Triangular Fibrocartilage Characterization with Ultrashort Echo Time-T2* MRI: Insights from a Healthy Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. MRI Protocol
2.3. UTE-T2* Measurement in the TFC Disc
2.4. Ulnar Variance Measurement
2.5. Statistical Analyses
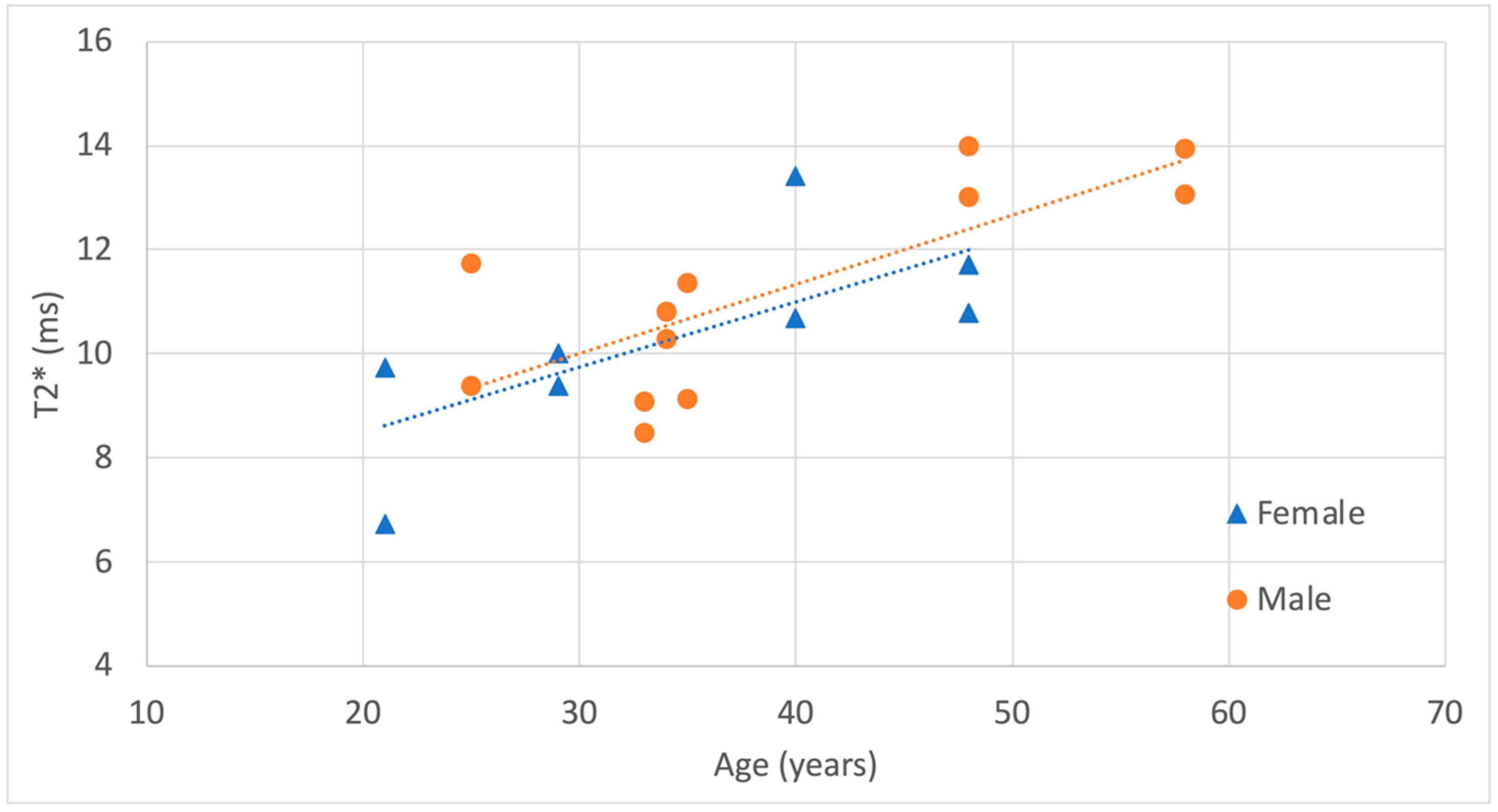
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| GRAPPA | GeneRalized Autocalibrating Partial Parallel Acquisition |
| PD | Proton Density |
| SPACE | Sampling Perfection with Application optimized Contrast using different flip angle Evolution |
| SPAIR | Spectral Attenuated Inversion Recover |
| TFC | Triangular Fibrocartilage |
| TFCC | Triangular Fibrocartilage Complex |
| UTE | Ultrashort echo time |
References
- Palmer, A.K.; Werner, F.W. The Triangular Fibrocartilage Complex of the Wrist—Anatomy and Function. J. Hand Surg. 1981, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, M.C.; Unglaub, F.; Mühldorfer-Fodor, M.; Pillukat, T.; Hahn, P.; Müller, L.P.; Spies, C.K. Update TFCC: Histology and Pathology, Classification, Examination and Diagnostics. Arch. Orthop. Trauma. Surg. 2015, 135, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Unglaub, F.; Thomas, S.B.; Kroeber, M.W.; Dragu, A.; Fellenberg, J.; Wolf, M.B.; Horch, R.E. Apoptotic Pathways in Degenerative Disk Lesions in the Wrist. Arthroscopy 2009, 25, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Unglaub, F.; Wolf, M.B.; Thome, M.A.; Germann, G.; Sauerbier, M.; Reiter, A. Correlation of Ulnar Length and Apoptotic Cell Death in Degenerative Lesions of the Triangular Fibrocartilage. Arthroscopy 2008, 24, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K. Triangular Fibrocartilage Complex Lesions: A Classification. J. Hand Surg. 1989, 14, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Jawed, A.; Ansari, M.T.; Gupta, V. TFCC Injuries: How We Treat? J. Clin. Orthop. Trauma. 