Ultrasound Phenotype-Based Approach to Treatment Choice in Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
Ultrasound Assessment
3. Suggestions for US Phenotypes
- Predominant intra-articular effusion (with minimal synovial proliferation (synovial sacs) and large intra-articular effusion;
- Predominant synovial proliferation, fat pads, and Hoffa synovitis (with a small amount of intra-articular effusion);
- Predominant cartilage destruction (with almost no synovitis and intra-articular effusion);
- Altered subchondral bone (superficial bone erosions or osteophytes);
- Changes in extra-articular soft tissue (ligaments, tendons, capsule, entheses, muscles, bursae, Baker’s cysts, menisci—for knee joints); periarticular cystic lesions (including meniscal);
- Crystal deposits (MSU, CPPD);
- Post-traumatic (fractures, cruciate ligament, menisci—for knee joints, tendons).
4. Examples of US Phenotypes
5. Statistical Analysis
6. Results
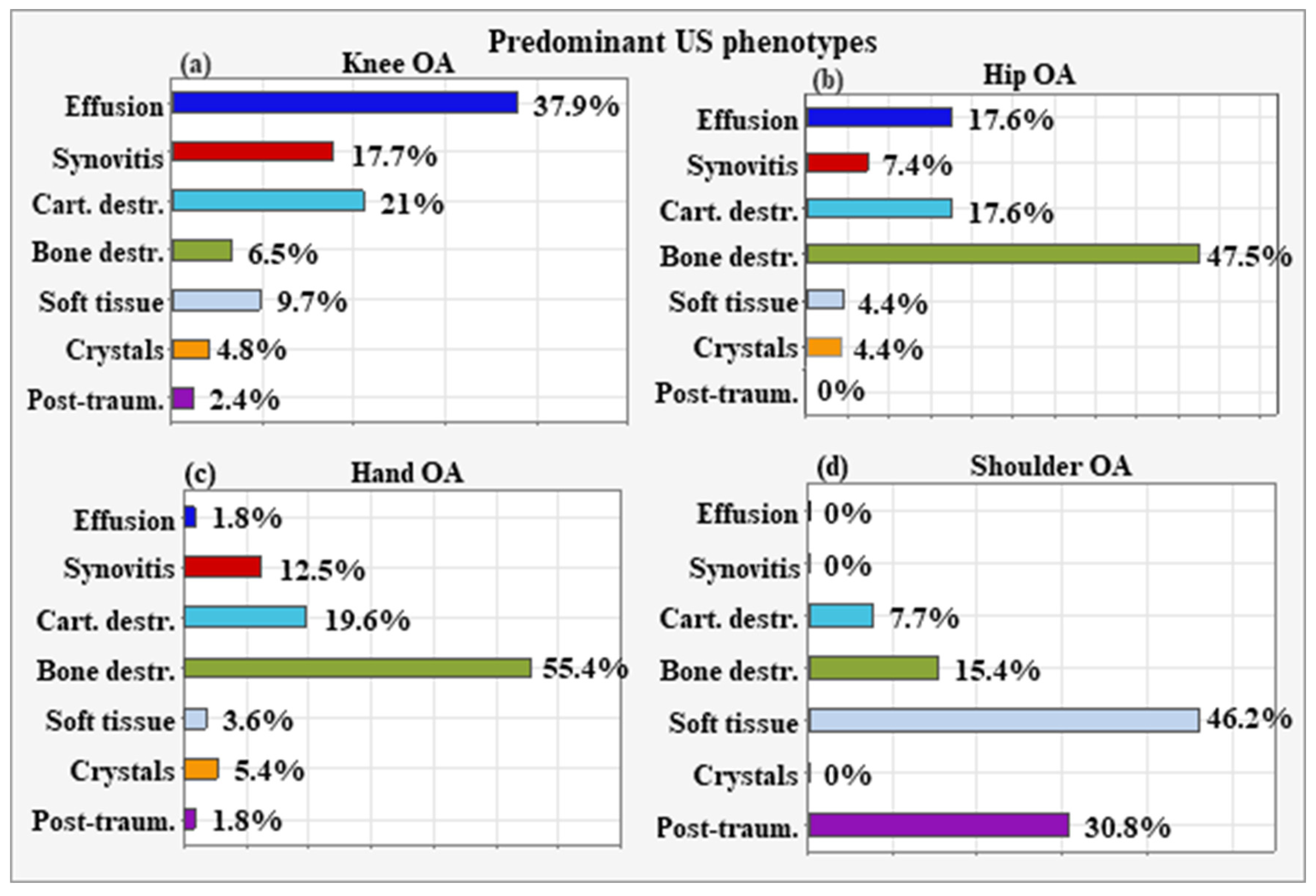
6.1. Predominant US Phenotypes According to OA Localization
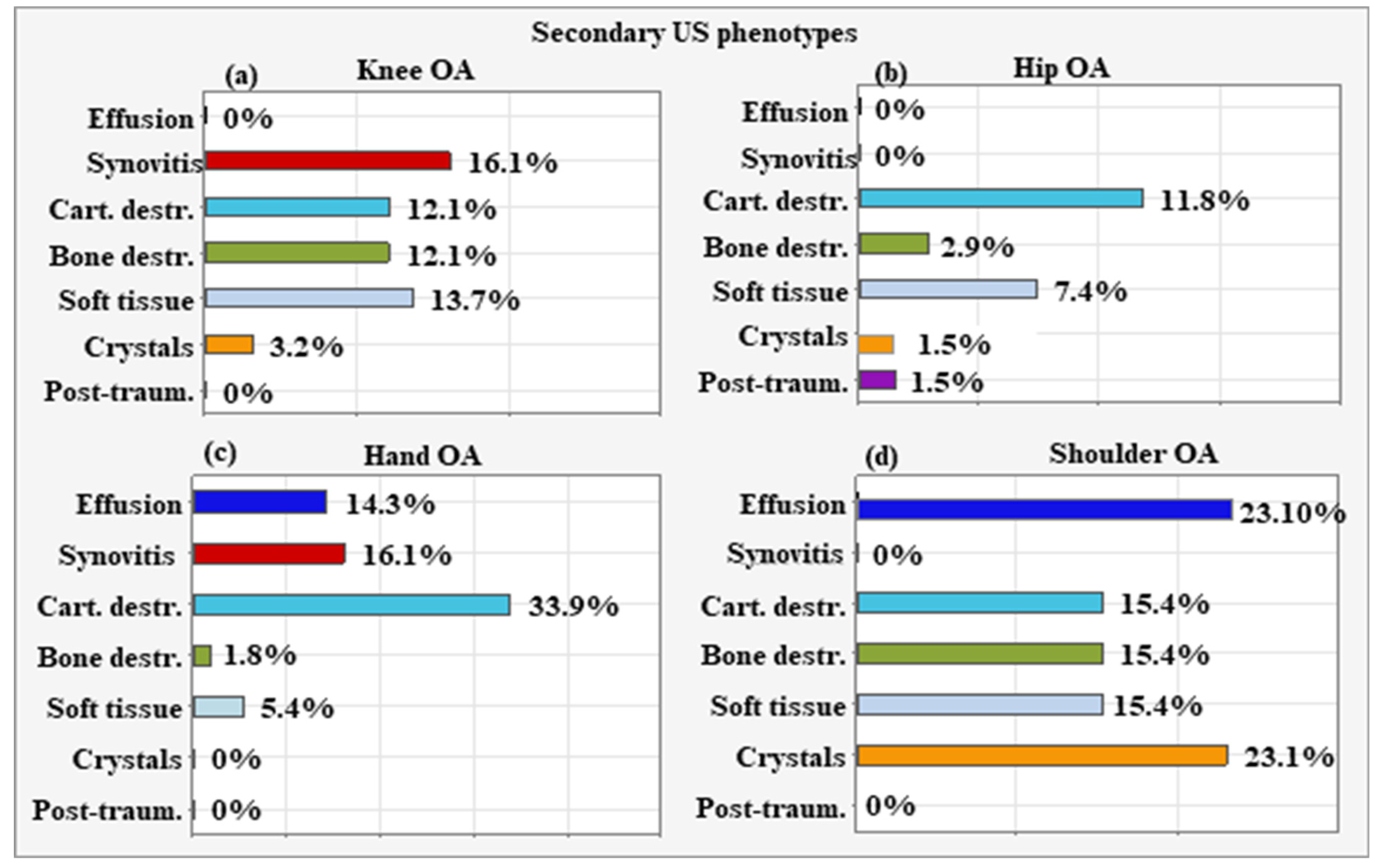
6.2. Secondary US Phenotypes According to the Localization of OA
7. Discussion
Relationship Between OA Clinical Phenotype and Type of Underlying Affected Joint Region
8. Therapeutic Algorithm for OA
9. Conclusions
10. Future Directions
11. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loeser, R.; Goldring, S.; Scanzello, C.; Goldring, M. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y. Osteoarthritis in year 2021: Biochemical markers. Osteoarthr. Cartil. 2022, 30, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.; Roman-Blas, J.A.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis: A progressive disease with changing phenotypes. Rheumatology 2014, 53, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Collins, J.E.; Neogi, T.; Crema, M.D.; Guermazi, A. Association of knee OA structural phenotypes to risk for progression: A secondary analysis from the Foundation for National Institutes of Health Osteoarthritis Biomarkers study (FNIH). Osteoarthr. Cartil. 