Serum p-Cresyl Sulfate Is Independently Associated with Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Analysis
2.3. Biochemical Investigations
2.4. Measurement of Serum PCS Levels by High-Performance Liquid Chromatography–Mass Spectrometry
2.5. Measurements of Blood Pressure (BP) and cfPWV
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARIC | Atherosclerosis Risk in Communities |
| AS | Aortic stiffness |
| AUC | Area under the curve |
| BMI | Body mass index |
| BP | Blood pressure |
| BUN | Blood urea nitrogen |
| CKD | Chronic kidney disease |
| CVD | CV disease |
| DBP | Diastolic blood pressure |
| DM | Diabetes mellitus |
| PCS | p-Cresyl sulfate |
| ROC | Receiver operating characteristic |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| UPCR | Urine protein-to-creatinine ratio |
References
- Navaneethan, S.D.; Schold, J.D.; Arrigain, S.; Jolly, S.E.; Nally, J.V. Cause-specific deaths in non-dialysis-dependent CKD. J. Am. Soc. Nephrol. 2015, 26, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N. Evolution of cardiovascular disease during the transition to end-stage renal disease. Semin. Nephrol. 2017, 37, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.M.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Yerram, P.; Karuparthi, P.R.; Hesemann, L.; Horst, J.; Whaley-Connell, A. Chronic kidney disease and cardiovascular risk. J. Am. Soc. Hypertens. 2007, 1, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.T.; Sippel, M.; Mukherjee, D. Interrelationship between chronic kidney disease and risk of cardiovascular diseases. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Chang, J.M.; Liu, W.C.; Tsai, Y.C.; Tsai, J.C.; Hsu, P.C.; Lin, T.H.; Lin, M.Y.; Su, H.M.; Hwang, S.J.; et al. Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 724–732. [Google Scholar] [CrossRef] [PubMed]
- London, G.M. Arterial stiffness in chronic kidney disease and end-stage renal disease. Blood Purif. 2018, 45, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.D.; Tanaka, H.; Ballew, S.H.; Sang, Y.; Heiss, G.; Coresh, J.; Matsushita, K. Associations between kidney disease measures and regional pulse wave velocity in a large community-based cohort: The Atherosclerosis Risk in Communities (ARIC) study. Am. J. Kidney Dis. 2018, 72, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R. Arterial stiffness in CKD: A review. Am. J. Kidney Dis. 2019, 73, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Obeid, H.; Soulat, G.; Mousseaux, E.; Laurent, S.; Stergiopulos, N.; Boutouyrie, P.; Segers, P. Numerical assessment and comparison of pulse wave velocity methods aiming at measuring aortic stiffness. Physiol. Meas. 2017, 38, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [PubMed]