2020, 11, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Tomaino, M.M.; Elfar, J. Ulnar Impaction Syndrome. Hand Clin. 2005, 21, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Spies, C.K.; Bruckner, T.; Müller, L.P.; Unglaub, F.; Eysel, P.; Löw, S.; Filbert, M.J. Long-Term Outcome after Arthroscopic Debridement of Palmer Type 2C Central Degenerative Lesions of the Triangular Fibrocartilage Complex. Arch. Orthop. Trauma Surg. 2021, 141, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Lee, S.-W.; Kim, D.-H.; Oh, W.-T.; Koh, I.-H.; Chun, Y.-M.; Choi, Y.-R. Prognostic Factors for Clinical Outcomes after Arthroscopic Treatment of Traumatic Central Tears of the Triangular Fibrocartilage Complex. Bone Jt. J. 2024, 106-B, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Portnoff, B.; Casey, J.C.; Thirumavalavan, J.; Abbott, E.; Faber, R.; Gil, J.A. Prevalence of Asymptomatic TFCC Tears on MRI: A Systematic Review. Hand Surg. Rehabil. 2024, 43, 101684. [Google Scholar] [CrossRef] [PubMed]
- Lauder, J.; Younis, F.; Khan, S.H. Imaging of Ulnar-Sided Wrist Pain. Br. J. Hosp. Med. 2019, 80, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Dineen, H.A.; Greenberg, J.A. Ulnar-Sided Wrist Pain in the Athlete. Clin. Sports Med. 2020, 39, 373–400. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, A.; Aoki, T.; Narimatsu, H.; Kuwahara, C.; Nozaki, A.; Menuki, K.; Sakai, A.; Korogi, Y. Ultrashort Time-to-Echo Quantitative Magnetic Resonance Imaging of the Triangular Fibrocartilage: Differences in Position. Eur. Radiol. 2019, 29, 3219–3223. [Google Scholar] [CrossRef] [PubMed]