2020, 28, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Bay-Jensen, A.-C.; Henrotin, Y.; Karsdal, M.; Mobasheri, A. The need for predictive, prognostic, objective and complementary blood-based biomarkers in osteoarthritis (OA). eBioMedicine 2016, 7, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Bay-Jensen, A.C.; Lories, R.J.; Abramson, S.; Spector, T.; Pastoureau, P.; Christiansen, C.; Attur, M.; Henriksen, K.; Goldring, S.R.; et al. The coupling of bone and cartilage turnover in osteoarthritis: Opportunities for bone antiresorptives and anabolics as potential treatments? Ann. Rheum. Dis. 2014, 73, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Roman-Blas, J.A.; Bruyère, O.; Cooper, C.; Kanis, J.; Maggi, S.; Rizzoli, R.; Reginster, J.Y. Clinical settings in knee osteoarthritis: Pathophysiology guides treatment. Maturitas 2017, 96, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Van Spil, W.E.; Kubassova, O.; Boesen, M.; Bay-Jensen, A.C.; Mobasheri, A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharmacol. 2019, 165, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Kloppenburg, M.; Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Wu, C.; Sun, B.; Hose, M.K.; Quan, A.; Hughes, T.H.; Boyle, D.; Kalunian, K.C. Inflammatory characteristics on ultrasound predict poorer long-term response to intraarticular corticosteroid injections in knee osteoarthritis. J. Rheumatol. 2010, 37, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Vrouwe, J.P.M.; Burggraaf, J.; Kloppenburg, M.; Stuurman, F.E. Challenges and opportunities of pharmacological interventions for osteoarthritis: A review of current clinical trials and developments. Osteoarthr. Cartil. Open 2021, 3, 100212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mobasheri, A.; van Spil, W.E.; Budd, E.; Uzieliene, I.; Bernotiene, E.; Bay-Jensen, A.C.; Larkin, J.; Levesque, M.C.; Gualillo, O.; Henrotin, Y. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: Biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr. Opin. Rheumatol. 