- Niwińska, M.M.; Chlabicz, S. Evaluation of arterial stiffness parameters measurement with noninvasive methods—A systematic review. Cardiol. Res. Pract. 2024, 2024, 4944517. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Harlacher, E.; Wollenhaupt, J.; Baaten, C.C.F.M.J.; Noels, H. Impact of uremic toxins on endothelial dysfunction in chronic kidney disease: A systematic review. Int. J. Mol. Sci. 2022, 23, 531. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Miyamoto, Y.; Enoki, Y.; Ishima, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Tanaka, M.; Matsushita, K.; Mori, Y.; et al. p-Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol. Res. Perspect. 2015, 3, e00092. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Maré, A.; De Leger, W.; Meijers, B.; Meijers, B.; Verhulst, A.; Evenepoel, P.; Evenepoel, P.; et al. Indoxyl sulfate and p-cresyl sulfate promote vascular calcification and associate with glucose intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Six, I.; Flissi, N.; Lenglet, G.; Louvet, L.; Kamel, S.; Gallet, M.; Massy, Z.A.; Liabeuf, S. Uremic toxins and vascular dysfunction. Toxins 2020, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Vanholder, R.; Van Biesen, W.; Pletinck, A.; Schepers, E.; Neirynck, N.; Speeckaert, M.M.; De Bacquer, D.; Verbeke, F. Free p-cresyl sulfate shows the highest association with cardiovascular outcome in chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.W.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, P. Current sample size conventions: Flaws, harms, and alternatives. BMC Med. 2010, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Wu, T.J.; Wu, D.A.; Hsu, B.G. Hypoadiponectinemia is associated with aortic stiffness in nondialysis diabetic patients with stage 3–5 chronic kidney disease. Vascular 2022, 30, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Y.; Hsu, B.G.; Lin, Y.L.; Wang, C.H.; Lai, Y.H. Serum adipocyte fatty acid-binding protein level is positively associated with aortic stiffness in nondialysis chronic kidney disease patients: A cross-sectional study. Medicine 2022, 101, e29558. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Chen, M.S.; Lin, Y.L.; Hsu, B.G. Resistin: A potential indicator of aortic stiffness in non-dialysis chronic kidney disease patients. Medicina 2023, 59, 1652. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, Y.L.; Lai, Y.H.; Wang, C.H.; Hsu, B.G. Serum p-cresyl sulfate level is an independent marker of peripheral arterial stiffness as assessed using brachial-ankle pulse wave velocity in patients with non-dialysis chronic kidney disease stage 3 to 5. Toxins 2022, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.Y.; Lin, Y.L.; Chen, Y.H.; Hung, S.C.; Tsai, J.P.; Hsu, B.G. Positive association of serum galectin-3 with the development of aortic stiffness of patients on peritoneal dialysis. J. Clin. Med. 2023, 12, 3519. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. Blood Press. 2014, 23, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pechlaner, R.; Cai, J.; Yuan, H.; Huang, Z.; Yang, G.; Wang, J.; Chen, Z.; Kiechl, S.; Xu, Q.; et al. Trajectories of age-related arterial stiffness in Chinese men and women. J. Am. Coll. Cardiol. 2020, 75, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Xu, L.; Li, F.; Xie, X.; Guan, X.; Zhan, X.; Wu, C.; Yang, H.; Wang, X.; Wang, Y.; et al. Structural and load-dependent arterial stiffness across the adult life span. J. Hypertens. 2025, 43, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial stiffness and cardiovascular risk in hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Shiina, K.; Takahashi, T.; Fujii, M.; Iwasaki, Y.; Matsumoto, H.; Yamashina, A.; Chikamori, T.; Tomiyama, H. Bidirectional longitudinal relationships between arterial stiffness and hypertension are independent of those between arterial stiffness and diabetes: A large-scale prospective observational study in employees of a Japanese company. J. Am. Heart Assoc. 2022, 11, e025924. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E. Arterial stiffness as a risk factor for clinical hypertension. Nat. Rev. Cardiol. 2018, 15, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Iseki, K.; Iseki, C.; Miyagi, T.; Kinjo, K.; Takishita, S. Increased pulse wave velocity is associated with low creatinine clearance and proteinuria in a screened cohort. Am. J. Kidney Dis. 2006, 47, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.R.; Roos, M.; Schmaderer, C.; Lutz, J.; Wang, J.G.; Heemann, U.; Baumann, M. Interrelationship between aortic stiffness and proteinuria in chronic kidney disease. J. Hum. Hypertens. 2010, 24, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Gong, J.; Wang, T.; Luo, L.; Lian, G.; Wang, H.; Chen, W.; Xie, L. Relationship between high-normal albuminuria and arterial stiffness in Chinese population. J. Clin. Hypertens. 2020, 22, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, D.; Nannipieri, M.; Armenia, S.; Taddei, S.; Solini, A.; Bruno, R.M. Non-albumin proteinuria marks tubular involvement and is associated with arterial stiffness in subjects affected by severe obesity. Obes. Res. Clin. Pract. 2023, 17, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R.; Townsend, R.R.; Fink, J.C.; Teal, V.; Anderson, C.A.M.; Appel, L.J.; Chen, J.; He, J.; Litbarg, N.; Ojo, A.O.; et al. Hemodynamic correlates of proteinuria in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Lin, C.C.; Huang, Y.Y.; Chen, J.Y.; Chen, J.H. Association of increased arterial stiffness and inflammation with proteinuria and left ventricular hypertrophy in non-diabetic hypertensive patients. Blood Press. 