- Geiger, D.; Bae, W.C.; Statum, S.; Du, J.; Chung, C.B. Quantitative 3D Ultrashort Time-to-Echo (UTE) MRI and Micro-CT (μCT) Evaluation of the Temporomandibular Joint (TMJ) Condylar Morphology. Skelet. Radiol. 2014, 43, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Dallaudière, B.; Trotier, A.; Ribot, E.; Verdier, D.; Lepreux, S.; Miraux, S.; Hauger, O. Three-Dimensional Ultrashort Echo Time (3D UTE) MRI of Achilles Tendon at 4.7T MRI with Comparison to Conventional Sequences in an Experimental Murine Model of Spondyloarthropathy. J. Magn. Reson. Imaging 2019, 50, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Finkenstaedt, T.; Biswas, R.; Abeydeera, N.A.; Siriwanarangsun, P.; Healey, R.; Statum, S.; Bae, W.C.; Chung, C.B. Ultrashort Time to Echo Magnetic Resonance Evaluation of Calcium Pyrophosphate Crystal Deposition in Human Menisci. Investig. Radiol. 2019, 54, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Dallaudiere, B.; Trotier, A.J.; Ribot, E.J.; Loubrie, S.; Miraux, S.; Hauger, O. Early Achilles Enthesis Involvement in a Murine Model of Spondyloarthropathy: Morphological Imaging with Ultrashort Echo-Time Sequences and Ultrasmall Superparamagnetic Iron Oxide (USPIO) Particle Evaluation in Macrophagic Detection. Contrast Media Mol. Imaging 2019, 2019, 2834273. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-L.; Liu, L.-H.; Rao, S.-X.; Wu, P.-Y.; Zhou, J.-J. Ultrashort Time-to-Echo T2* and T2* Relaxometry for Evaluation of Lumbar Disc Degeneration: A Comparative Study. BMC Musculoskelet. Disord. 2022, 23, 524. [Google Scholar] [CrossRef] [PubMed]