2019, 31, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, W.M.; Maillefert, J.F.; Hunter, D.J.; Katz, J.N.; Conaghan, P.G.; Losina, E. Responsiveness to change and reliability of measurement of radiographic joint space width in osteoarthritis of the knee: A systematic review. Osteoarthr. Cartil. 2011, 19, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wluka, A.E.; Jones, G.; Ding, C.; Cicuttini, F.M. Use magnetic resonance imaging to assess articular cartilage. Ther. Adv. Musculoskelet. Dis. 2012, 4, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Hayashi, D.; Eckstein, F.; Hunter, D.J.; Duryea, J.; Roemer, F.W. Imaging of osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 67–105. [Google Scholar] [CrossRef] [PubMed]
- Eymard, F.; Foltz, V.; Chemla, C.; Gandjbakhch, F.; Etchepare, F.; Fautrel, B.; Pascal, R.; Tomi Anne, L.; Gaujoux-Viala, C.; Chevalier, X. MRI and ultrasonography for detection of early interphalangeal osteoarthritis. Jt. Bone Spine 2022, 89, 105370. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Sasaki, E.; Chiba, D.; Oyama, T.; Ota, S.; Ishibashi, H.; Yamamoto, Y.; Tsuda, E.; Sawada, K.; Jung, S. Effusion detected by ultrasonography and overweight may predict the risk of knee osteoarthritis in females with early knee osteoarthritis: A retrospective analysis of Iwaki cohort data. BMC Musculoskelet. Disord. 2022, 23, 1021. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Sasaki, E.; Chiba, D.; Yamamoto, Y.; Tsuda, E.; Ishibashi, Y. Synovitis detected by ultrasonography predict the risk of developing knee osteoarthritis in early knee osteoarthritis from the IWAKI cohort study. Osteoarthr. Cartil. 2022, 30, S23–S24. [Google Scholar] [CrossRef]
- Nevalainen, M.T.; Uusimaa, A.P.; Saarakkala, S. The ultrasound assessment of osteoarthritis: The current status. Skeletal Radiol. 2023, 52, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.; Watt, F.E.; Vincent, T.L.; Dziedzic, K. Hand osteoarthritis: Clinical phenotypes, molecular mechanisms and disease management. Nat. Rev. Rheumatol. 