2007, 16, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Lentini, P.; Briet, M.; Castellino, P.; House, A.A.; London, G.M.; Malatino, L.; McCullough, P.A.; Mikhailidis, D.P.; Boutouyrie, P. Arterial stiffness in the heart disease of CKD. J. Am. Soc. Nephrol. 2019, 30, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.J.; Wei, R.; Zhao, J.; Su, T.Y.; Li, Q.; Yang, X.; Chen, X. Albuminuria and endothelial dysfunction in patients with non-diabetic chronic kidney disease. Med. Sci. Monit. 2017, 23, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, L.; Lentini, P.; Sessa, C.; Castellino, N.; D’Anca, A.; Torrisi, I.; Marcantoni, C.; Castellino, P.; Santoro, D.; Zanoli, L. Inflammation and arterial stiffness as drivers of cardiovascular risk in kidney disease. Cardiorenal. Med. 2024, 15, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Kanno, Y.; Takenaka, T. Central blood pressure and chronic kidney disease. World J. Nephrol. 2016, 5, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Campbell, K.L.; Johnson, D.W.; Stanton, T.; Vesey, D.A.; Coombes, J.S.; Weston, K.S.; Hawley, C.M.; McWhinney, B.; Ungerer, J.P.J.; et al. Protein-bound uremic toxins, inflammation and oxidative stress: A cross-sectional study in stage 3–4 chronic kidney disease. Arch. Med. Res. 2014, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.J.; Ni, J.W.; Ding, F.H.; Fang, Y.H.; Wang, X.Q.; Wang, H.B.; Chen, X.N.; Chen, N.; Zhan, W.W.; Lu, L.; et al. p-Cresyl sulfate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in apoE−/− mice. Kidney Int. 2016, 89, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; D’Haese, P.C.; Verhulst, A. Molecular and cellular mechanisms that induce arterial calcification by indoxyl sulfate and p-cresyl sulfate. Toxins 2020, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Kuypers, D.; Augustijns, P.; Meijers, B. Metabolism, protein binding, and renal clearance of microbiota–derived p-cresol in patients with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Mair, R.D.; Lee, S.; Plummer, N.S.; Sirich, T.L.; Meyer, T.W. Impaired tubular secretion of organic solutes in advanced chronic kidney disease. J. Am. Soc. Nephrol. 2021, 32, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.F.; Hsieh, C.Y.; Liou, J.C.; Liu, S.H.; Hung, C.F.; Lu, K.C.; Lin, C.C.; Wu, C.C.; Ka, S.M.; Wen, L.L.; et al. Scavenging intracellular ROS attenuates p-cresyl sulfate-triggered osteogenesis through MAPK signaling pathway and NF-κB activation in human arterial smooth muscle cells. Toxins 2020, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Saigusa, D.; Mishima, E.; Uchida, T.; Miura, D.; Morikawa-Ichinose, T.; Kisu, K.; Sekimoto, A.; Saito, R.; Oe, Y.; et al. Impact of the oral adsorbent AST-120 on organ-specific accumulation of uremic toxins: LC-MS/MS and MS imaging techniques. Toxins 2017, 10, 19. [Google Scholar] [CrossRef] [PubMed]
