein |
| ILs | interleukins |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-alpha |
| BMI | body mass index |
| L/A ratio | leptin to adiponectin ratio |
| VDRs | Vitamin D receptors |
Appendix A
| PubMed |
|---|
| (“Vitamin D”[Mesh] OR “Vitamin D” OR cholecalciferol OR “25(OH)D3” OR “vit D” OR “vitamin D3” OR “25-hydroxy-vitamin D” OR “25-hydroxy vitamin D” OR “25-hydroxyvitamin D” OR “(25(OH)D)”) |
| AND |
| (administration OR supplementation) |
| AND |
| (“Pediatric obesity”[Mesh] OR “pediatric obesity” OR “childhood obesity” OR “obesity in children” OR “obesity in adolescents” OR “children with obesity” OR “adolescents with obesity” OR “obese children” OR “obese adolescents” OR “obese children” OR (“Obesity”[Mesh] AND (preschool OR child OR kid OR kids OR minors OR boy OR boys OR girl* OR childhood OR boyhood OR pediatric OR paediatric OR preteen OR schoolchild* OR underage OR adolescent OR juvenile OR teenager OR pubescen*)) OR (“Obesity, Morbid”[Mesh] AND (preschool OR child OR kid OR kids OR minors OR boy OR boys OR girl* OR childhood OR boyhood OR pediatric OR paediatric OR preteen OR schoolchild* OR underage OR adolescent OR juvenile OR teenager OR pubescen*)) OR (“Obesity, Abdominal”[Mesh] AND (preschool OR child OR kid OR kids OR minors OR boy OR boys OR girl* OR childhood OR boyhood OR pediatric OR paediatric OR preteen OR schoolchild* OR underage OR adolescent OR juvenile OR teenager OR pubescen*)) OR (Obesity AND (preschool OR child OR kid OR kids OR minors OR boy OR boys OR girl* OR childhood OR boyhood OR pediatric OR paediatric OR preteen OR schoolchild* OR underage OR adolescent OR juvenile OR teenager OR pubescen*)) OR (“Overweight”[Mesh] AND (preschool OR child OR kid OR kids OR minors OR boy OR boys OR girl* OR childhood OR boyhood OR pediatric OR paediatric OR preteen OR schoolchild* OR underage OR adolescent OR juvenile OR teenager OR pubescen*)) OR (Overweight AND (preschool OR child OR kid OR kids OR minors OR boy OR boys OR girl* OR childhood OR boyhood OR pediatric OR paediatric OR preteen OR schoolchild* OR underage OR adolescent OR juvenile OR teenager OR pubescen*)) OR (“Adipose Tissue”[Mesh] AND (preschool OR child OR kid OR kids OR minors OR boy OR boys OR girl* OR childhood OR boyhood OR pediatric OR paediatric OR preteen OR schoolchild* OR underage OR adolescent OR juvenile OR teenager OR pubescen*)) OR (“adipose tissue” AND (preschool OR child OR kid OR kids OR minors OR boy OR boys OR girl* OR childhood OR boyhood OR pediatric OR paediatric OR preteen OR schoolchild* OR underage OR adolescent OR juvenile OR teenager OR pubescen*))) |
| Embase |
| ((‘child’/exp OR ‘child’) OR (‘minor (person)’/exp OR ‘minor (person)’) OR adolescen* OR boy OR boys OR boyhood OR child* OR girl OR juvenile OR kid OR kids OR minors OR paediatric OR pediatric OR preschool* OR preteen OR pubescen* OR choolchild* OR teenage? OR underage) |
| AND |
| (‘obesity’/exp OR ‘obesity’ OR obes* OR ‘overweight’/exp OR overweight* OR ‘adiposity’/exp OR adiposity OR ‘adipose tissue’/exp OR ‘adipose tissue’) |
| AND |
| (‘vitamin d’/exp OR ‘vitamin d’ OR ((‘vitamin’/exp OR vitamin) AND d$) OR ‘colecalciferol’/exp OR ‘colecalciferol’ OR (25 AND hydroxyvitamin AND d$) OR (25 AND hydroxy AND vitamin AND d$) OR cholecalciferol OR cholecalciferol? OR (25 AND oh AND d$) OR (vit AND d$)) |
| AND |
| (‘supplementation’/exp OR ‘supplementation’ OR ‘administration of drugs, food and chemicals’/exp OR ‘administration of drugs, food and chemicals’) |
| Cochrane Library |
| ID Search |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 May 2025).