2018, 14, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Doherty, M.; Leeb, B.F.; Alekseeva, L.; Arden, N.K.; Bijlsma, J.W.; Dinçer, F.; Dziedzic, K.; Häuselmann, H.J.; Herrero-Beaumont, G.; et al. EULAR evidence based recommendations for the management of hand osteoarthritis: Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann. Rheum. Dis. 2007, 66, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.L.; Grainger, A.J.; Tanner, S.F.; Shelley, D.M.; Pease, C.; Emery, P.; McGonagle, D. High-resolution magnetic resonance imaging for the assessment of hand osteoarthritis. Arthritis Rheum. 2005, 52, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Singh, A. Imaging and Biochemical Markers for Osteoarthritis. Diagnostics 2021, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Karalilova, R.; Kazakova, M.; Batalov, A.; Sarafian, V. Correlation between protein YKL-40 and ultrasonographic findings in active knee osteoarthritis. Med. Ultrason. 2018, 20, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Terenzi, R.; Karalilova, R.; Lepri, G.; Bruni, C.; Bellando-Randone, S.; Manetti, M.; Romano, E.; Melchiorre, D.; Blagojevic, J.; Wang, Y.; et al. Enthesopathy and involvement of synovio-entheseal complex in systemic sclerosis: An ultrasound pilot study. Rheumatology 2020, 59, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.T.; Nestorova, R.; Batalov, A. Diagnostic value of musculoskeletal ultrasound in patients with low back pain—A review of the literature. Med. Ultrason. 2018, 20, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.; Botushanov, A.; Batalov, Z.; Karalilova, R.; Batalov, A. High-resolution musculoskeletal ultrasonography and elastography for eosinophilic fasciitis diagnosis and follow-up: A case-based review. Rheumatol. Int. 2023, 43, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Mamoto, K.; Di Carlo, M.; Salaffi, F. Clinical utility and potential of ultrasound in osteoarthritis. Radiol. Med. 2019, 124, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Guermazi, A.; Demehri, S.; Wirth, W.; Kijowski, R. Imaging in Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 913–934. [Google Scholar] [CrossRef] [PubMed]