- Finkenstaedt, T.; Siriwananrangsun, P.; Masuda, K.; Bydder, G.M.; Chen, K.C.; Bae, W.C. Ultrashort Time-to-Echo MR Morphology of Cartilaginous Endplate Correlates with Disc Degeneration in the Lumbar Spine. Eur. Spine J. 2023, 32, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lee, Y.H.; Song, H.-T.; Suh, J.-S. Comparison of T2∗ Mapping between Regular Echo Time and Ultrashort Echo Time with 3D Cones at 3 Tesla for Knee Meniscus. Medicine 2018, 97, e13443. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.C.; Tafur, M.; Chang, E.Y.; Du, J.; Biswas, R.; Kwack, K.-S.; Healey, R.; Statum, S.; Chung, C.B. High-Resolution Morphologic and Ultrashort Time-to-Echo Quantitative Magnetic Resonance Imaging of the Temporomandibular Joint. Skelet. Radiol. 2016, 45, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Nebelung, S.; Tingart, M.; Pufe, T.; Kuhl, C.; Jahr, H.; Truhn, D. Ex Vivo Quantitative Multiparametric MRI Mapping of Human Meniscus Degeneration. Skelet. Radiol. 2016, 45, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Rosset, A.; Spadola, L.; Ratib, O. OsiriX: An Open-Source Software for Navigating in Multidimensional DICOM Images. J. Digit. Imaging 2004, 17, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Serfaty, A.; Costa, H.P.; Foelker, C.E.; Filho, E.N.K.; Souza, F.F.; Bordalo-Rodrigues, M. Evaluation of Ulnar Variance on Wrist MR Imaging: Is It a Reliable Measure? Skelet. Radiol. 2020, 49, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Branco, D.F.; Bouvet, C.; Hamard, M.; Beaulieu, J.Y.; Poletti, P.A.; Boudabbous, S. Reliability of Radio-Ulnar and Carpal Alignment Measurements in the Wrist between Radiographs and 3D Imaging. Eur. J. Radiol. 2022, 154, 110417. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Williams, A.A.; Coyle, C.H.; Bowers, M.E. Early Diagnosis to Enable Early Treatment of Pre-Osteoarthritis. Arthritis Res. Ther. 2012, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, I.; Stahl, R.; Cheng, J.; Li, X.; Huber, M.B.; Luke, A.; Majumdar, S.; Link, T.M. Meniscal Measurements of T1ρ and T2 at MR Imaging in Healthy Subjects and Patients with Osteoarthritis. Radiology 2008, 249, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Sanal, H.T.; Bae, W.C.; Pauli, C.; Du, J.; Statum, S.; Znamirowski, R.; Sah, R.L.; Chung, C.B. 3T Mri of the temporomandibular joint disc: Feasibility of novel quantitative mr evaluation using histologic and biomechanical reference standards. J. Orofac. Pain 2011, 25, 345. [Google Scholar] [PubMed]
- Kakimoto, N.; Shimamoto, H.; Chindasombatjaroen, J.; Tsujimoto, T.; Tomita, S.; Hasegawa, Y.; Murakami, S.; Furukawa, S. Comparison of the T2 Relaxation Time of the Temporomandibular Joint Articular Disk between Patients with Temporomandibular Disorders and Asymptomatic Volunteers. Am. J. Neuroradiol. 2014, 35, 1412–1417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Semisch, M.; Hagert, E.; Garcia-Elias, M.; Lluch, A.; Rein, S. Histological Assessment of the Triangular Fibrocartilage Complex. J. Hand Surg. Eur. Vol. 2016, 41, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Milz, S.; Sicking, B.; Sprecher, C.M.; Putz, R.; Benjamin, M. An Immunohistochemical Study of the Triangular Fibrocartilage Complex of the Wrist: Regional Variations in Cartilage Phenotype. J. Anat. 2007, 211, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sato, S. Load transmission through the wrist joint: A biomechanical study comparing the normal and pathological wrist. Nihon Seikeigeka Gakkai Zasshi 1995, 69, 470–483. [Google Scholar] [PubMed]