- Hamjane, N.; Benyahya, F.; Nourouti, N.G.; Mechita, M.B.; Barakat, A. Cardiovascular diseases and metabolic abnormalities associated with obesity: What is the role of inflammatory responses? A systematic review. Microvasc. Res. 2020, 131, 104023. [Google Scholar] [CrossRef]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.M.B.; Oliveira, P.D.; Wehrmeister, F.C.; Gonçalves, H.; Assunção, M.C.F.; Tovo-Rodrigues, L.; Ferreira, G.D.; Oliveira, I.O. Association between interleukin-6, C-reactive protein and adiponectin with adiposity: Findings from the 1993 pelotas (Brazil) birth cohort at 18 and 22 years. Cytokine 2018, 110, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell death and inflammation during obesity: “Know my methods, WAT(son)”. Cell Death Differ. 2023, 30, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Aamir, A.B.; Kumari, R.; Latif, R.; Ahmad, S.; Rafique, N.; Salem, A.M.; Alasoom, L.I.; Alsunni, A.; Alabdulhadi, A.S.; Chander, S. Effects of intermittent fasting and caloric restriction on inflammatory biomarkers in individuals with obesity/overweight: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2025, 26, e13838. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators Linking Adipose Tissue, Inflammation and Immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic Syndrome: Pathophysiology, Management, and Modulation by Natural Compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Lima, G.B.; Figueiredo, N.; Kattah, F.M.; Oliveira, E.S.; Horst, M.A.; Dâmaso, A.R.; Oyama, L.M.; Whitton, R.G.M.; de Souza, G.I.M.H.; Lima, G.C.; et al. Serum Fatty Acids and Inflammatory Patterns in Severe Obesity: A Preliminary Investigation in Women. Biomedicines 2024, 12, 2248. [Google Scholar] [CrossRef]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. 2019, 10, 103. [Google Scholar] [CrossRef]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.F. Recent insights into vitamin D, adipocyte, and adipose tissue biology. Obes. Rev. 2022, 23, e13453. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T Cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Zhou, Q.; Qin, S.; Zhang, J.; Zhon, L.; Pen, Z.; Xing, T. 1,25(OH)2D3 Induces Regulatory T Cell Differentiation by Influencing the VDR/PLC-g1/TGF-b1/ Pathway. Mol. Immunol. 2017, 91, 156–164. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The Role of Vitamin D in Autoimmune Diseases: Could Sex Make the Difference? Biol. Sex. Differ. 2021, 12, 12. [Google Scholar] [CrossRef]
- Todosenko, N.; Vulf, M.; Yurova, K.; Khaziakhmatova, O.; Mikhailova, L.; Litvinova, L. Causal Links between Hypovitaminosis D and Dysregulation of the T Cell Connection of Immunity Associated with Obesity and Concomitant Pathologies. Biomedicines 2021, 9, 1750. [Google Scholar] [CrossRef]
- Fenercioglu, A.K. The Anti-Inflammatory Roles of Vitamin D for Improving Human Health. Curr. Issues Mol. Biol. 2024, 46, 13514–13525. [Google Scholar] [CrossRef] [PubMed]
- Bendix, M.; Dige, A.; Deleuran, B.; Dahlerup, J.F.; Jørgensen, S.P.; Bartels, L.E.; Husted, L.B.; Harsløf, T.; Langdahl, B.; Agnholt, J. Flow Cytometry Detection of Vitamin D Receptor Changes During Vitamin D Treatment in Crohn’s Disease. Clin. Exp. Immunol. 2015, 181, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M.; Witkowska-Sędek, E.; Rumińska, M.; Stelmaszczyk-Emmel, A.; Sobol, M.; Majcher, A.; Pyrżak, B. Vitamin D Effects on Selected Anti-Inflammatory and Pro-Inflammatory Markers of Obesity-Related Chronic Inflammation. Front. Endocrinol. 2022, 13, 920340. [Google Scholar] [CrossRef]
- Cheung, M.M.; Dall, R.D.; Shewokis, P.A.; Altasan, A.; Volpe, S.L.; Amori, R.; Singh, H.; Sukumar, D. The effect of combined magnesium and vitamin D supplementation on vitamin D status, systemic inflammation, and blood pressure: A randomized double-blinded controlled trial. Nutrition 2022, 99–100, 111674. [Google Scholar] [CrossRef]