- Oo, W.M.; Linklater, J.M.; Bennell, K.L.; Pryke, D.; Yu, S.; Fu, K.; Wang, X.; Duong, V.; Hunter, D.J. Are OMERACT Knee Osteoarthritis Ultrasound Scores Associated With Pain Severity, Other Symptoms, and Radiographic and Magnetic Resonance Imaging Findings? J. Rheumatol. 2021, 48, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Bruyn, G.A.; Naredo, E.; Damjanov, N.; Bachta, A.; Baudoin, P.; Hammer, H.B.; Lamers-Karnebeek, F.B.; Moller Parera, I.; Richards, B.; Taylor, M.; et al. Ultrasound Task Force. An OMERACT reliability exercise of inflammatory and structural abnormalities in patients with knee osteoarthritis using ultrasound assessment. Ann. Rheum. Dis. 2016, 75, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; D’Agostino, M.A.; Le Bars, M.; Baron, G.; Schmidely, N.; Wakefield, R.; Ravaud, P.; Grassi, W.; Martin-Mola, E.; So, A.; et al. Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: Results from a large, 3-year, prospective EULAR study. Ann. Rheum. Dis. 2010, 69, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Keen, H.I.; Hensor, E.M.; Wakefield, R.J.; Mease, P.J.; Bingham, C.O., 3rd; Conaghan, P.G. Ultrasound assessment of response to intra-articular therapy in osteoarthritis of the knee. Rheumatology 2015, 54, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Roemer, F.W.; Eckstein, F.; Samuels, J.; Guermazi, A. Imaging of OA—From disease modification to clinical utility. Best. Pract. Res. Clin. Rheumatol. 2020, 34, 101588. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Alarcón, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Gray, R. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990, 33, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Alarcon, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Gray, R. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991, 34, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Bruyn, G.A.; Iagnocco, A.; Naredo, E.; Balint, P.V.; Gutierrez, M.; Hammer, H.B.; Collado, P.; Filippou, G.; Schmidt, W.A.; Jousse-Joulin, S.; et al. OMERACT Definitions for Ultrasonographic Pathologies and Elementary Lesions of Rheumatic Disorders 15 Years On. J. Rheumatol. 2019, 46, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Dell’Isola, A.; Allan, R.; Smith, S.L.; Marreiros, S.S.; Steultjens, M. Identification of clinical phenotypes in knee osteoarthritis: A systematic review of the literature. BMC Musculoskelet. Disord. 2016, 17, 425. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Saarakkala, S.; Finnilä, M.; Karsdal, M.A.; Bay-Jensen, A.C.; van Spil, W.E. Recent advances in understanding the phenotypes of osteoarthritis. F1000Research 2019, 8, F1000 Faculty Rev-2091. [Google Scholar] [CrossRef] [PubMed]
- Deveza, L.A.; Melo, L.; Yamato, T.P.; Mills, K.; Ravi, V.; Hunter, D.J. Knee osteoarthritis phenotypes and their relevance for outcomes: A systematic review. Osteoarthr. Cartil. 2017, 25, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, E.; Stankova, T.; Batalov, K.; Staynova, R.; Nonchev, B.; Bivolarska, A.; Karalilova, R. Association of serum and synovial adipokines (chemerin and resistin) with inflammatory markers and ultrasonographic evaluation scores in patients with knee joint osteoarthritis—A pilot study. Rheumatol. Int. 2024, 44, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Dvir-Ginzberg, M.; Reich, E. Chopping off the chondrocyte proteome. Biomarkers 2015, 20, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Kiso, T.; Okada, Y.; Kawata, S.; Shichiji, K.; Okumura, E.; Hatsumi, N.; Matsuura, R.; Kaminaga, M.; Kuwano, H.; Okumura, E. Ultrasound-based radiomics and machine learning for enhanced diagnosis of knee osteoarthritis: Evaluation of diagnostic accuracy, sensitivity, specificity, and predictive value. Eur. J. Radiol. Open 2025, 14, 100649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Agostino, V.; Sorriento, A.; Cafarelli, A.; Donati, D.; Papalexis, N.; Russo, A.; Lisignoli, G.; Ricotti, L.; Spinnato, P. Ultrasound Imaging in Knee Osteoarthritis: Current Role, Recent Advancements, and Future Perspectives. J. Clin. Med. 2024, 13, 4930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorriento, A.; Cafarelli, A.; Spinnato, P.; Russo, A.; Lisignoli, G.; Rabusseau, F.; Cabras, P.; Dumont, E.; Ricotti, L. Design, Development and Validation of a Knee Brace to Standardize the US Imaging Evaluation of Knee Osteoarthritis. IEEE J. Transl. Eng. Health Med. 2021, 10, 1800308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
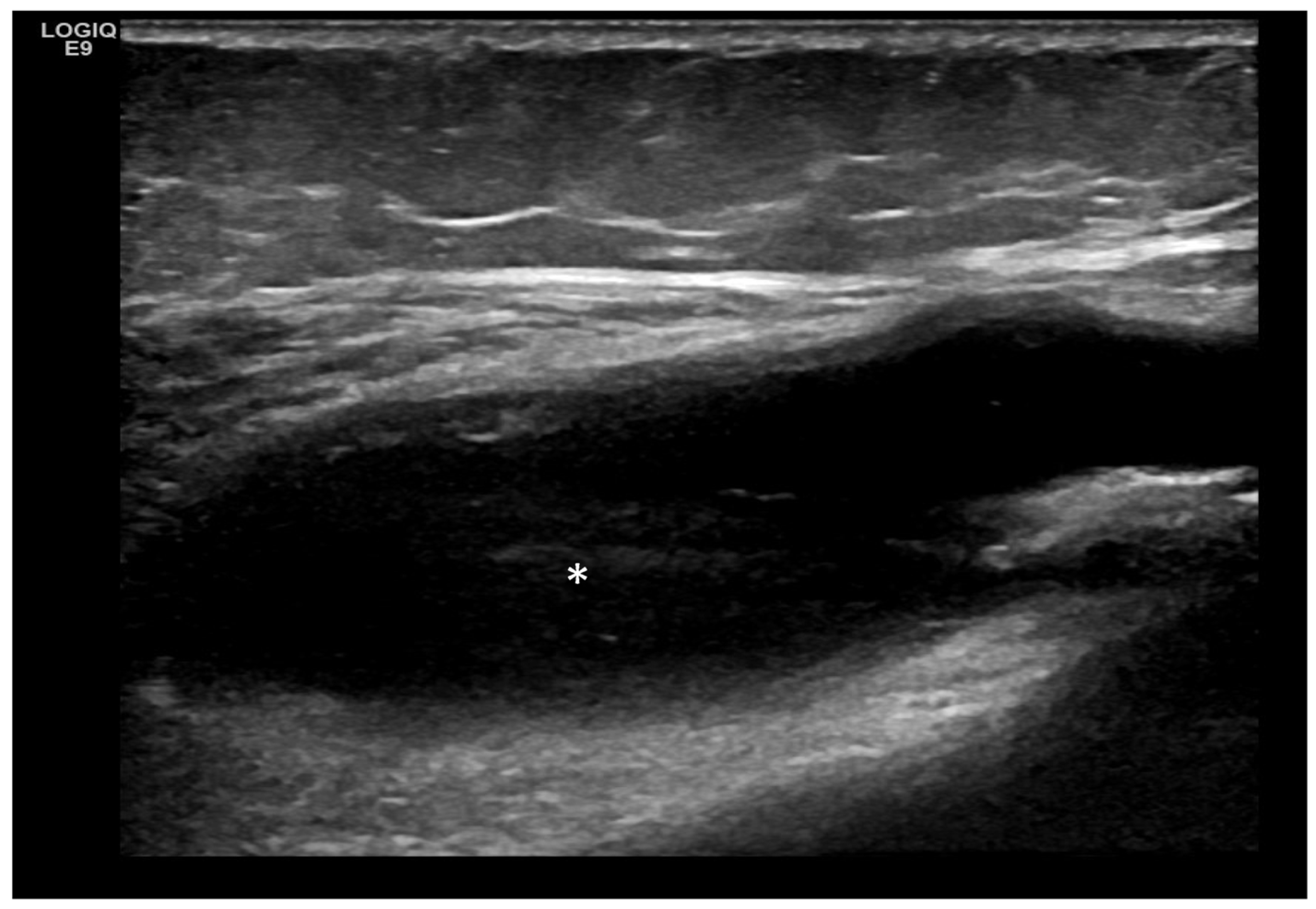
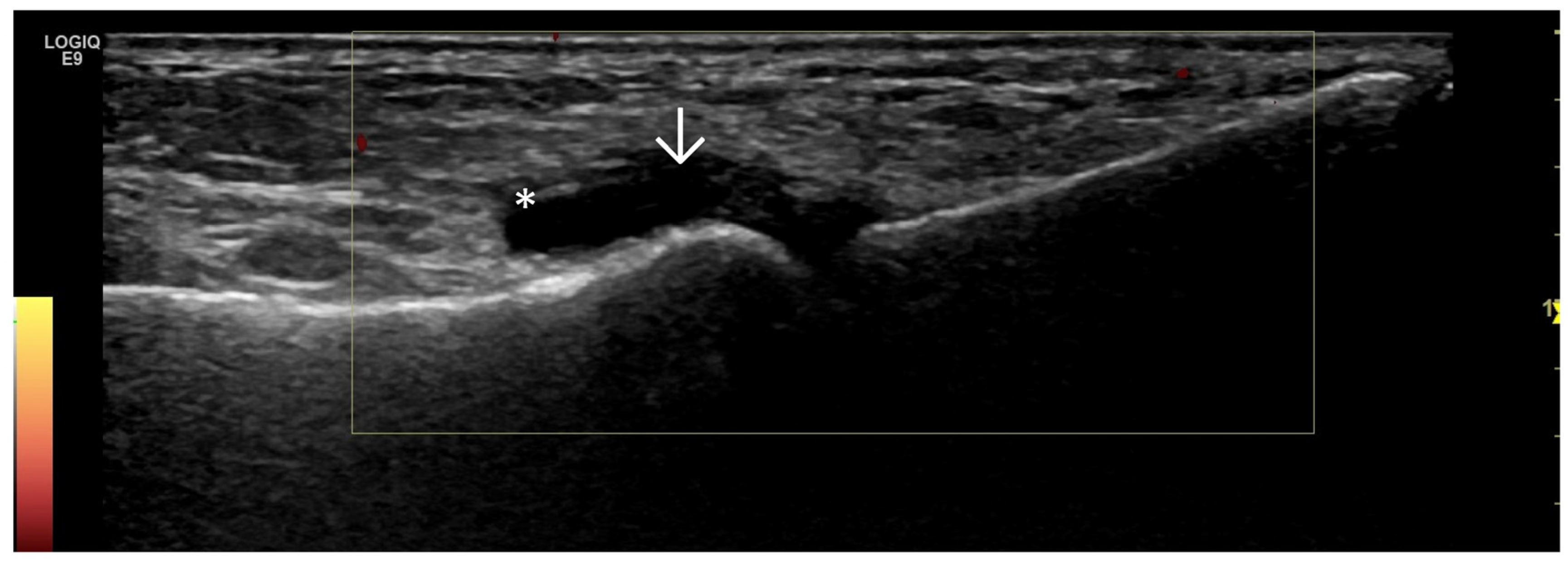

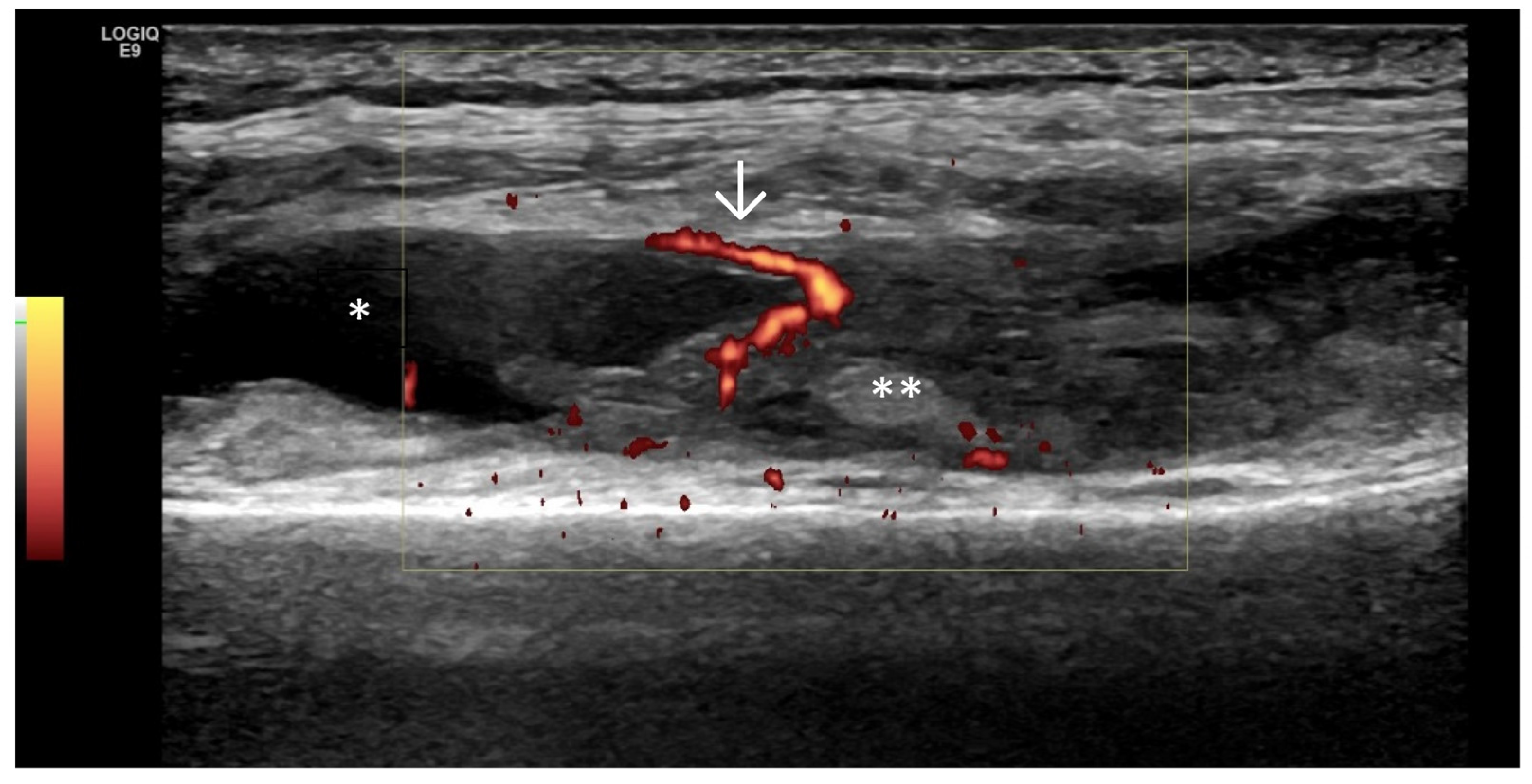
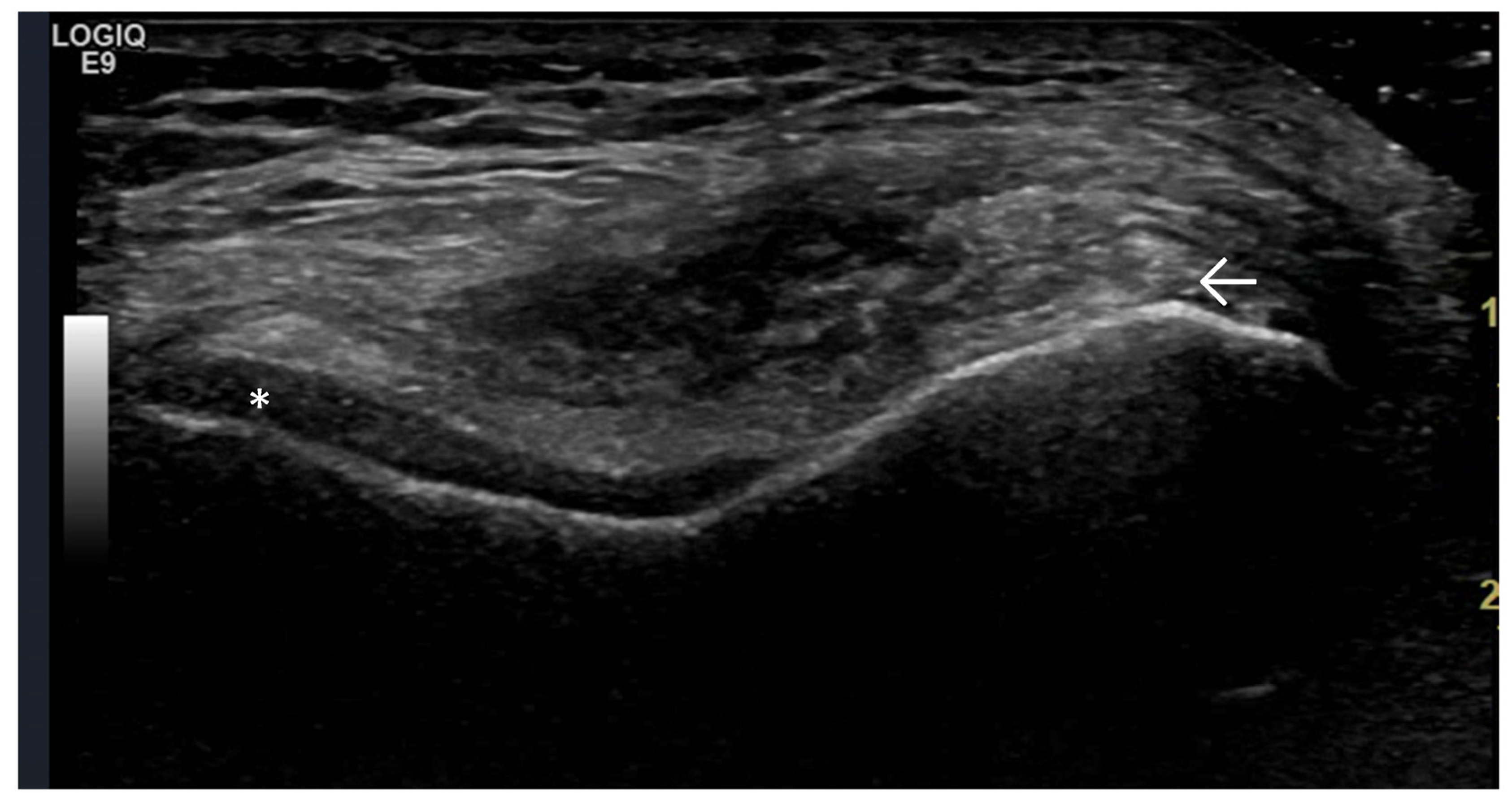
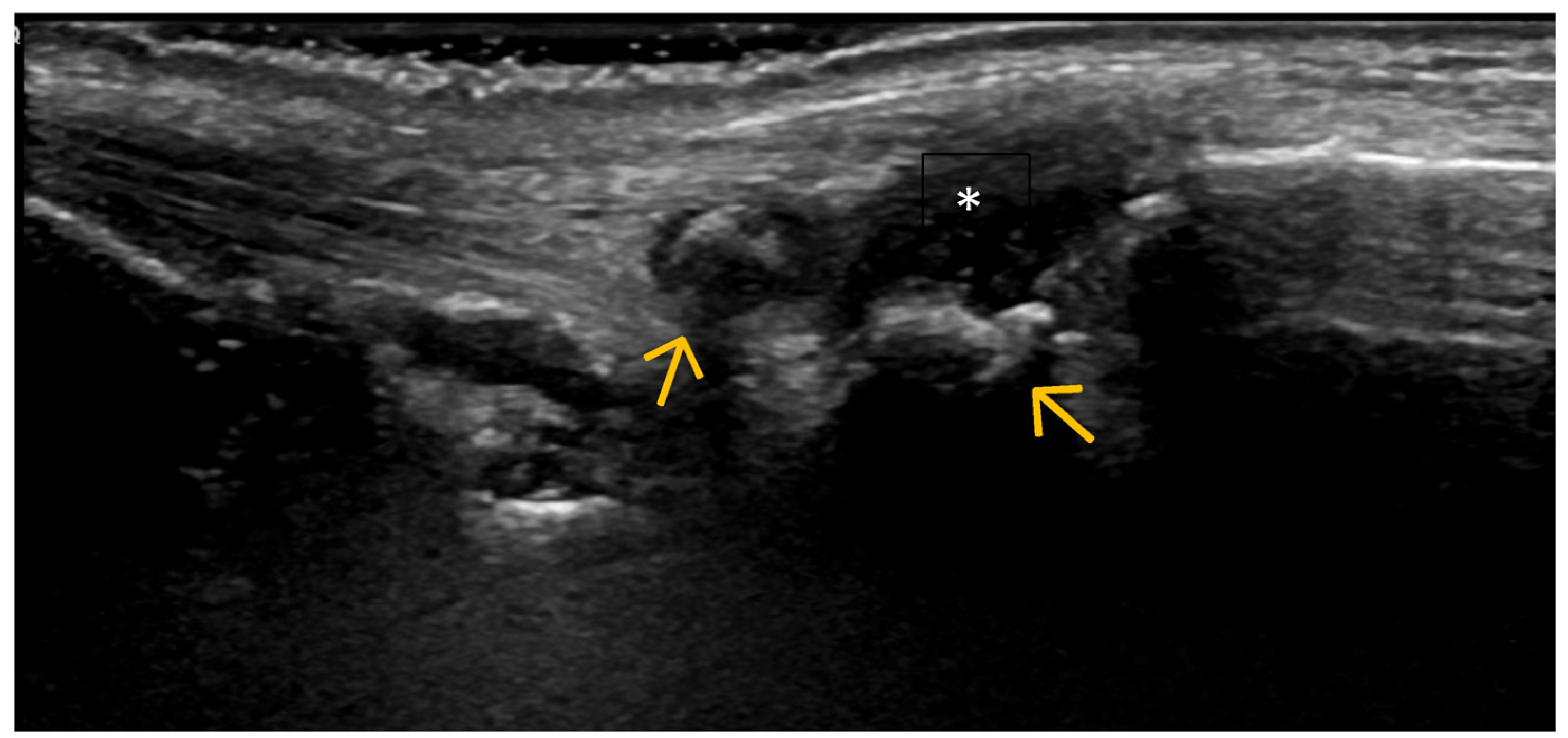
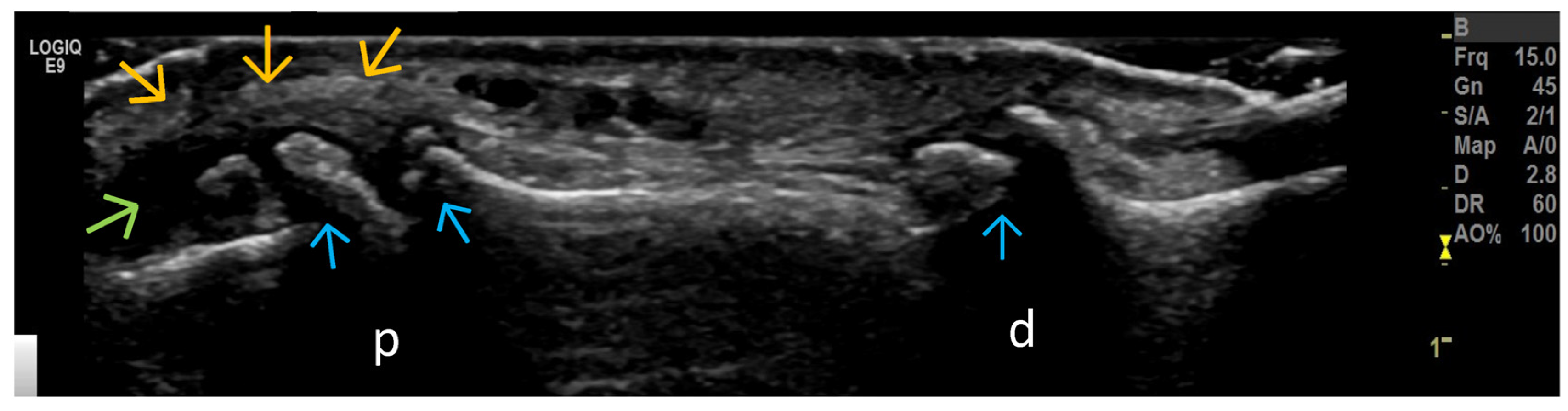
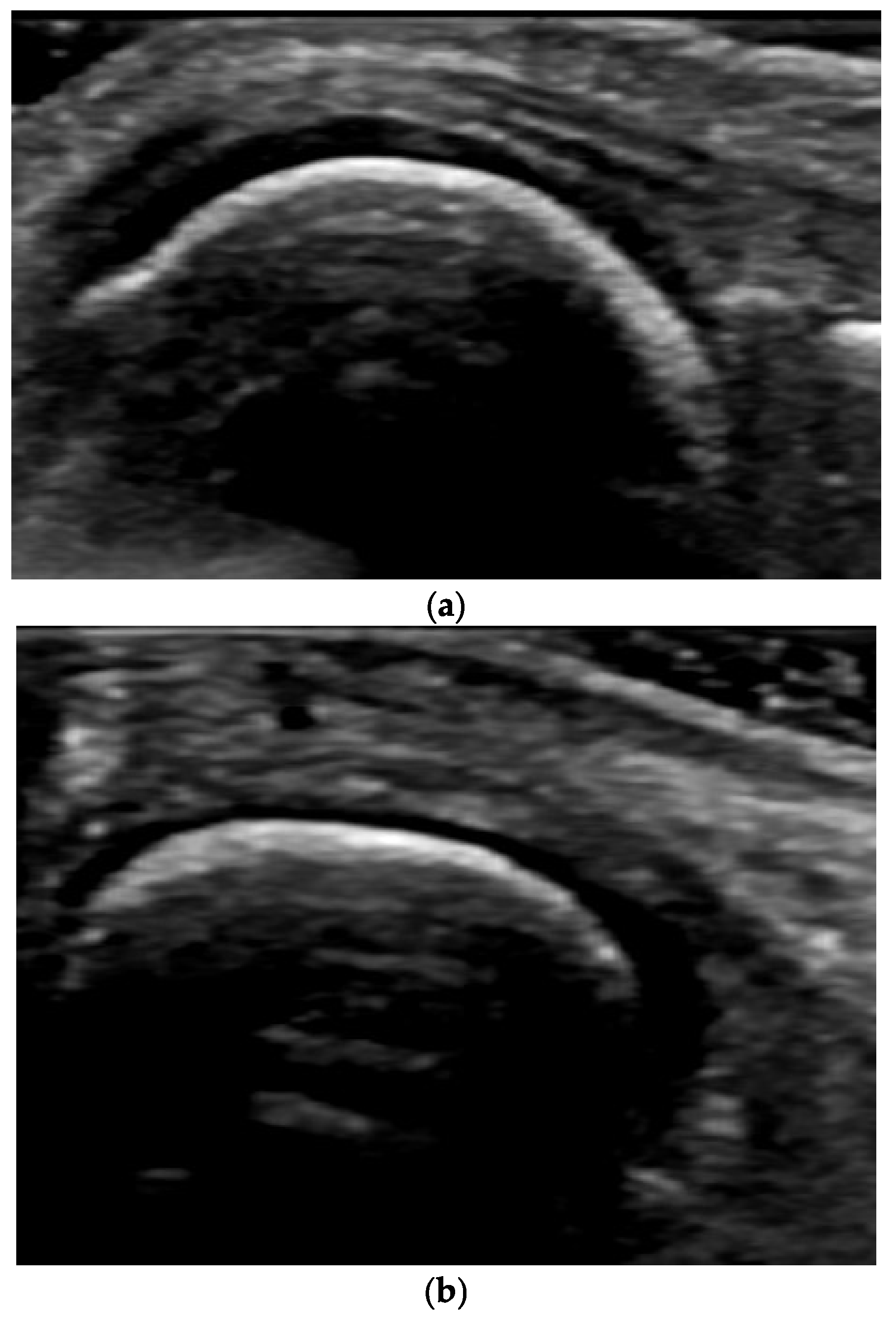
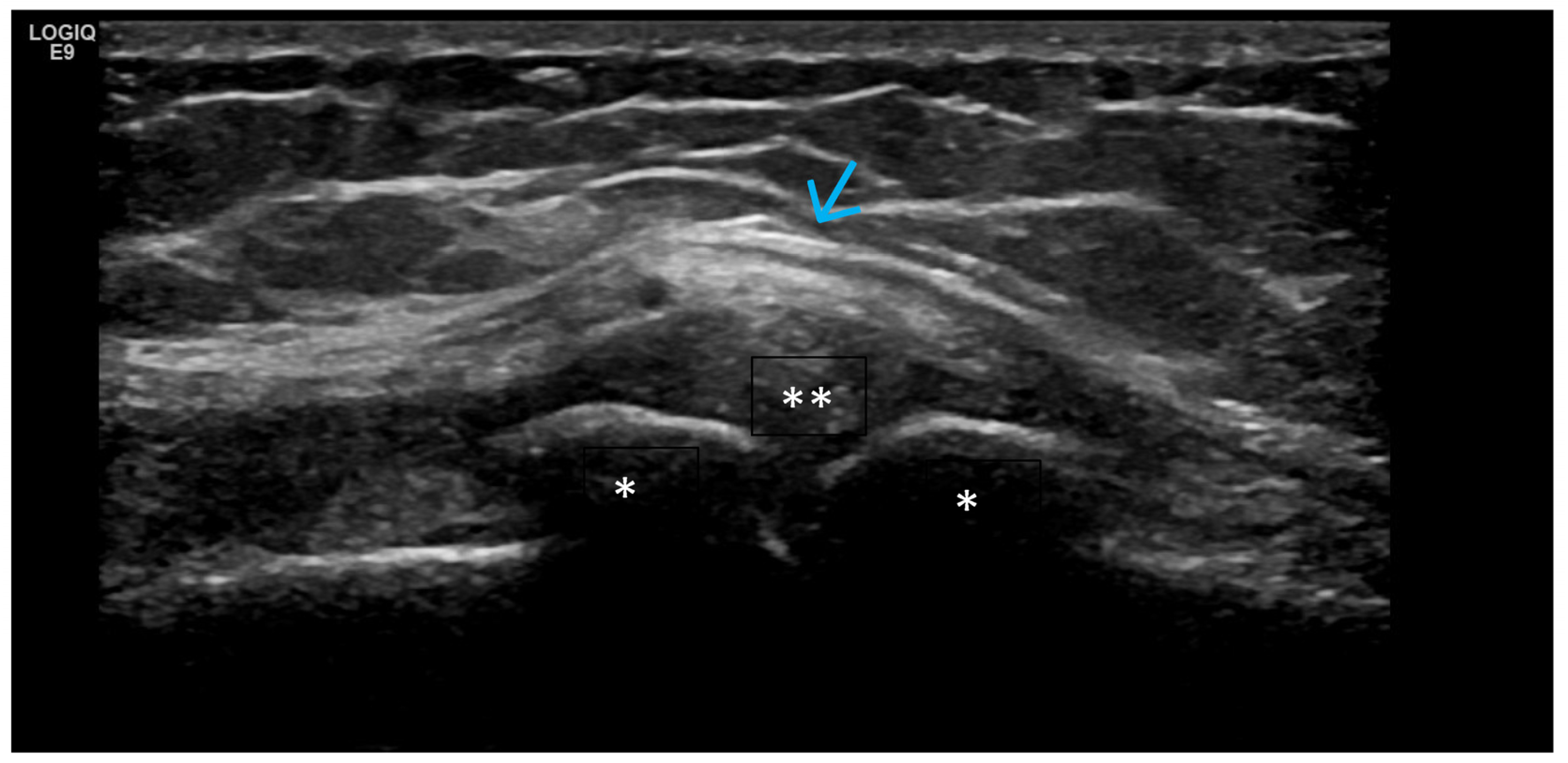
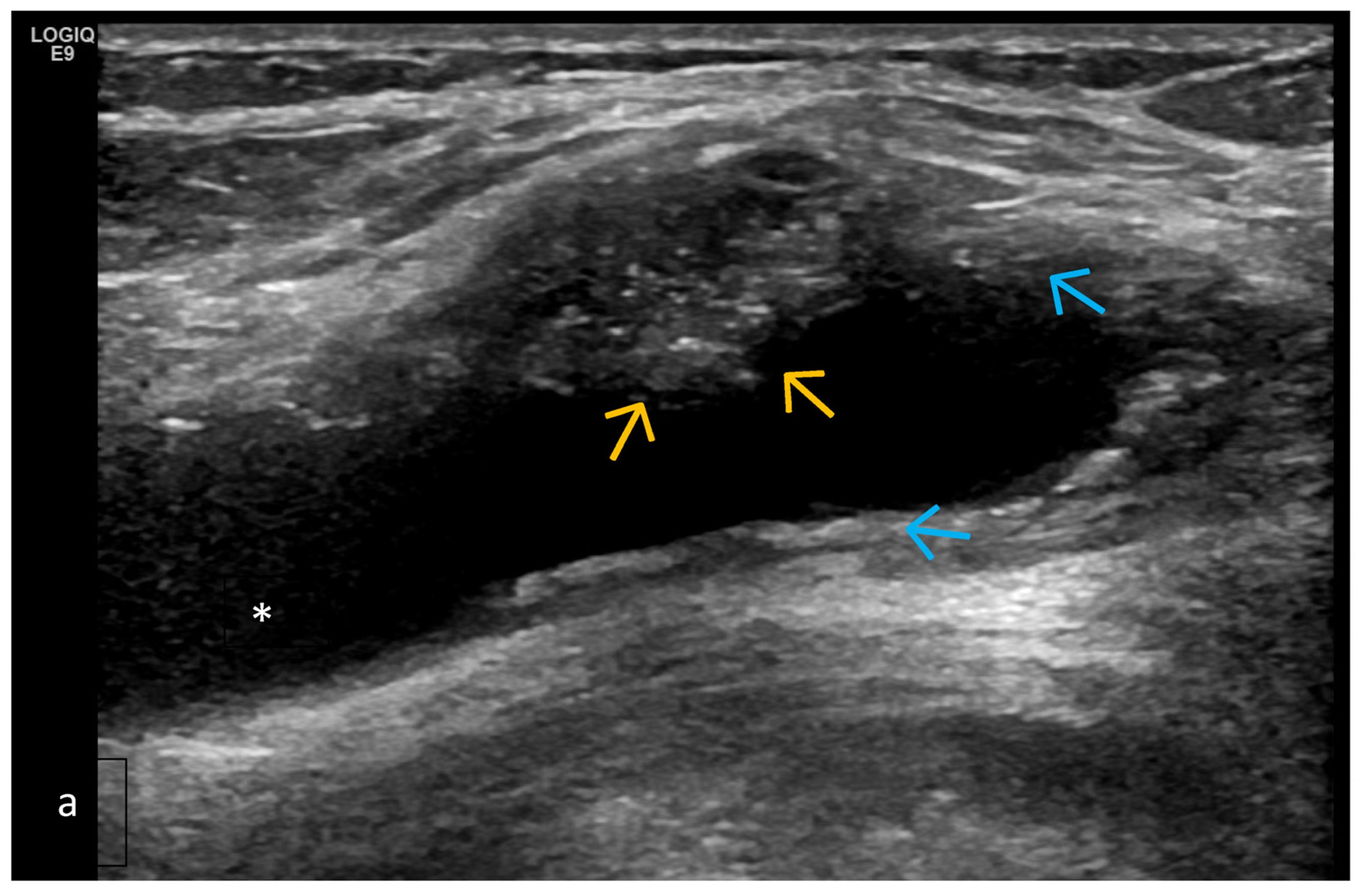
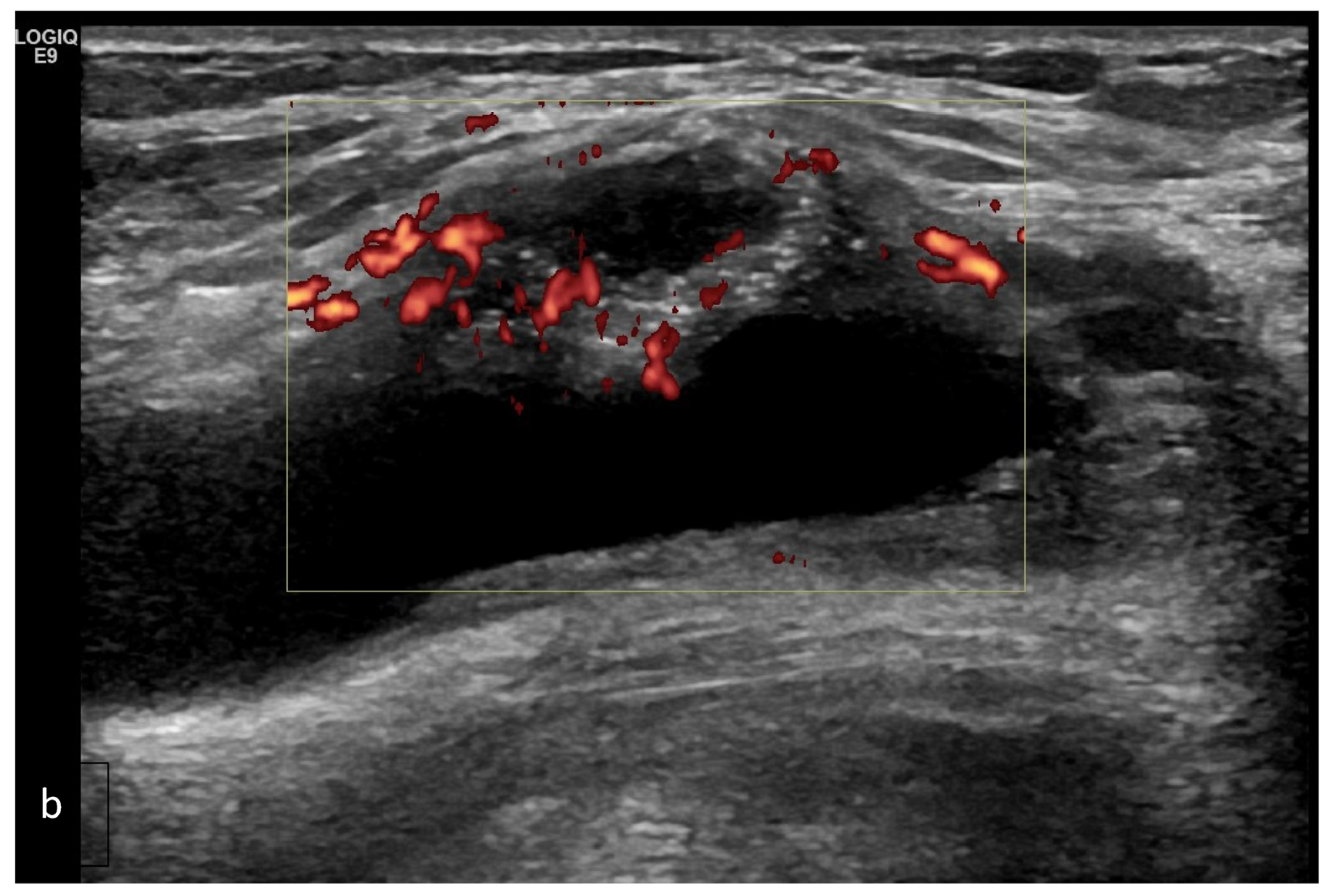
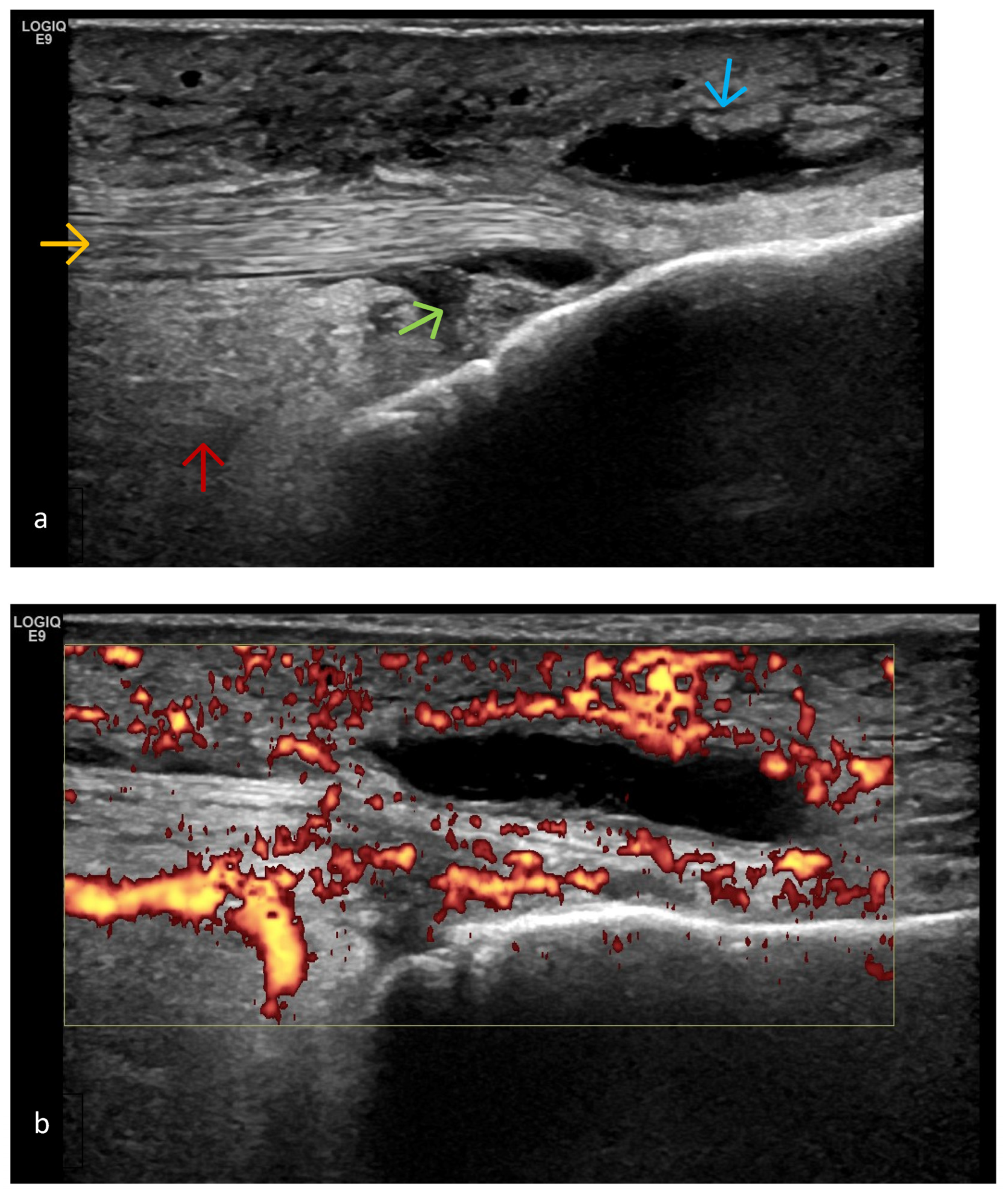
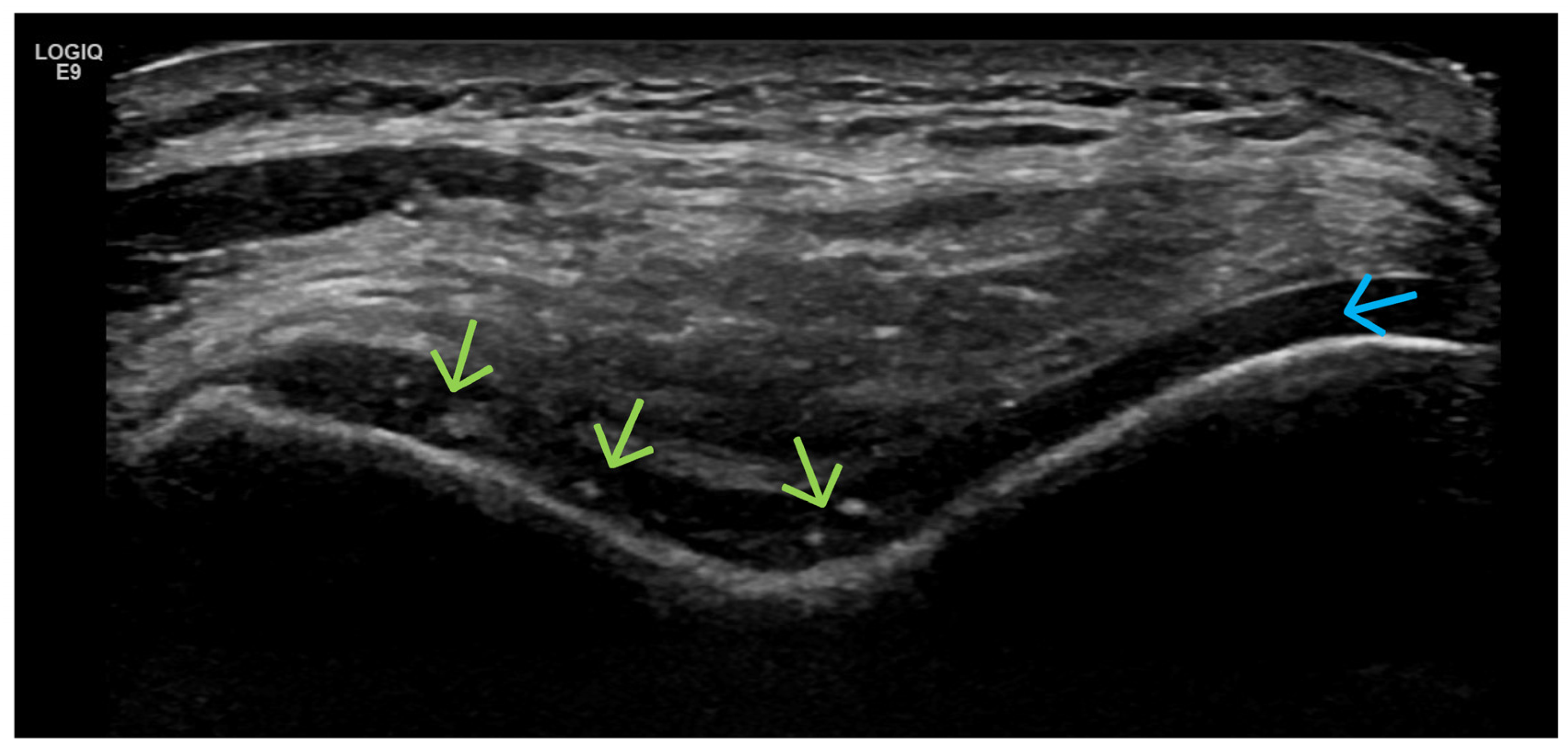

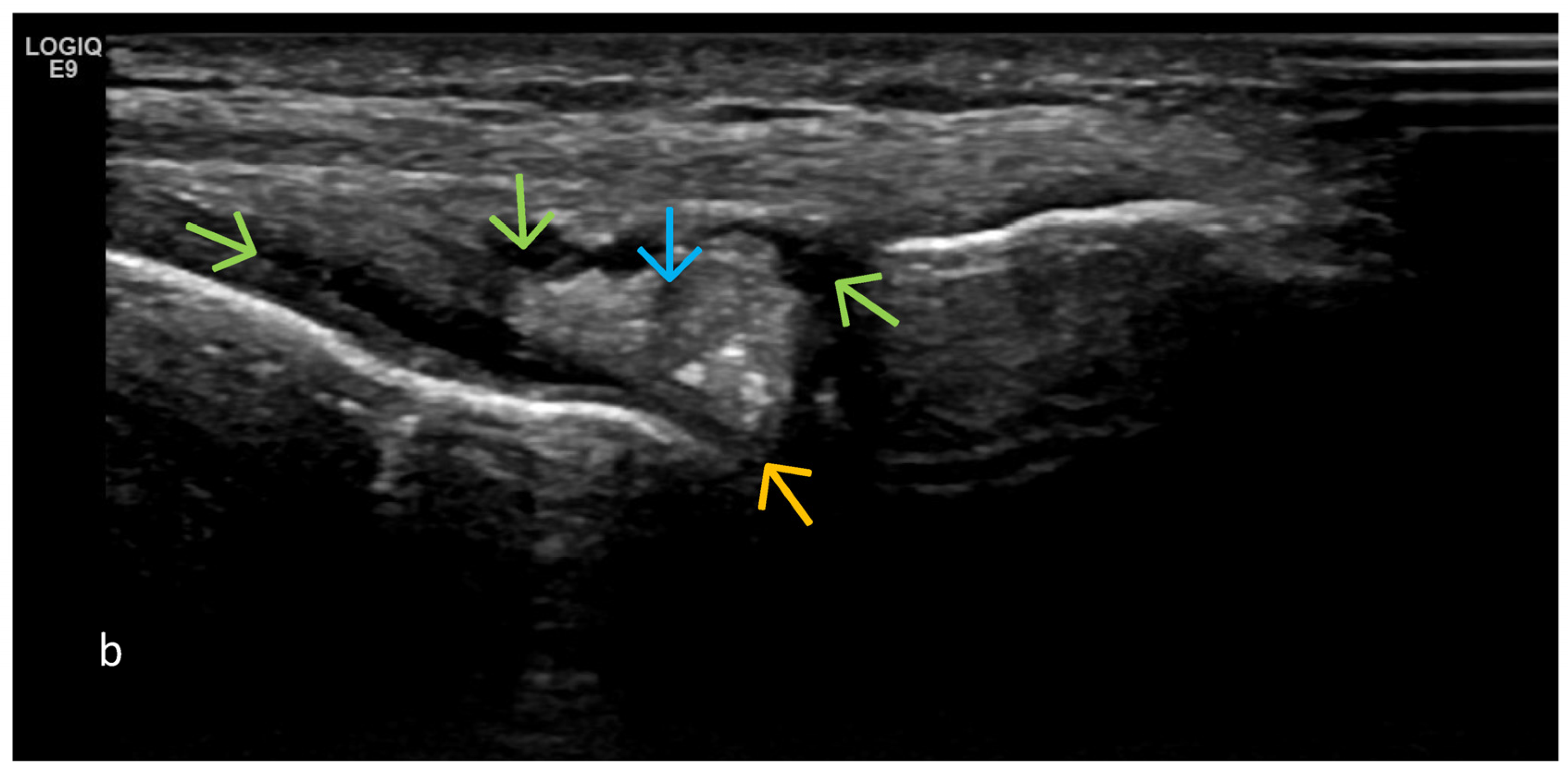
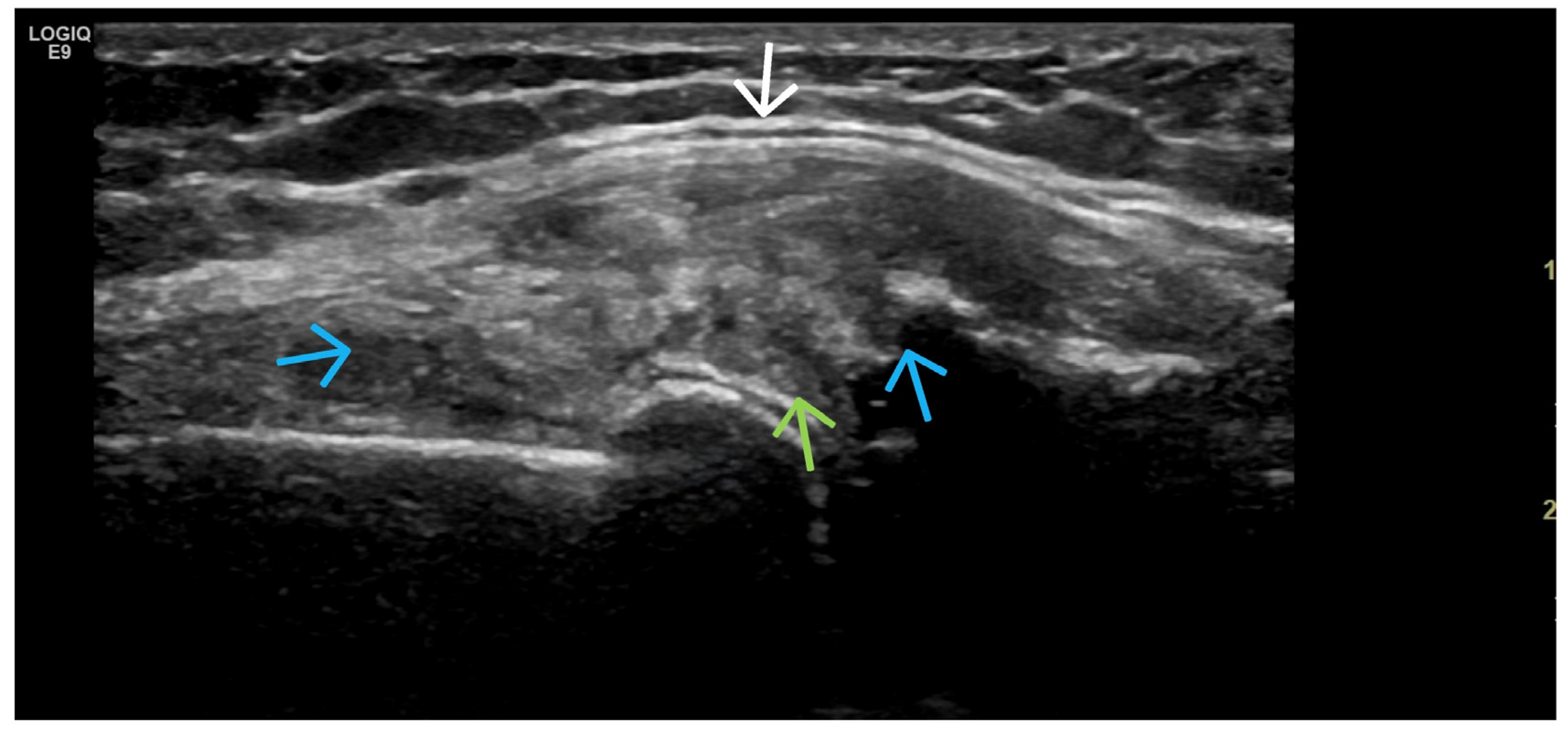
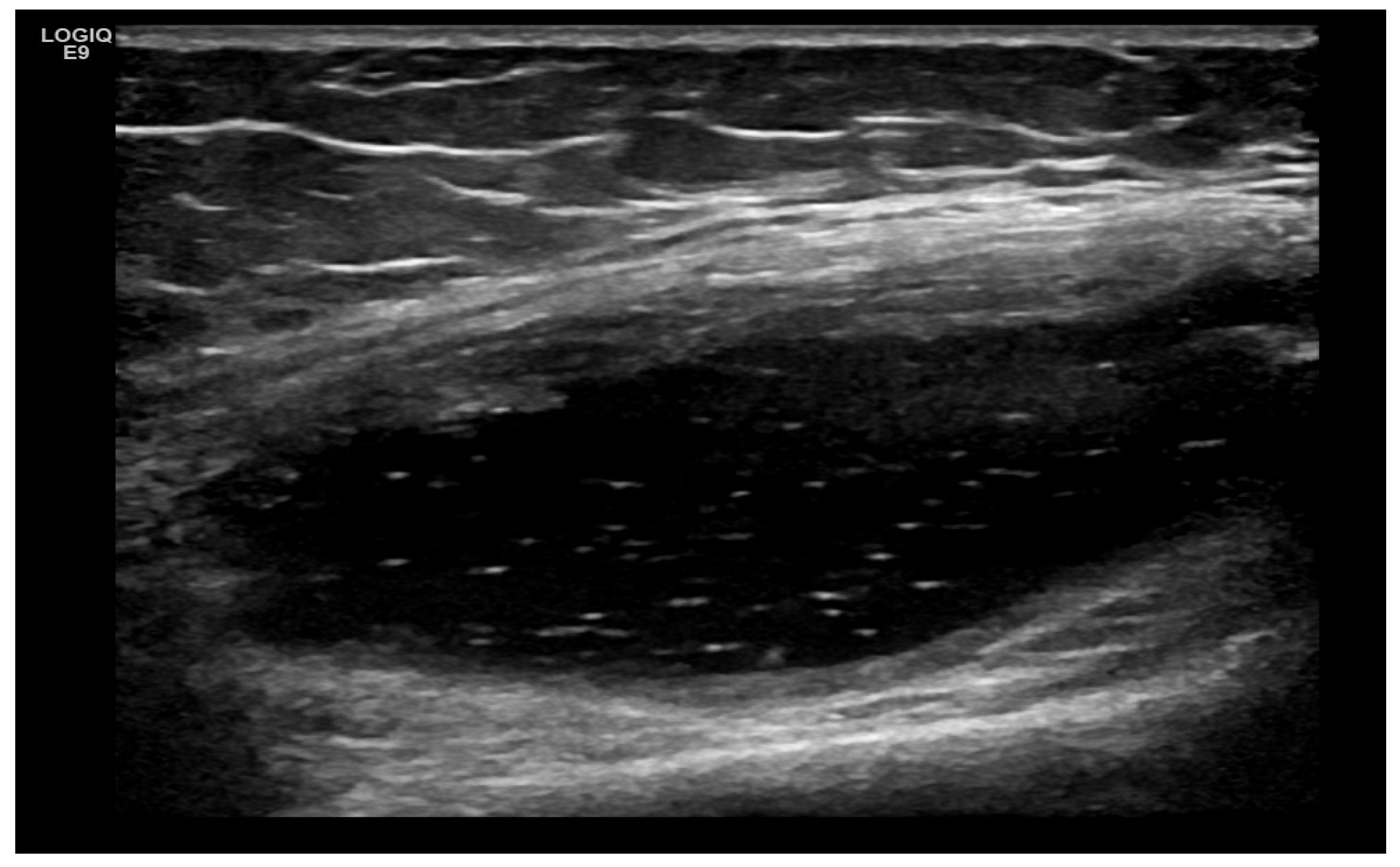
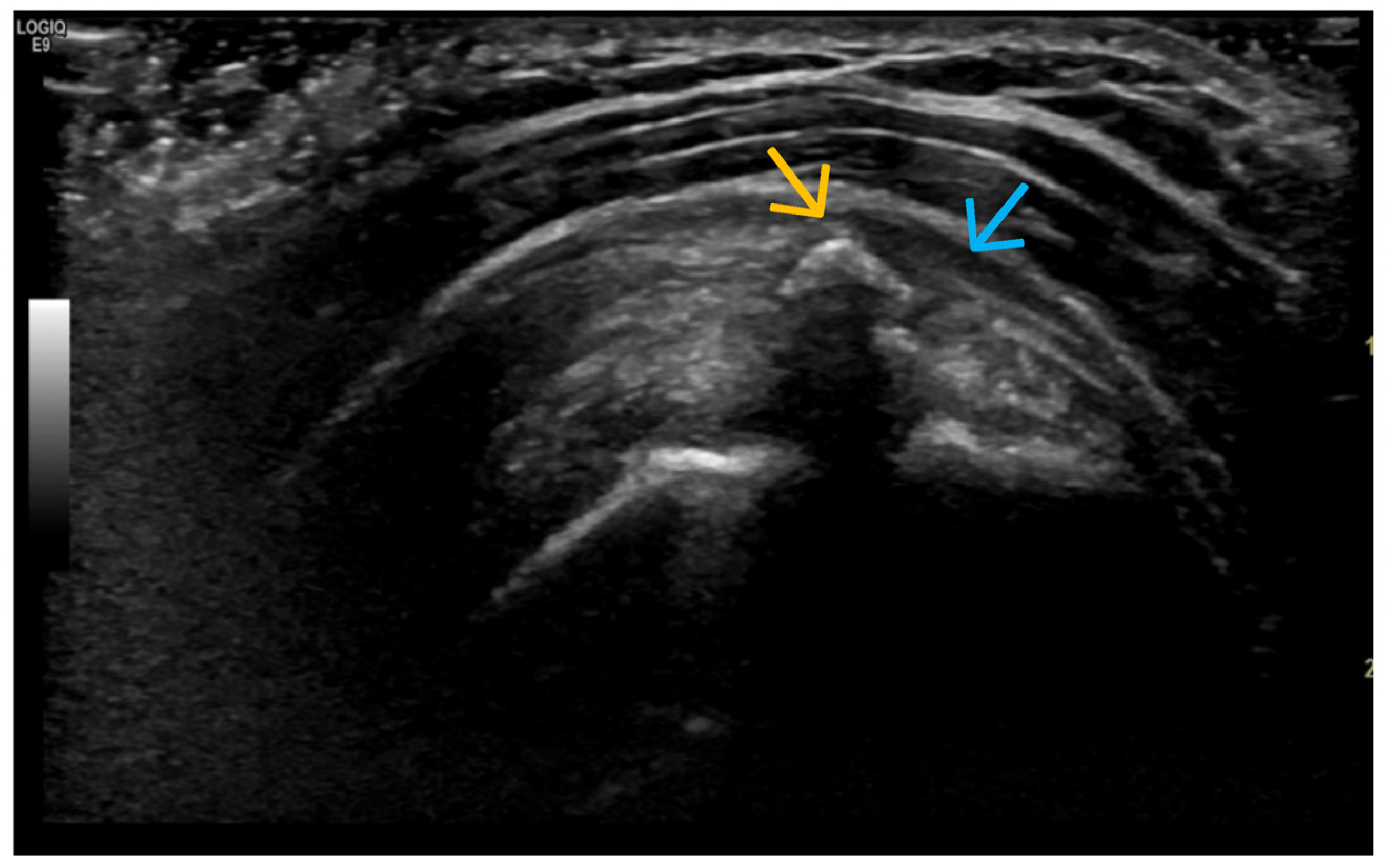
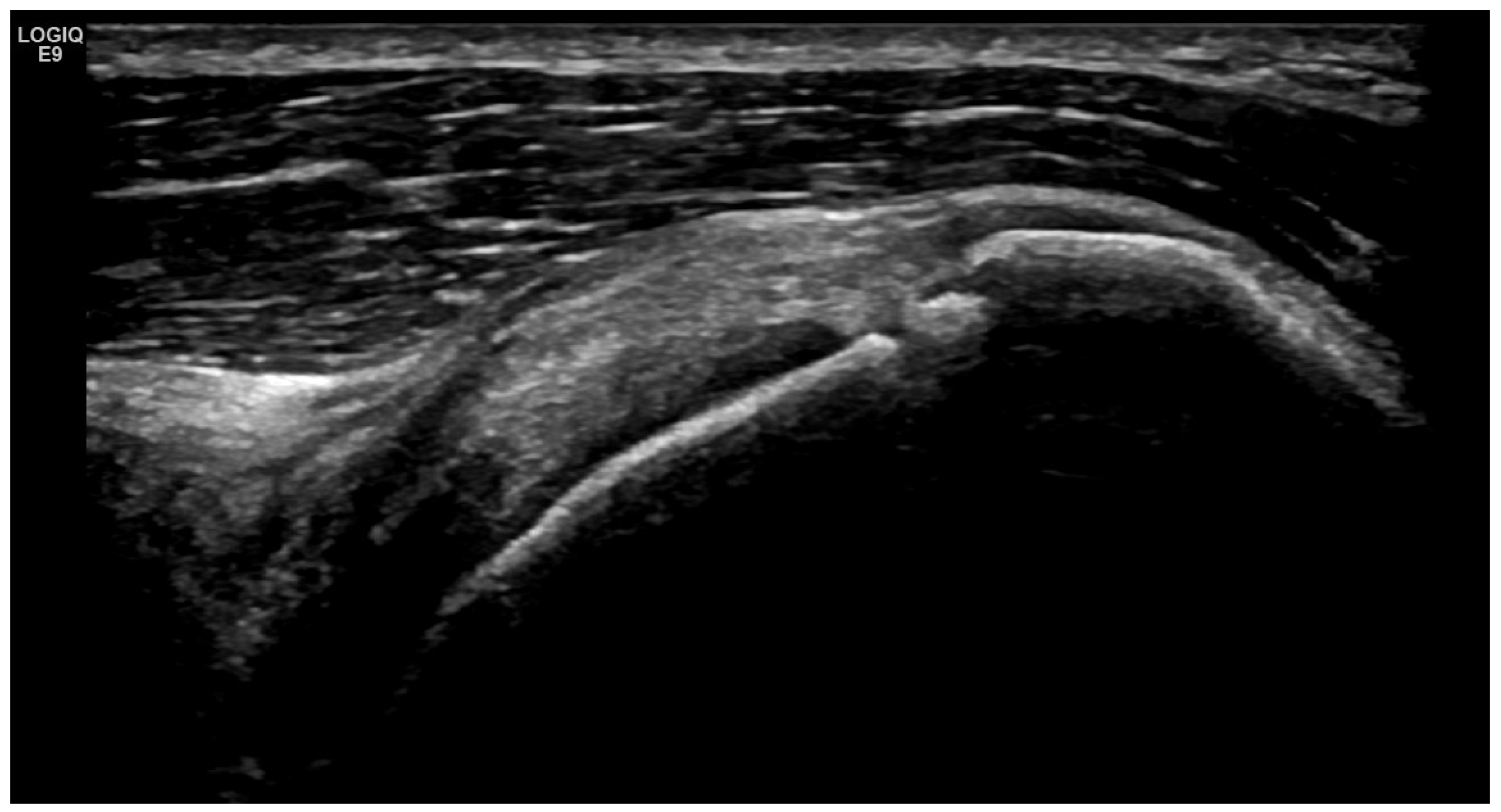
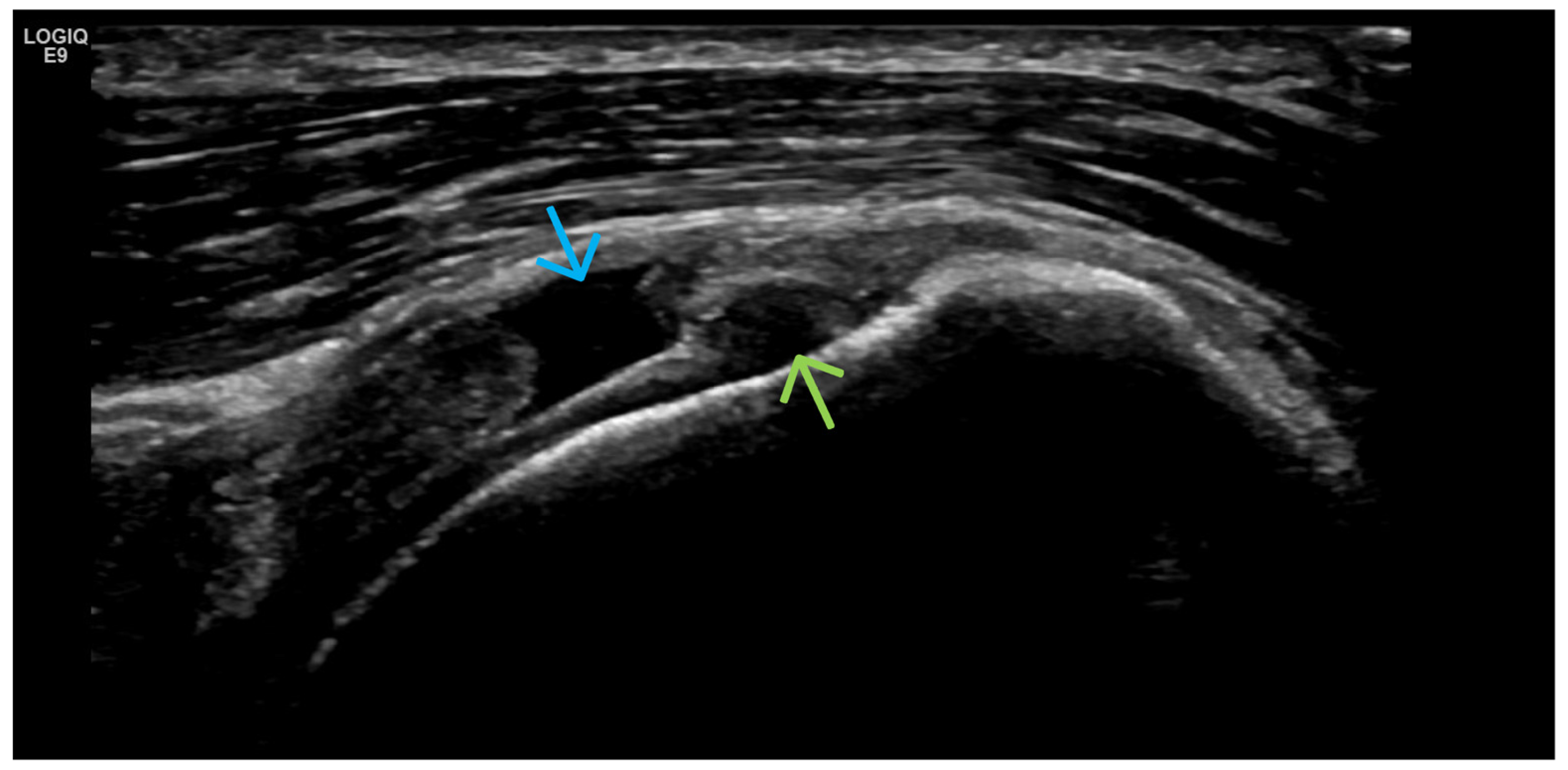
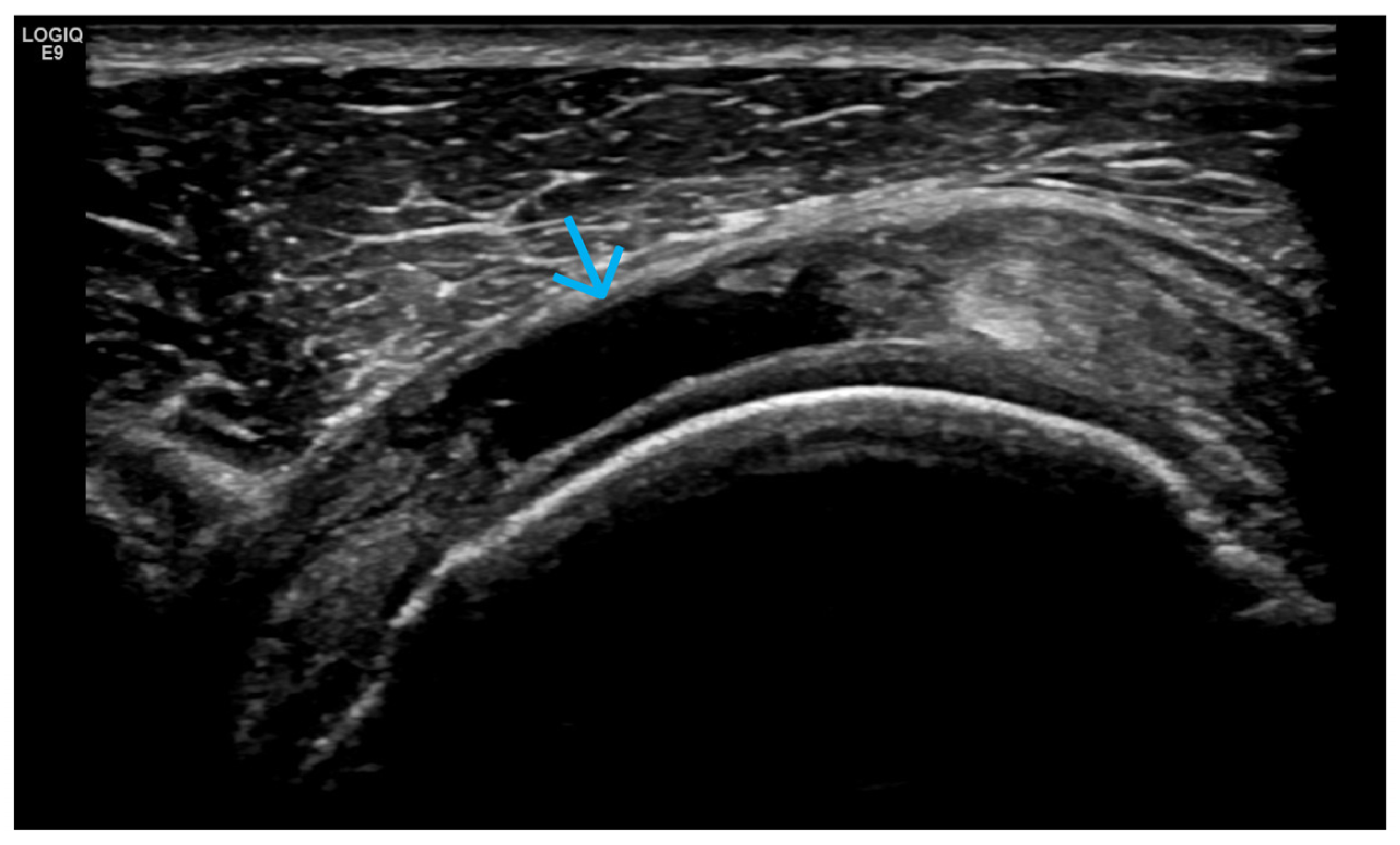
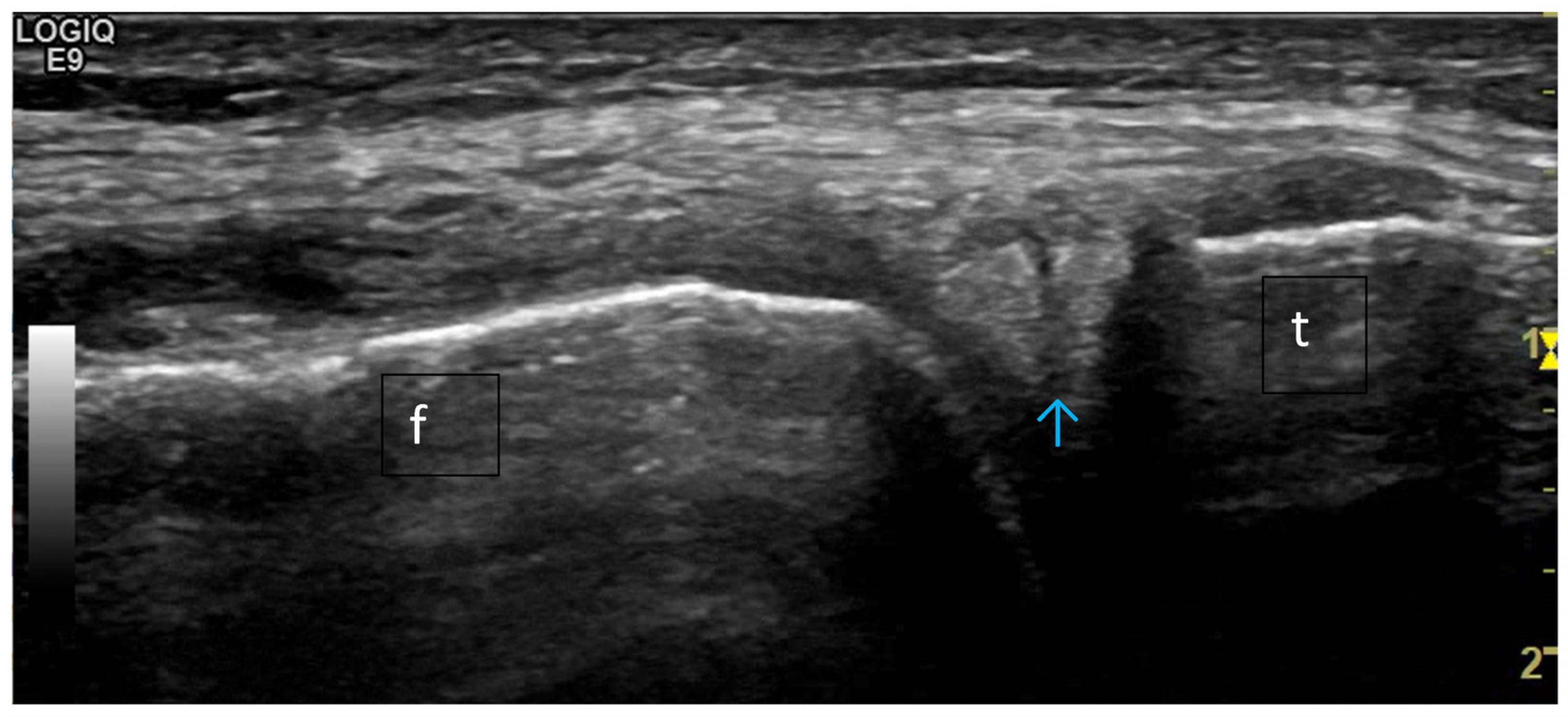
| Targeted Structure | Grade, Type |
|---|---|
| 1. Cartilage | 0, I, II, III grade |
| 2. Bone | Osteophytes (0, I, II, III grade) and erosions |
| 3. Synovium | 0, I, II, III grade |
| 4. Synovial hypertrophy | + or − |
| 5. Doppler activity | With/without Doppler activity |
| 6. Hoffa synovitis—degrees | For knee joint |
| 7. Joint effusion | + or − |
| 8. Periarticular soft tissue | |
| 9. Ligaments | |
| 10. Bursae | |
| 11. Menisci (extrusion, cysts) | For knee joint |
| 12. Tendinopathy | |
| 13. Entheses | |
| 14. Compression of peripheral nerves | |
| 15. Deposition in the joint and periarticular soft tissue | Monosodium urate crystals (MSU), calcium pyrophosphate (CPP) crystals |