- Harley, B.J.; Pereria, M.L.; Werner, F.W.; Kinney, D.A.; Sutton, L.G. Force Variations in the Distal Radius and Ulna: Effect of Ulnar Variance and Forearm Motion. J. Hand Surg. 2015, 40, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Qian, Y.; Bear, D.; Chu, C.R. Assessing Degeneration of Human Articular Cartilage with Ultra-Short Echo Time (UTE) T2* Mapping. Osteoarthr. Cartil. 2010, 18, 539. [Google Scholar] [CrossRef] [PubMed]
- Baum, T.; Joseph, G.B.; Karampinos, D.C.; Jungmann, P.M.; Link, T.M.; Bauer, J.S. Cartilage and Meniscal T2 Relaxation Time as Non-Invasive Biomarker for Knee Osteoarthritis and Cartilage Repair Procedures. Osteoarthr. Cartil. 2013, 21, 1474. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.; Preacher, K.J. On Effect Size. Psychol. Methods 2012, 17, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Bydder, M.; Rahal, A.; Fullerton, G.D.; Bydder, G.M. The Magic Angle Effect: A Source of Artifact, Determinant of Image Contrast, and Technique for Imaging. J. Magn. Reson. Imaging 2007, 25, 290–300. [Google Scholar] [CrossRef] [PubMed]
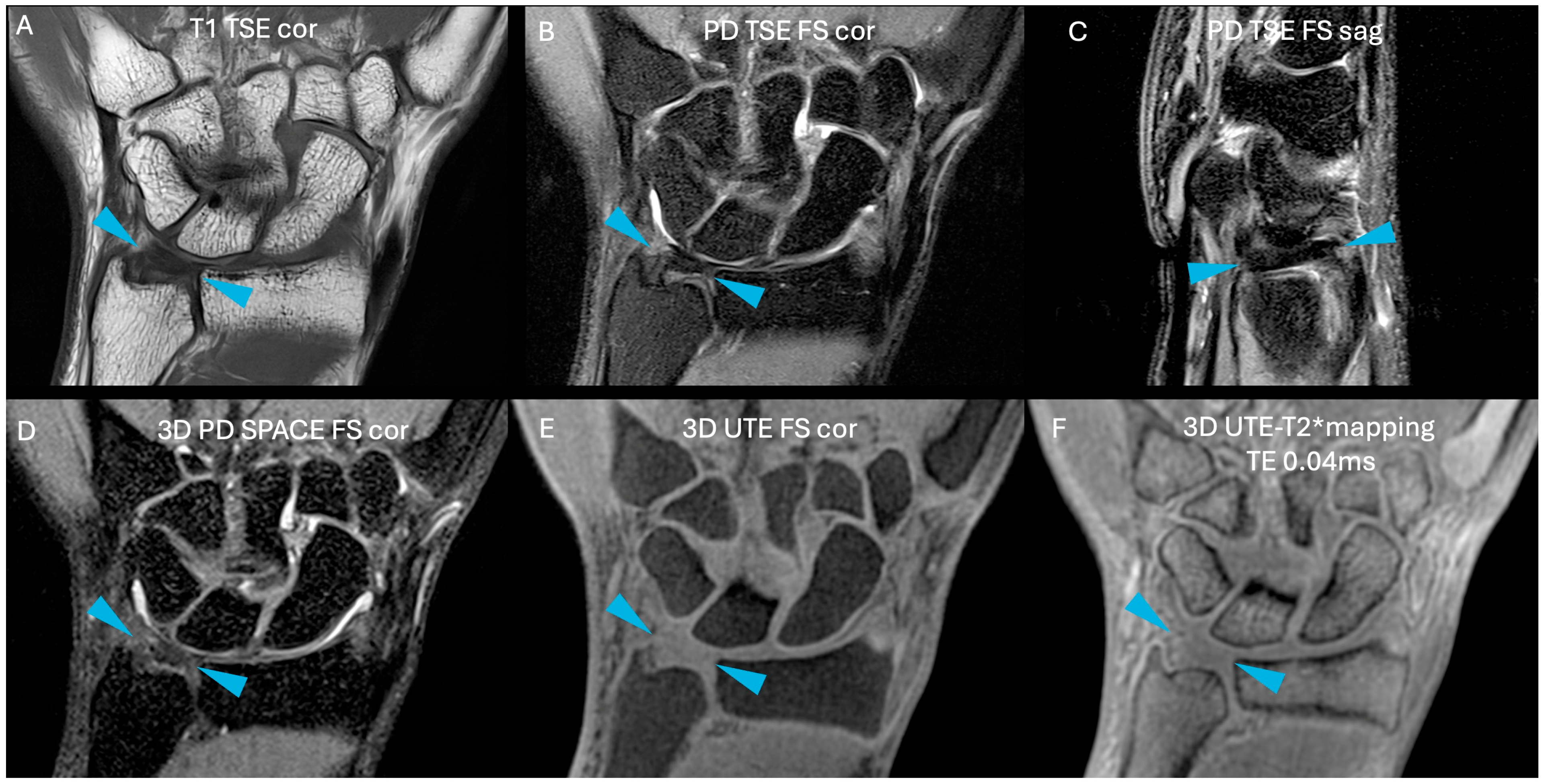
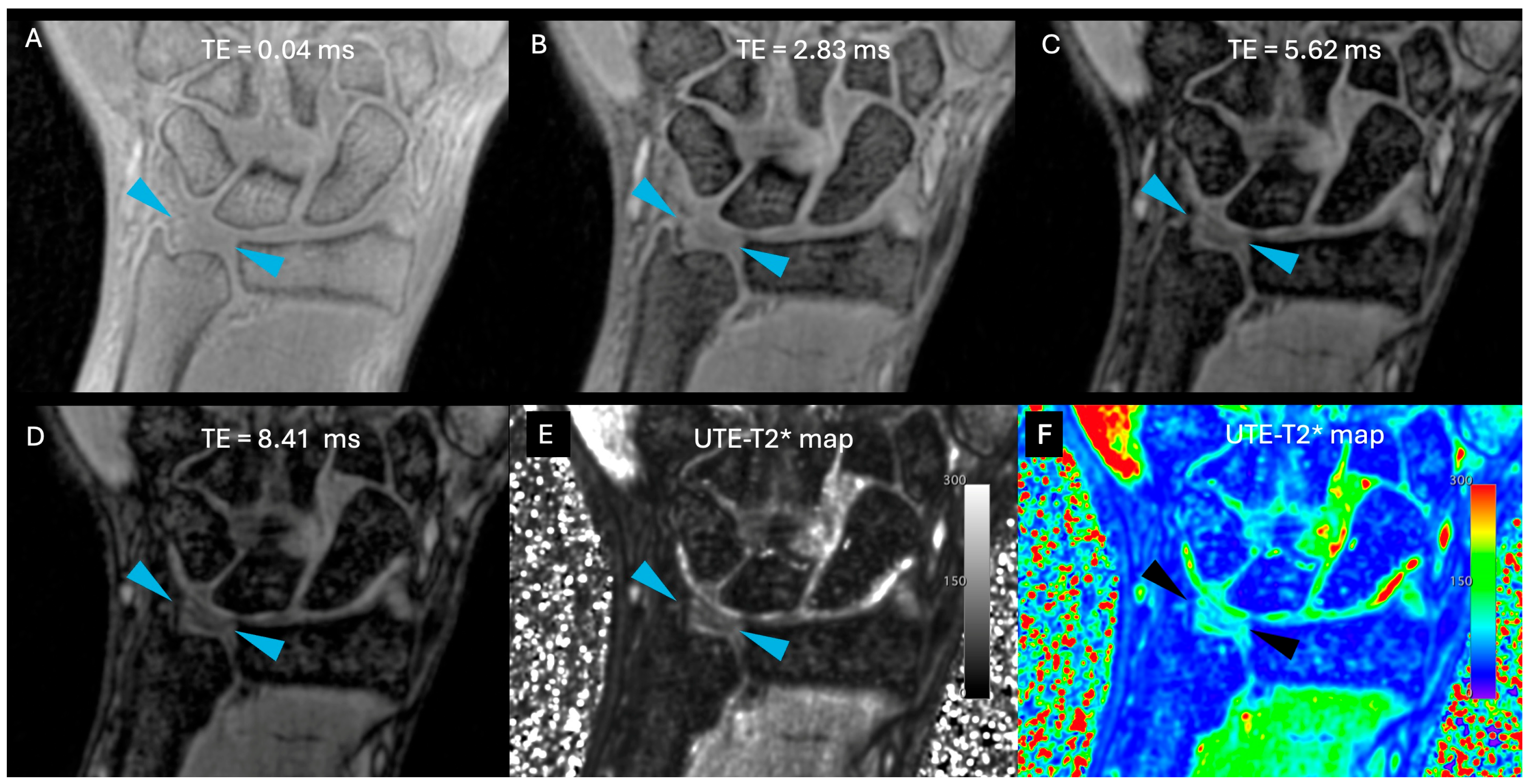
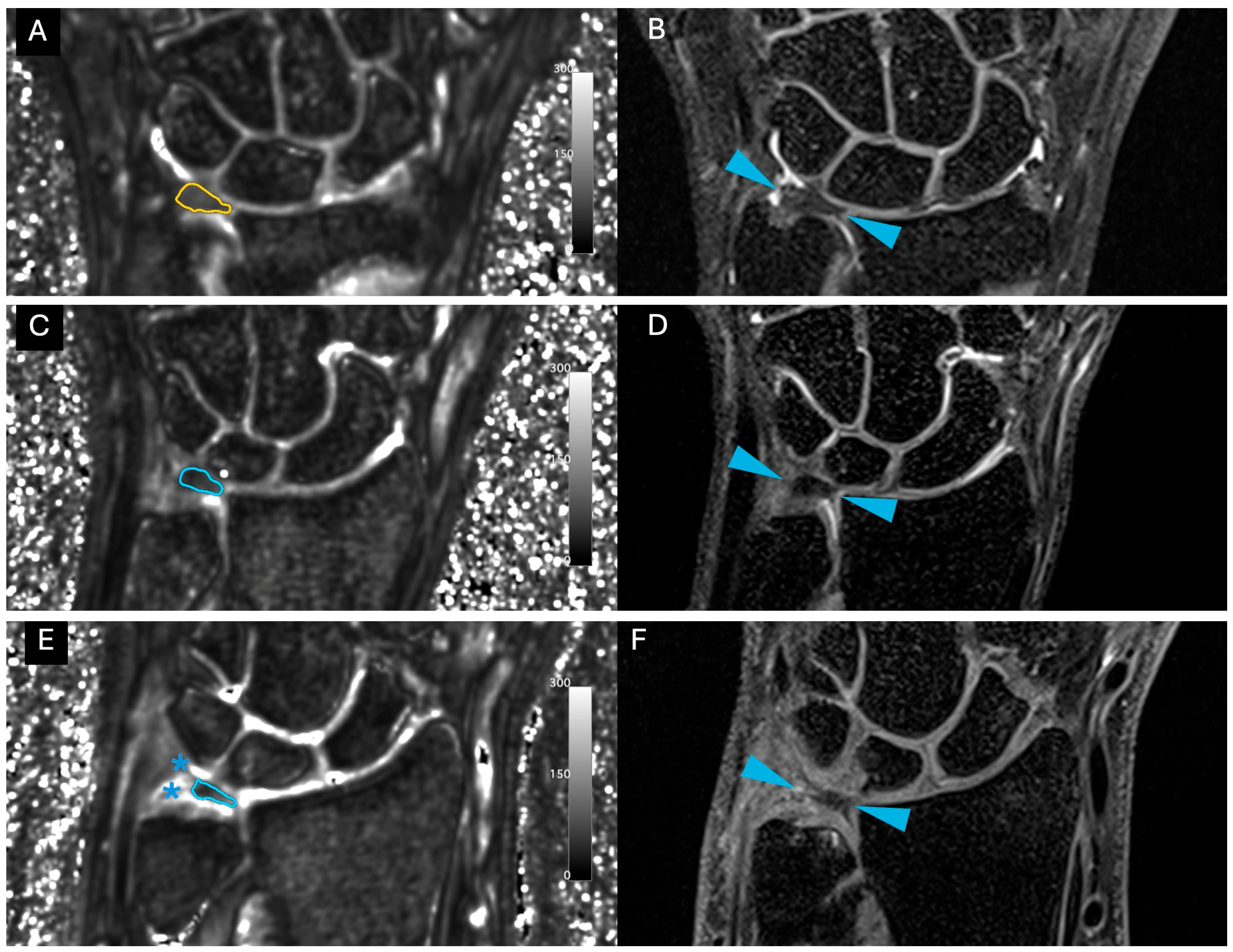
| Sequence | T1 TSE cor | PD TSE FS cor | PD TSE FS sag | 3D PD SPACE FS cor | 3D UTE FS cor | 3D UTE-T2* Mapping |
|---|---|---|---|---|---|---|
| FOV (mm) | 90 | 90 | 90 | 110 | 110 | 110 |
| slice thickness (mm) | 2.5 | 2.5 | 2.5 | 0.5 | 0.4 | 0.5 |
| TE (ms) | 13 | 37 | 37 | 44 | 0.04 | 0.04, 2.38, 5.62, 8.41 |
| TR (ms) | 505 | 2200 | 2200 | 900 | 5.7 | 13.7 |
| Averages | 2 | 2 | 2 | 1.4 | 1 | 1 |