- Bellia, A.; Garcovich, C.; D’Adamo, M.; Lombardo, M.; Tesauro, M.; Donadel, G.; Gentileschi, P.; Lauro, D.; Federici, M.; Lauro, R.; et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern. Emerg. Med. 2013, 8, 33–40. [Google Scholar] [CrossRef]
- Park, C.Y.; Han, S.N. Vitamin D and obesity. Adv. Food Nutr. Res. 2024, 109, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and development. Vital. Health Stat. 11 2002, 11, 1–190. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Belenchia, A.M.; Tosh, A.K.; Hillman, L.S.; Peterson, C.A. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 774–781. [Google Scholar] [CrossRef]
- Nader, N.S.; Aguirre Castaneda, R.; Wallace, J.; Singh, R.; Weaver, A.; Kumar, S. Effect of vitamin D3 supplementation on serum 25(OH)D, lipids and markers of insulin resistance in obese adolescents: A prospective, randomized, placebo-controlled pilot trial. Horm. Res. Paediatr. 2014, 82, 107–112. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef]
- Cai, B.; Luo, X.; Zhang, P.; Luan, Y.; Cai, X.; He, X. Effect of vitamin D supplementation on markers of cardiometabolic risk in children and adolescents: A meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2800–2814. [Google Scholar] [CrossRef]
- Corsello, A.; Macchi, M.; D’Oria, V.; Pigazzi, C.; Alberti, I.; Treglia, G.; De Cosmi, V.; Mazzocchi, A.; Agostoni, C.; Milani, G.P. Effects of vitamin D supplementation in obese and overweight children and adolescents: A systematic review and meta-analysis. Pharmacol. Res. 2023, 192, 106793. [Google Scholar] [CrossRef]
- Rajakumar, K.; Moore, C.G.; Khalid, A.T.; Vallejo, A.N.; Virji, M.A.; Holick, M.F.; Greenspan, S.L.; Arslanian, S.; Reis, S.E. Effect of vitamin D3 supplementation on vascular and metabolic health of vitamin D-deficient overweight and obese children: A randomized clinical trial. Am. J. Clin. Nutr. 2020, 111, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Wilson, D.M.; Bachrach, L.K. Large Doses of Vitamin D Fail to Increase 25-Hydroxyvitamin D Levels or to Alter Cardiovascular Risk Factors in Obese Adolescents: A Pilot Study. J. Adolesc. Health 2015, 57, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, D.B.D.L.; Adikaram, S.G.S.; Atapattu, N.; Kendaragama, K.M.D.L.D.; Senevirathne, J.T.N.; Jayasekera, H.D.; Wickramasinghe, V.P. Vitamin D supplementation in obese Sri Lankan children: A randomized controlled trial. BMC Pediatr. 2020, 20, 426. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gomez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef]
- Mendoza-Herrera, K.; Florio, A.A.; Moore, M.; Marrero, A.; Tamez, M.; Bhupathiraju, S.N.; Mattei, J. The Leptin System and Diet: A Mini Review of the Current Evidence. Front. Endocrinol. 2021, 12, 749050. [Google Scholar] [CrossRef]
- Fernández-Vallejo, B.; Monteagudo, F.J.; Romero, L.; Aznárez, M.I.L.; Cobas, M.d.C.R.; Pérez-Martínez, L. Cross-Sectional Analysis of IL-6, TNF-α, Adiponectin, Leptin, and Klotho Serum Levels in Relation to BMI Among Overweight and Obese Children Aged 10–14 in La Rioja, Spain. Children 2025, 12, 89. [Google Scholar] [CrossRef]
- Iikuni, N.; Lam, Q.L.; Lu, L.; Matarese, G.; La Cava, A. Leptin and Inflammation. Curr. Immunol. Rev. 2008, 4, 70–79. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Perakakis, N.; Mantzoros, C.S. Evidence from clinical studies of leptin: Current and future clinical applications in humans. Metabolism 2024, 161, 156053. [Google Scholar] [CrossRef]