| Synovitis grade (0–3) Synovial hypertrophy (+/−) Effusion (+/−) PD (+) Cartilage grade (0–3) Osteophyte grade (0–3) Meniscal extrusion grade (0–2) |
| KOA (n = 123) | Hip OA (n = 67) | Hand OA (n = 56) | Shoulder OA (n = 13) | |
|---|---|---|---|---|
| Gender (M/F) | 43/80 | 39/28 | 15/41 | 7/6 |
| Age (±SD), years | 58.1 ± 6.7 | 61.3 ± 5.2 | 48.7 ± 3.9 | 49.8 ± 4.5 |
| Duration of disease, years | 7.6 ± 5.5 | 5.5 ± 6.7 | 6.3 ± 4.6 | 5.0 ± 4.1 |
| BMI median, kg/m2 | 28.1 (24.5–34.6) | 27.5 (23.2–34.7) | 24.2 (19.8–26.6) | 23.5 (20.1–27.3) |
| ESR (mm/h) | 13.8 (5–42) | 14.2 (6–28) | 16.9 (10–44) | 12.7 (8–22) |
| CRP (mg/L) | 3.4 (1.1–7.2) | 2.8 (1.0–5.2) | 4.4 (2.8–6.2) | 2.2 (0.8–5.3) |
| MSK US Subtype | Therapeutic Approach |
|---|---|
|
|
| Arthrocentesis with evacuation of synovial fluid and subsequent administration of intra-articular corticosteroids |
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karalilova, R.; Popova, V.; Batalov, K.; Kolev, D.; Kodzhaahmed, L.; Petrova-Stoyankova, D.; Tepeliev, N.; Kostova, T.; Mekenyan, L.; Batalov, Z. Ultrasound Phenotype-Based Approach to Treatment Choice in Osteoarthritis. Life 2025, 15, 1140. https://doi.org/10.3390/life15071140
Karalilova R, Popova V, Batalov K, Kolev D, Kodzhaahmed L, Petrova-Stoyankova D, Tepeliev N, Kostova T, Mekenyan L, Batalov Z. Ultrasound Phenotype-Based Approach to Treatment Choice in Osteoarthritis. Life. 2025; 15(7):1140. https://doi.org/10.3390/life15071140
Chicago/Turabian StyleKaralilova, Rositsa, Velichka Popova, Konstantin Batalov, Dimitar Kolev, Lyatif Kodzhaahmed, Dimitrina Petrova-Stoyankova, Nikola Tepeliev, Tsvetelina Kostova, Lili Mekenyan, and Zguro Batalov. 2025. "Ultrasound Phenotype-Based Approach to Treatment Choice in Osteoarthritis" Life 15, no. 7: 1140. https://doi.org/10.3390/life15071140
APA StyleKaralilova, R., Popova, V., Batalov, K., Kolev, D., Kodzhaahmed, L., Petrova-Stoyankova, D., Tepeliev, N., Kostova, T., Mekenyan, L., & Batalov, Z. (2025). Ultrasound Phenotype-Based Approach to Treatment Choice in Osteoarthritis. Life, 15(7), 1140. https://doi.org/10.3390/life15071140