| flip angle (excitation) | 90 | 90 | 90 | PD Var §§ | 6 | 5 |
| matrix | 384 | 320 | 320 | 224 | 304 | 240 |
| in-plane resolution (mm) | 0.1 | 0.1 | 0.1 | 0.2 | 0.4 | 0.5 |
| Deep Resolve § | Yes | Yes | Yes | No | No | No |
| Parallel imaging technique | GRAPPA | GRAPPA | GRAPPA | Compressed Sensing | none | none |
| Parallel imaging acceleration factor | 3 | 2 | 2 | 5 | - | - |
| Fat suppression | none | Fat saturation | Fat saturation | SPAIR | Fat saturation | none |
| Bandwidth (Hz/px) | 303 | 252 | 252 | 413 | 715 | 906 |
| Acquisition time (min:s) | 02:32 | 02:00 | 02:00 | 04:18 | 03:51 | 05:57 |
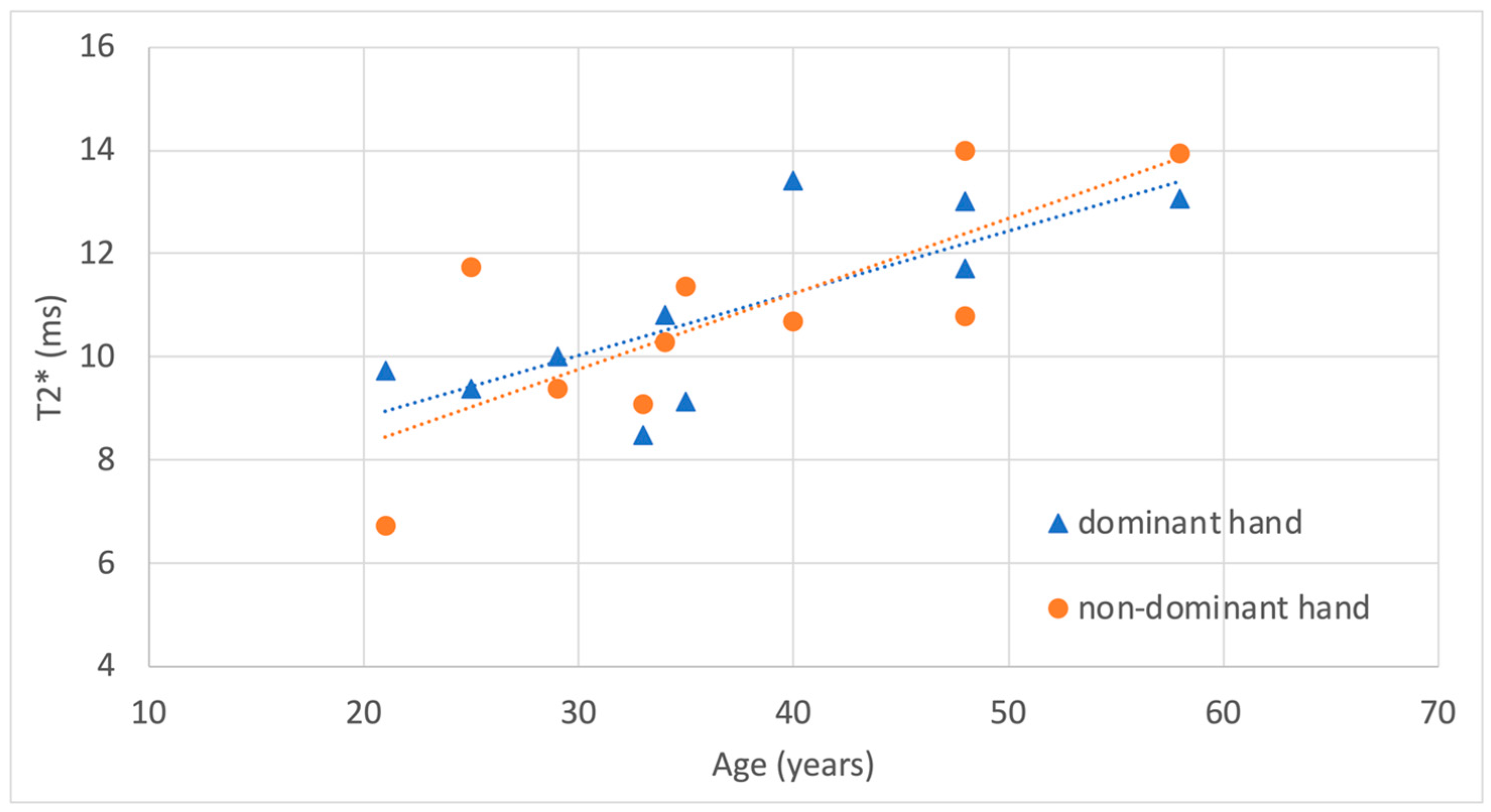
| Subject | Sex | Age (years) | Weight (kg) | Height (cm) | BMI | Side | ROI Volume (cm3) | Mean T2* (ms) | SD T2* (ms) | Dominant (D) or Non-Dominant (ND) Hand |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 34 | 83 | 180 | 26 | Right | 0.220 | 10.8 | 3.47 | D |
| 1 | M | 34 | 83 | 180 | 26 | Left | 0.105 | 10.3 | 2.65 | ND |
| 2 | M | 35 | 71 | 171 | 24 | Right | 0.188 | 9.1 | 3.14 | D |
| 2 | M | 35 | 71 | 171 | 24 | Left | 0.234 | 11.4 | 2.95 | ND |
| 3 | M | 33 | 57 | 170 | 23 | Right | 0.233 | 8.5 | 2.52 | D |
| 3 | M | 33 | 57 | 170 | 23 | Left | 0.255 | 9.1 | 2.32 | ND |
| 4 | M | 48 | 95 | 181 | 29 | Right | 0.121 | 13.0 | 6.64 | D |
| 4 | M | 48 | 95 | 181 | 29 | Left | 0.139 | 14.0 | 5.48 | ND |
| 5 | F | 21 | 58 | 168 | 21 | Right | 0.127 | 9.7 | 2.92 | D |
| 5 | F | 21 | 58 | 168 | 21 | Left | 0.175 | 6.7 | 2.24 | ND |
| 6 | M | 58 | 88 | 182 | 27 | Right | 0.188 | 13.1 | 5.63 | D |
| 6 | M | 58 | 88 | 182 | 27 | Left | 0.168 | 13.9 | 6.95 | ND |
| 7 | F | 29 | 57 | 165 | 21 | Right | 0.200 | 10.0 | 3.11 | D |
| 7 | F | 29 | 57 | 165 | 21 | Left | 0.210 | 9.4 | 2.53 | ND |