- Kishida, K.; Funahashi, T.; Shimomura, I. Adiponectin as a routine clinical biomarker. Best. Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 119–130. [Google Scholar] [CrossRef]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.A.; Isaksen, V.T.; Moen, O.S.; Wilsgaard, L.; Remijn, M.; Paulssen, E.J.; Florholmen, J.; Goll, R. Leptin to adiponectin ratio—A surrogate biomarker for early detection of metabolic disturbances in obesity. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Portincasa, P.; Colina, I.; Gómez-Ambrosi, J. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci. Rep. 2017, 7, 6619. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- Brock, K.; Huang, W.Y.; Fraser, D.R.; Ke, L.; Tseng, M.; Stolzenberg-Solomon, R.; Peters, U.; Ahn, J.; Purdue, M.; Mason, R.S.; et al. Low Vitamin D Status is Associated with Physical Inactivity, Obesity and Low Vitamin D Intake in a Large US Sample of Healthy Middle-Aged Men and Women. J. Steroid Biochem. Mol. Biol. 2010, 121, 462–466. [Google Scholar] [CrossRef]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The Link Between Obesity and Low Circulating 25-Hydroxyvitamin D Concentrations: Considerations and Implications. Int J Obes (Lond). 2012, 36, 387–396. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological Functions of Vitamin D in Adipose Tissue. J. Steroid Biochem. Mol. Biol. 2017, 165 Pt B, 369–381. [Google Scholar] [CrossRef]
- Hajhashemy, Z.; Shahdadian, F.; Ziaei, R.; Saneei, P. Serum vitamin D levels in relation to abdominal obesity: A systematic review and dose-response meta-analysis of epidemiologic studies. Obes. Rev. 2021, 22, e13134. [Google Scholar] [CrossRef]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.S.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Alzohily, B.; AlMenhali, A.; Gariballa, S.; Munawar, N.; Yasin, J.; Shah, I. Unraveling the complex interplay between obesity and vitamin D metabolism. Sci. Rep. 2024, 14, 7583. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
| References (Authors, Year of Publication, Country) | Subjects/Age/Study Design | Baseline 25(OH)D in the Vitamin D3 Group (ng/mL) | Supplementation Strategy (Dose and Duration) | 25(OH)D During/at the End of the Intervention | Evaluation of Inflammatory Biomarkers |
|---|---|---|---|---|---|
| Belenchia et al. 2013, USA [27] | 35 obese adolescents (n = 18 vitamin D3 group, n = 17 placebo group)/9–19 years/ randomized double-blind, placebo-controlled trial | 19.2 ± 6.3 | 4000 IU/day Duration: 6 months | After 3 months, no subjects in the vitamin D3 group were 25(OH)D deficient. At 6 months, 93% of participants in the vitamin D3 group were 25(OH)D sufficient. | CRP, IL-6, TNF-α, and adiponectin remained unchanged after 3 and 6 months (placebo compared with control group). After 3 months no change in leptin. After 6 months, significant decrease in the leptin to adiponectin ratio in the vitamin D3 group compared with the placebo group. |
| Nader et al., 2014, USA [28] | 44 obese adolescents (n = 20 vitamin D3 group, n = 24 placebo group)/12–18 years/ randomized double-blind, placebo-controlled trial | 25.8 ± 5.9 | 2000 IU/day Duration: 12 weeks | After 12 weeks, 25(OH)D increased in the vitamin D3 group to a median of 31 ng/mL. Ten of the 20 participants in the vitamin D3 group achieved 25(OH)D > 30 ng/mL at 3 months. | No changes in hs-CRP in the vitamin D3 group compared with the placebo group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska, M.; Witkowska-Sędek, E. Effects of Vitamin D3 Supplementation on Inflammatory Markers in Overweight and Obese Children and Adolescents: A Systematic Review. Life 2025, 15, 1142. https://doi.org/10.3390/life15071142
Krajewska M, Witkowska-Sędek E. Effects of Vitamin D3 Supplementation on Inflammatory Markers in Overweight and Obese Children and Adolescents: A Systematic Review. Life. 2025; 15(7):1142. https://doi.org/10.3390/life15071142
Chicago/Turabian StyleKrajewska, Maria, and Ewelina Witkowska-Sędek. 2025. "Effects of Vitamin D3 Supplementation on Inflammatory Markers in Overweight and Obese Children and Adolescents: A Systematic Review" Life 15, no. 7: 1142. https://doi.org/10.3390/life15071142
APA StyleKrajewska, M., & Witkowska-Sędek, E. (2025). Effects of Vitamin D3 Supplementation on Inflammatory Markers in Overweight and Obese Children and Adolescents: A Systematic Review. Life, 15(7), 1142. https://doi.org/10.3390/life15071142