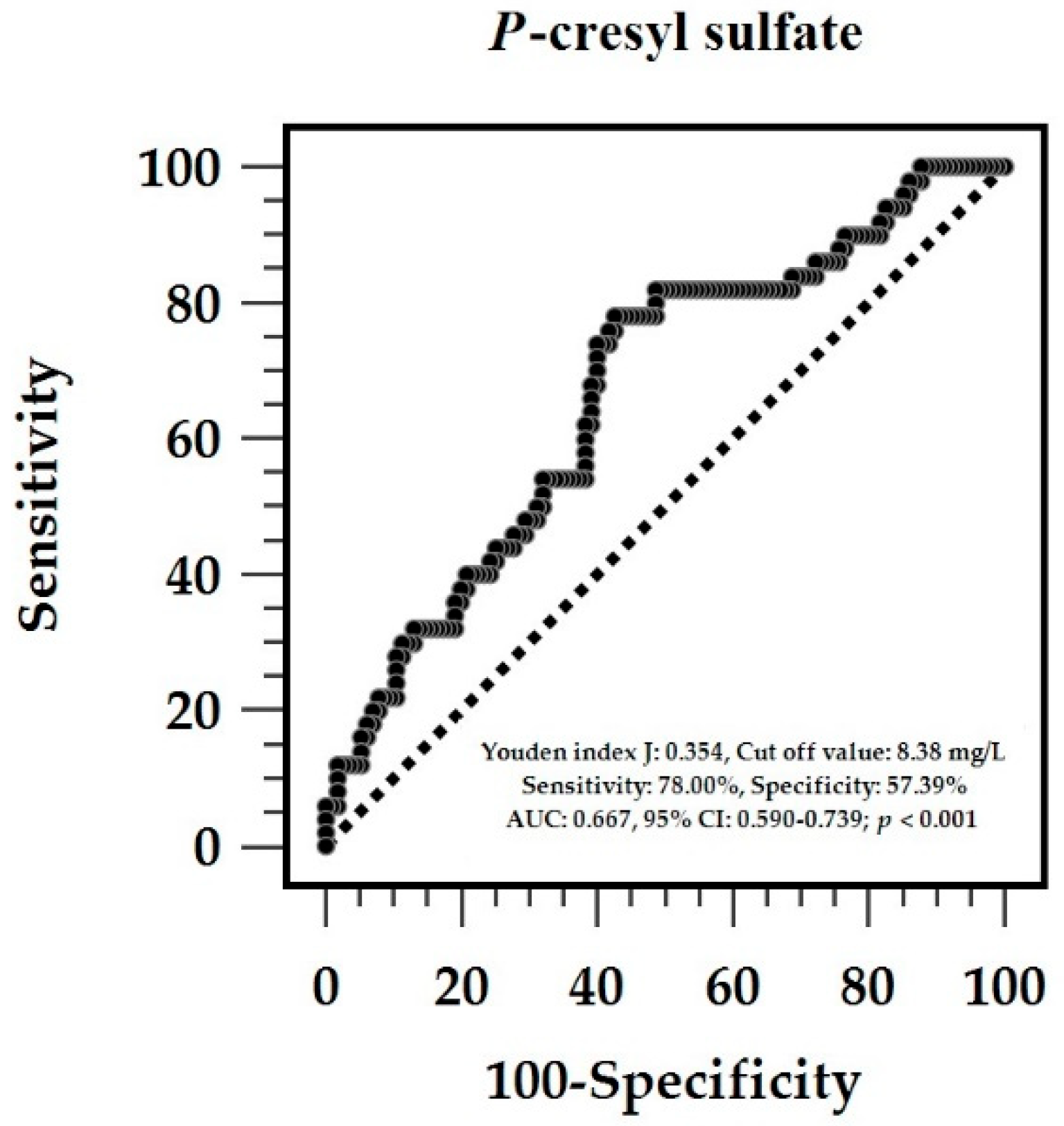
| Characteristics | All Patients (n = 165) | Control Group (n = 115) | Aortic Stiffness Group (n = 50) | p-Value |
|---|---|---|---|---|
| Age (years) | 65.71 ± 13.98 | 63.07 ± 13.87 | 71.78 ± 12.35 | <0.001 * |
| Body mass index (kg/m2) | 26.01 ± 4.65 | 25.63 ± 4.73 | 26.90 ± 4.38 | 0.108 |
| cfPWV (m/s) | 9.26 ± 2.94 | 7.74 ± 1.45 | 12.75 ± 2.49 | <0.001 * |
| SBP (mmHg) | 147.27 ± 24.45 | 144.24 ± 22.46 | 154.22 ± 27.50 | 0.016 * |
| DBP (mmHg) | 83.88 ± 13.78 | 83.26 ± 12.86 | 85.30 ± 15.75 | 0.384 |
| Total cholesterol (mg/dL) | 159.10 ± 40.59 | 156.56 ± 40.94 | 164.96 ± 39.56 | 0.223 |
| Triglyceride (mg/dL) | 119.0 (90.00–165.00) | 116.00 (87.00–162.00) | 133.50 (97.00–178.50) | 0.119 |
| LDL-C (mg/dL) | 87.01 ± 33.72 | 84.94 ± 34.41 | 91.78 ± 31.92 | 0.232 |
| Fasting glucose (mg/dL) | 110.00 (98.50–139.50) | 108.00 (97.00–132.00) | 122.00 (100.75–166.00) | 0.008 * |
| Blood urea nitrogen (mg/dL) | 29.00 (22.00–43.50) | 28.00 (22.00–44.00) | 32.00 (22.75–43.25) | 0.631 |
| Creatinine (mg/dL) | 1.70 (1.30–2.55) | 1.70 (1.20–2.60) | 1.80 (1.40–2.43) | 0.419 |
| eGFR (mL/min) | 38.76 ± 24.96 | 40.13 ± 25.66 | 35.56 ± 23.18 | 0.279 |
| Spot UPCR (g/g) | 0.53 (0.19–1.20) | 0.45 (0.16–1.08) | 0.65 (0.24–1.95) | 0.048 * |
| Total p-Cresyl sulfate (1 mg/L) | 8.64 (6.25–12.63) | 7.68 (6.08–11.63) | 10.45 (8.53–15.34) | 0.001 * |
| Female, n (%) | 77 (46.7) | 56 (48.7) | 21 (42.0) | 0.428 |
| Diabetes mellitus, n (%) | 69 (41.8) | 42 (36.5) | 27 (54.0) | 0.036 * |
| Hypertension, n (%) | 134 (81.2) | 93 (80.9) | 41 (82.0) | 0.864 |
| Glomerulonephritis, n (%) | 41 (24.8) | 31 (27.0) | 10 (20.0) | 0.342 |
| ARB use, n (%) | 103 (62.4) | 75 (65.2) | 28 (56.0) | 0.261 |
| β-blocker use, n (%) | 46 (27.9) | 32 (27.8) | 14 (28.0) | 0.982 |
| CCB use, n (%) | 68 (41.2) | 45 (39.1) | 23 (46.0) | 0.410 |
| Statin use, n (%) | 80 (48.5) | 54 (47.0) | 26 (52.0) | 0.551 |
| Fibrate use, n (%) | 47 (28.5) | 30 (26.1) | 17 (34.0) | 0.301 |
| CKD stage 1–2 | 26 (15.8) | 20 (17.4) | 6 (12.0) | 0.775 |
| CKD stage 3 | 65 (39.4) | 46 (40.0) | 19 (38.0) | |
| CKD stage 4 | 44 (26.7) | 29 (25.2) | 15 (30.0) | |
| CKD stage 5 | 30 (18.2) | 20 (17.4) | 10 (20.0) |
| Clinical Variables | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Total p-Cresyl sulfate, 1 mg/L | 1.097 | 1.024–1.175 | 0.008 * |
| Age, 1 year | 1.057 | 1.025–1.090 | <0.001 * |
| Systolic blood pressure, 1 mmHg | 1.006 | 0.989–1.023 | 0.477 |