| 8 | F | 48 | 58 | 160 | 23 | Right | 0.115 | 11.7 | 3.68 | D |
| 8 | F | 48 | 58 | 160 | 23 | Left | 0.131 | 10.8 | 2.55 | ND |
| 9 | F | 40 | 62 | 169 | 22 | Right | 0.162 | 13.4 | 3.90 | D |
| 9 | F | 40 | 62 | 169 | 22 | Left | 0.131 | 10.7 | 3.64 | ND |
| 10 | M | 25 | 79 | 179 | 25 | Right | 0.268 | 9.4 | 3.60 | D |
| 10 | M | 25 | 79 | 179 | 25 | Left | 0.171 | 11.7 | 4.34 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudabbous, S.; Bouredoucen, H.; Ferreira Branco, D.; Sommer, S.; Hilbert, T.; Poletti, P.-A.; Salomir, R.; Delattre, B.M.A. Triangular Fibrocartilage Characterization with Ultrashort Echo Time-T2* MRI: Insights from a Healthy Cohort. Life 2025, 15, 1117. https://doi.org/10.3390/life15071117
Boudabbous S, Bouredoucen H, Ferreira Branco D, Sommer S, Hilbert T, Poletti P-A, Salomir R, Delattre BMA. Triangular Fibrocartilage Characterization with Ultrashort Echo Time-T2* MRI: Insights from a Healthy Cohort. Life. 2025; 15(7):1117. https://doi.org/10.3390/life15071117
Chicago/Turabian StyleBoudabbous, Sana, Hicham Bouredoucen, David Ferreira Branco, Stefan Sommer, Tom Hilbert, Pierre-Alexandre Poletti, Rares Salomir, and Bénédicte Marie Anne Delattre. 2025. "Triangular Fibrocartilage Characterization with Ultrashort Echo Time-T2* MRI: Insights from a Healthy Cohort" Life 15, no. 7: 1117. https://doi.org/10.3390/life15071117
APA StyleBoudabbous, S., Bouredoucen, H., Ferreira Branco, D., Sommer, S., Hilbert, T., Poletti, P.-A., Salomir, R., & Delattre, B. M. A. (2025). Triangular Fibrocartilage Characterization with Ultrashort Echo Time-T2* MRI: Insights from a Healthy Cohort. Life, 15(7), 1117. https://doi.org/10.3390/life15071117