| Diabetes mellitus, present | 1.275 | 0.487–3.366 | 0.624 |
| Fasting glucose, 1 mg/dL | 1.006 | 0.996–1.017 | 0.214 |
| Spot UPCR, 1 g/g | 1.108 | 0.871–1.410 | 0.404 |
| Clinical Variables | Carotid–Femoral Pulse Wave Velocity (m/s) | ||||
|---|---|---|---|---|---|
| Univariate Regression | Multivariate Regression | ||||
| r | p-Value | β | Adjusted R2 Change | p-Value | |
| Age (years) | 0.328 | <0.001 * | 0.311 | 0.102 | <0.001 * |
| Body mass index (kg/m2) | 0.107 | 0.170 | – | – | – |
| SBP (mmHg) | 0.282 | <0.001 * | 0.164 | 0.058 | 0.001 * |
| DBP (mmHg) | 0.126 | 0.106 | – | – | – |
| Total cholesterol (mg/dL) | 0.021 | 0.793 | – | – | – |
| Log-Triglyceride (mg/dL) | 0.025 | 0.754 | – | – | – |
| LDL-C (mg/dL) | 0.023 | 0.772 | – | – | – |
| Log-Glucose (mg/dL) | 0.166 | 0.033 * | – | – | – |
| Log-BUN (mg/dL) | 0.084 | 0.283 | – | – | – |
| Log-Creatinine (mg/dL) | 0.142 | 0.068 | – | – | – |
| eGFR (mL/min) | −0.150 | 0.054 | – | – | – |
| Log-UPCR (g/g) | 0.191 | 0.014 * | 0.148 | 0.015 | 0.046 * |
| Log-PCS (mg/L) | 0.275 | <0.001 * | 0.181 | 0.032 | 0.007 * |
| Variables | Spearman’s Correlation Coefficient | p-Value |
|---|---|---|
| Age (years) | 0.087 | 0.269 |
| Body mass index (kg/m2) | 0.079 | 0.313 |
| SBP (mmHg) | 0.261 | 0.001 * |
| DBP (mmHg) | 0.120 | 0.124 |
| Total cholesterol (mg/dL) | −0.031 | 0.689 |
| Log-Triglyceride (mg/dL) | 0.009 | 0.910 |
| LDL-C (mg/dL) | 0.068 | 0.383 |
| Log-Glucose (mg/dL) | 0.135 | 0.083 |
| Log-BUN (mg/dL) | 0.164 | 0.035 * |
| Log-Creatinine (mg/dL) | 0.209 | 0.007 * |
| eGFR (mL/min) | −0.170 | 0.029 * |
| Log-UPCR | 0.167 | 0.032 * |
| Carotid–femoral PWV (m/s) | 0.275 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chern, Y.-B.; Chia, K.L.; Liu, C.-H.; Lin, Y.-L.; Tsai, J.-P.; Hsu, B.-G. Serum p-Cresyl Sulfate Is Independently Associated with Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients. Life 2025, 15, 1116. https://doi.org/10.3390/life15071116
Chern Y-B, Chia KL, Liu C-H, Lin Y-L, Tsai J-P, Hsu B-G. Serum p-Cresyl Sulfate Is Independently Associated with Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients. Life. 2025; 15(7):1116. https://doi.org/10.3390/life15071116
Chicago/Turabian StyleChern, Yahn-Bor, Ken Lee Chia, Chin-Hung Liu, Yu-Li Lin, Jen-Pi Tsai, and Bang-Gee Hsu. 2025. "Serum p-Cresyl Sulfate Is Independently Associated with Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients" Life 15, no. 7: 1116. https://doi.org/10.3390/life15071116
APA StyleChern, Y.-B., Chia, K. L., Liu, C.-H., Lin, Y.-L., Tsai, J.-P., & Hsu, B.-G. (2025). Serum p-Cresyl Sulfate Is Independently Associated with Aortic Stiffness in Non-Dialysis Chronic Kidney Disease Patients. Life, 15(7), 1116. https://doi.org/10.3390/life15071116








